Abstract
Epilepsy, a prevalent neurological disorder characterized by high morbidity, frequent recurrence, and potential drug resistance, profoundly affects millions of people globally. Understanding the microscopic mechanisms underlying seizures is crucial for effective epilepsy treatment, and a thorough understanding of the intricate neural circuits underlying epilepsy is vital for the development of targeted therapies and the enhancement of clinical outcomes. This review begins with an exploration of the historical evolution of techniques used in studying neural circuits related to epilepsy. It then provides an extensive overview of diverse techniques employed in this domain, discussing their fundamental principles, strengths, limitations, as well as their application. Additionally, the synthesis of multiple techniques to unveil the complexity of neural circuits is summarized. Finally, this review also presents targeted drug therapies associated with epileptic neural circuits. By providing a critical assessment of methodologies used in the study of epileptic neural circuits, this review seeks to enhance the understanding of these techniques, stimulate innovative approaches for unraveling epilepsy's complexities, and ultimately facilitate improved treatment and clinical translation for epilepsy.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epilepsy, a neurological disorder characterized by recurrent and unprovoked seizures, is a result of anomalous neural activities in the brain (Beghi et al. 2015; Beghi 2020). Globally, this disorder currently affects an estimated 65 million individuals (Akyuz et al. 2021). Understanding the microscopic mechanisms underlying seizures is crucial for effective epilepsy treatment, although the etiology of epilepsy is notably complex. It encompasses a vast array of modifications in neural circuits, which span from molecular alterations to network-level changes (Laxpati et al. 2014). Neural circuits are intricate connections formed by neurons of diverse properties and functions within the brain (Zhang et al. 2021b; Lerner et al. 2016). Neurons with distinct properties and functions establish complex connections in various forms, constituting neural circuits and neural networks at different levels (Malezieux et al. 2023). These activities take various forms, such as series, parallel, feedforward and feedback (positive or negative) (Sharpee et al. 2016). In recent years, the emergence of advanced techniques, such as viral tracing, optogenetics, and chemogenetics, has facilitated a deeper understanding of the intricate neural networks implicated in epilepsy (** neural circuits, which is due to their ability to replicate within infected neurons and to label hierarchical neural circuits through self-amplification (Kuypers and Ugolini 1990; Martin and Dolivo 1983). Subsequently, the emergence of an attenuated strain of PRV demonstrated highly specific retrograde transneuronal infections (Enquist 2002; Loewy 1998). Further refinements to the Bartha PRV have resulted in a potent tool for retrograde viral tracing, lending itself to the analysis of neural circuits in non-primate species (Jia et al. 2019). Genetic modifications to the VSV and HSV have also enhanced their utility in neural circuits studies (van den Pol et al. 2009; Beier et al. 2011, 2013). In recent years, advancements in the rabies virus modification techniques have made it an invaluable tool, capable of expressing numerous essential genes to correlate circuits to function (Sun et al. 2019c; Suzuki et al. 2019).
Optogenetics and chemogenetics are two modulatable techniques with extensive applications in manipulating neural circuits. Their widespread acceptance and application in neural circuit studies have been instrumental in our current understanding of the neural circuits involved in epilepsy. The first chemogenetic approach was demonstrated in 1911, with its great potential presented (Strader et al. 1991). Then, the second-generation tools called receptors activated exclusively by synthetic ligands, engineered from native G-protein-coupled receptors, were described (Coward et al. 1998; Chang et al. 2007). Since designer receptors exclusively activated by designer drugs (DREADDs) were first pointed out in 2007, DREADDs-based approaches have been widely adopted and improved (Armbruster et al. 2007; Vardy et al. 2015). In parallel to the development of DREADDs, ion channel-based approaches were also developed (Lerchner et al. 1998; Nietz et al. 2022). In conclusion, calcium imaging is instrumental in identifying the network nodes outside the seizure locus that are responsible for seizure initiation, propagation, and termination. It plays a significant role in the detection of calcium ions in organisms at cellular and even subcellular structure levels, providing high spatial and temporal resolution.
In Vivo Microdialysis
In vivo microdialysis serves as an essential technique for sampling the immediate vicinity of the catheter tip to identify abnormal concentrations of specific molecules in the extracellular milieu of the brain (Zestos et al. 2019). These abnormal concentrations can provide insights into the etiology of numerous brain diseases, including Huntington's disease, Parkinson's disease, Alzheimer's disease, schizophrenia, traumatic brain injury, stroke, depression, and epilepsy (Di Giovanni et al. 2009; Zestos et al. 2019; Todd and Butterworth 2001; Bjorkli et al. 2021; McAdoo and Wu 2008) (Table 4).
In vivo microdialysis has emerged as an essential tool in revealing key aspects of epilepsy, particularly in showing how treatment can influence seizure activity (Zestos et al. 2019). This technique has been instrumental in tracing changes in endogenous substances, such as excitatory and inhibitory neurotransmitters and their metabolites, at various stages: before, during, and after seizures. Luna-Munguia et al. integrated state-of-the-art microdialysis techniques with an innovative model of ictogenesis, enabling simultaneous monitoring of 24 molecules during ictogenesis to discern the chemical shifts associated with epileptogenesis and ictogenesis (Luna-Munguia et al. 2019). Similarly, Fukuyama et al. utilized multiprobe microdialysis in conjunction with ultra-high-performance liquid chromatography to examine transmission abnormalities of L-glutamate and GABA in the thalamocortical pathway of transgenic rats bearing rat S286L-mutant Chrna4 (S286L-TG) (Fukuyama et al. 2020b). In a separate study, the same team implanted a concentric direct-insertion type dialysis probe in the orbitofrontal cortex of rats after post-AMPA-evoked stimulation (Fukuyama et al. 2020a). Dhaher et al. merged brain microdialysis with mass spectrometry to investigate seizure initiation, seizure propagation, and extracellular brain levels of glutamate and GABA (Dhaher et al. 2021). Besides, Shimoda et al. employed a microdialysis probe inserted into the CA3 hippocampus via a guide cannula, preserving collected samples using liquid chromatography-tandem mass spectrometry (Shimoda et al. 2022).
The main limitations of in vivo microdialysis are the size of the probe and the damage to the tissue caused by inserting the probe. Besides, since certain neurotransmitters are low in brain dialysate, samples must be taken at regular intervals, a time scale which is far removed from the time scale of neuronal events (Holmgaard et al. 2010; Zestos et al. 2019). For in vivo neurochemical monitoring, microdialysis is often paired with chromatographic, electrophoretic, or enzymatic assays (Tan et al. 2021). Of these, liquid chromatography, mass spectrometry, and their tandem applications are most frequently employed for a detailed examination of in vivo neurochemistry during seizures (Kennedy 2013). Given the challenges related to optogenetic neurochemical monitoring, such as the requirement for high sampling rate analytical methods and in vivo neurochemical monitoring during optogenetic neuronal stimulation, the fusion of optogenetics with microdialysis sampling might offer an enlightening approach to measure neurochemical alterations before, during, and after controlled epileptic seizures (Parrot et al. 2015).
Fiber Photometry
In recent years, fiber photometry has become a popular technique for in vivo neural recording, favored for its simplicity and specificity (Legaria et al. 2022). Its primary aim is to elucidate the real-time functions of distinct neural pathways in mammalian behavior by optically recording natural neural activity in genetically specified and connectivity-defined projections (Zhang et al. 2021a). The sensitivity of this recording technique enables the in vivo direct measurement of coordinated neuronal afferent activities projecting to a specific downstream target deep within the brain of a mammal engaged in behavior. This is achieved by the application of genetically encoded Ca2+ indicators and advanced photometric devices (Legaria et al. 2022). Table 5 summarizes the applications of Fiber photometry techniques in epilepsy neural circuits in recent years.
Utilizing the power of fiber photometry-based calcium recording, Zhang et al. analyzed the relationship between epileptic seizures and brain calcium activities in freely behaving mice, presenting a simple procedure with minimal damage to brain tissues (Zhang et al. 2019b). Additionally, Cheng et al. integrated optogenetics and DBS with fiber photometry, using a fiber photometry system (Nan**g Thinkertech), during hippocampal kindling (Chen et al. 2020). They examined the role of dorsal raphe and its 5-HTergic neurons in epilepsy by bandpass filtering and collecting GCaMP fluorescence with a photomultiplier tube. In a separate study, researchers combined fiber photometry with c-fos map** to identify numerous hyperactivated CR neurons in the PIL. This approach helped to investigate the causal roles of these neurons in hippocampal seizures (Qi et al. 2022). To further illustrate the utility of fiber photometry, Xu et al. combined in vitro c-fos staining with in vivo fiber photometry recordings to monitor activity changes in the paraventricular thalamic nucleus (Xu et al. 2022). Besides, Murphy et al. pioneered a new tool termed the Fiber Photometry Coupled Focused Ultrasound System (PhoCUS), which allows for simultaneous monitoring of focused ultrasound effects on neural activities of subcortical genetically targeted cell types in freely behaving animals (Murphy et al. 2022). This advancement provides a new way to investigate focused ultrasound effects on specific cell types in deep brain regions.
Evidently, fiber photometry offers a promising method for advancing studies into the pathogenesis of epilepsy and epileptiform signal transmission. This is attributable to its exceptional sensitivity and high-resolution detection capabilities, especially regarding calcium signal detection in multiple brain regions. However, similar to electrophysiology, fiber photometry faces the problems of low resolution and limited range of brain area monitoring, and its difficulty in controlling the depth of captured signals, which are worthy of subsequent research and improvement (Mineur and Picciotto 2023).
Virus Tracing Technology
Due to the collaborative efforts across various disciplines, particularly the combined progress in molecular biology, virology, microscopy, computer science, and genetics, effective advancements have been achieved in the methodological aspects of neuron tracing. Viral tracing techniques are a hallmark product of this progress. In the past twenty years, viral tracing techniques have garnered a surge of interest in neural circuitry studies, especially in epilepsy studies (Table 6). They exhibit a unique ability to deliver tracers in a cell-type-specific and circuit-selective fashion, employing cis-, retrograde, and trans-synaptic methods (Liu et al. 2022).
Innovative studies have applied rabies tracing techniques to explore neural circuits relevant to epilepsy with some success. Studer et al. utilized rabies virus-mediated retrograde synaptic tracing to investigate a mouse model of atonic epilepsy (Studer et al. 2022). Their results showed that cortical neurons, located in both the superficial and deep layers of the whisker primary somatosensory cortex, demonstrated significantly increased and abnormal connectivity in a genetic model of anhedonic epilepsy. This suggests that the enhanced structural connectivity patterns of these neurons might be a crucial pathological basis for increased neuronal synchronization and the generation of anhedonic seizures. In sum, this research offers new insights into the relationship between neural hypersynchronization and abnormal structural connectivity in neuronal networks during epileptic seizures.
Rabies virus-mediated presumptive retrograde trans-synaptic tracing is frequently combined with other tracing techniques for improved performance, such as alternative viral tracing methods, optogenetics, and chemogenetics (Islam et al. 2022; Kato and Kobayashi 2020; Wang et al. 2020). In an experimental rat model of medial temporal lobe epilepsy (mTLE) induced by trichothecene, Du et al. implemented a dual viral tracing strategy, which combined retroviral birth dating with rabies virus-mediated presumptive retrograde trans-synaptic tracing (Du et al. 2017). They observed substantial remodeling of the hippocampal circuit following epileptogenic injury, causing DGCs to generate significant excitatory monosynaptic inputs, such as local recurrence and widespread feedback loops. A similar outcome was achieved by Zhou et al., who integrated rabies virus tracing techniques with chemogenetic approaches (Zhou et al. 2019). Future research could consider utilizing other tracing techniques or examining unexplored subtypes of interneurons.
Recently, Wang et al. integrated optogenetics and chemogenetics with a combination of in vivo and in vitro electrophysiology, as well as retrograde rabies virus tracing (Wang et al. 2020). This innovative combination helped to elucidate a direct septo-hippocampal cholinergic circuit, which efficiently mitigates seizures by instigating growth inhibitor inhibition in an animal model of TLE. This study improves our understandings of alterations in seizure circuits and their precise spatiotemporal controls. Despite these advancements, few studies have documented brain circuits activities during seizures through recombinant adenoviral methodologies. Currently, lentiviruses and rabies viruses are widely used in the tracing of neural circuits (Kato and Kobayashi 2020; Deng et al. 2022). It is crucial to choose different viral vectors based on specific research objectives.
There are also several limitations in virus tracing technologies. Due to the specificity of the vector, it often faces biosafety issues such as neurotoxicity (Sun et al. 2021; Christenson Wick et al. 2017). Chen et al. investigated the complex circuitry of TLE, suggesting that the long-range SNr-PF inhibitory circuit is involved in regulating seizures in TLE and the deactivation of this circuit could lessen seizure severity (Chen et al. 2020). In a comparative study of pathological changes in the medial septum's (MS) hippocampal circuits between TLE patients and healthy individuals, Wang et al. discovered that direct MS-hippocampal cholinergic projections mediated antiepileptic effects by preferentially targeting hippocampal GABAergic neurons, underscoring the specific role of the MS-hippocampal cholinergic circuit in TLE (Wang et al. 2020). Additionally, aberrant sparse activity of hippocampal dentate gyrus (DG) granule cells (GCs) has been found to contribute to TLE, with recent optogenetic evidence indicating that the axons of glutamatergic mossy cells play a pivotal role in this process (Botterill et al. 2019). These studies hold significant potential for precisely targeting epilepsy.
Furthering the integration of optogenetics with viral tracing techniques, Sere et al. investigated the role of lateral hypothalamic neurons (LH) in dyscalculia epilepsy (Sere et al. 2021). The study illustrated that LH neurons extend to the dorsal raphe nucleus (DRN) in the brainstem, with a subset being GABAergic. It confirmed the presence of long-distance subcortical modulation of serotonergic neuromodulation during anhedonic seizures, enhancing our understanding of seizure complexities. It is known that pulmonary portal mossy cells can influence hippocampal circuits function, and substantial loss of these cells is associated with alterations in the same circuits during epileptic states. Optogenetics has been instrumental in delineating this involved microcircuitry (Butler et al. 2022). Seeking to clarify how abnormal neural circuits contribute to epilepsy, Yang et al. examined circuit mechanisms in a mouse model of focal cortical malformations through optogenetic stimulation (Yang et al. 2021). Their findings revealed site-specific and cell-type-specific synaptic reorganization within epileptic cortical circuits, highlighting the need for more extensive research to understand the prevalence and overall landscape of these abnormal circuits. Besides, recent work has demonstrated that calretinin (CR) neurons in the posterior intralaminar thalamic nucleus (PIL) exhibit varying influences on hippocampal seizures via distinct downstream circuits, adding to our understanding of the PIL-lateral amygdala CR circuit (Qi et al. 2022).
Optogenetics offers the ability to accurately activate or inhibit-specific neurons, serving as a research tool and potential therapeutic strategy for managing aberrant neuronal activities. GABAergic neurons have been proposed as novel targets for optogenetic modulation of temporal lobe seizures. As the scope of animal studies and clinical treatment broadens, optogenetics holds considerable promise in elucidating epileptogenesis, seizure onset, and pathological circuits, which could potentially open avenues for alternative therapeutic strategies such as gene therapy. The study of neuronal interactions during seizures with the aid of optogenetics has garnered increasing interest and is expected to become a central area of investigation in epilepsy research.
Optogenetics also faces some dilemmas. The expression of photosensitive proteins is not uniform across neuronal populations, which can lead to heterogeneity in the magnitude and scope of optogenetic manipulation (Melonakos et al. 2020). In addition, the simultaneous action of a large range of light stimuli on a population of neurons may cause circuits to show non-physiological patterns of activity (Peralvárez-Marín and Garriga 2016). And because of the complexity of neural circuits, optogenetics is more difficult to apply to the primate brain, which limits its possible wide application (Merlin and Vidyasagar 2023).
Chemogenetics
Similar to optogenetics, chemogenetics is widely applied in neuroscience for the modulation of specific neurons or neural circuits. Chemogenetics is a method which utilizes gene technology and artificially designed drugs to alter the function of neurons (Poth et al. 2021; Magnus et al. 2019). It is commonly employed to explore the function and activity dependence of neurons. This technique involves the introduction of engineered receptors or channels, which selectively respond to synthetic ligands, thereby modulating cellular signaling pathways. Table 8 summarizes the recent applications of chemogenetics in the neural circuits of epilepsy. Chemogenetics can either be direct, through the utilization of synthetic ligand-gated ion channels, or indirect, by leveraging DREADDs, tools derived from G-protein-coupled receptors (Oyrer et al. 2018). DREADDs have gained popularity as tools of chemogenetics in studying the neural circuits of epilepsy due to their capability to either inhibit or excite neurons. This is achieved through the expression of designer receptors which respond to exogenous compounds such as clozapine N-oxide or olanzapine.
Chemogenetic tools have proven instrumental in the selective activation or silencing of pathways in a specific population of neurons. It has greatly facilitated the investigation of anatomically constrained neural circuits in epilepsy and the development of high-resolution circuit maps associated with seizures. Chemogenetics has illuminated the circuit-based mechanisms underlying seizures. For instance, Kahn et al. used DREADDs to manipulate the activity of dentate gyrus cells (DGCs) to target the aggregate output of the dentate gyrus, thereby demonstrating the importance of fine-tuning activity within this region for cognitive performance (Kahn et al. 2019). In addition, Zhou et al. have underscored the crucial role of newborn DGCs in the hippocampus in the formation of epileptic neural circuits and the initiation of spontaneous recurrent seizures (Zhou et al. 2019). Through the application of chemogenetic methodologies, circuits such as the nigra-parafascicular disinhibitory circuit, which regulates seizures in TLE, and the dHPC dorsal lateral septum circuit that impairs risk assessment behavior, have been identified and evaluated (Chen et al. 2020; Cao et al. 2022). Projections from the mediodorsal nucleus to the prelimbic cortex have also been implicated in the propagation of amygdala-kindled seizures (Wicker et al. 2022). An increasing number of studies are leveraging chemogenetics for the specific activation and inhibition of neurons associated with particular circuits, thus assessing the impact of chemogenetic interventions on epileptic activity (Berglind et al. 2018). Furthermore, develo** novel DREADDs ligands and investigating new mouse models are subjects of current research, and improvements in these areas are anticipated (Desloovere et al. 2022).
Interneurons have garnered attention as pivotal components for understanding the neural circuits involved in epilepsy, with particular focus on the three main interneuron populations in the rodent hippocampus: parvalbumin (PV), somatostatin (SST), and vasoactive intestinal peptide expressing interneurons (Miles et al. 1996; Paz and Huguenard 2015; Lovett-Barron et al. 2012). Cǎlin et al. utilized excitatory DREADDs to activate different interneuron subtypes, finding that their ability to increase postsynaptic inhibition in principal neurons and reduce epileptiform synchronization in neuronal networks varies. However, all these subtypes may serve as effective anticonvulsant strategies (Călin et al. 2018). Besides, Panthi and Leitch focused on the activation of PV + interneurons using DREADDs, and they administered clozapine N-oxide to activate these interneurons (Panthi and Leitch 2021). Their study suggested that FFI of PV + interneurons within cortico-thalamocortical microcircuits could inspire new advancements in anti-absence seizure treatment. A separate study emphasized the role of DG PV-INs, proposing that the inhibitory function of PV-INs could be a promising therapeutic target for seizure modulation (Mattis et al. 2022). Notably, GqDREADD-mediated chemogenetic activation of wild-type SST interneurons could lead to an increase in spontaneous excitability and susceptibility to depolarization block, thereby contributing directly to seizures in vivo and highlighting the substantial role of SST interneurons in seizure events (Wengert et al. 2021).
Moreover, the field of chemogenetics is proving to be groundbreaking in making strides toward clinical translation in the treatment of epilepsy. Gene therapy, which enables the manipulation of neuronal activities in the epileptic focus while simultaneously preserving function, appears as a promising alternative to traditional pharmacological methods and surgical interventions for epilepsy. By expressing proteins that can be regulated by drugs, chemogenetics can serve as a titratable therapy that could potentially overcome challenges associated with traditional therapies that are limited by fixed gene dose, expression of that gene, and the distribution of transfected cells (Kullmann et al. 2014; Walker and Kullmann 2020). Zhang and Wang have summarized and elucidated the benefits of chemogenetics for gene therapy and several studies have demonstrated the potential effectiveness of chemogenetics in epilepsy treatment, thereby supporting its prospective clinical translation in the future (Zhang and Wang 2021).
Chemogenetics could result in minimal damages to animals. After viral expression in the brain, each experiment only requires the injection of drugs into muscles or veins to inhibit or excite a specific brain region, making it highly suitable for translational medical research (Song et al. 2022). However, compared to optogenetics, chemogenetics also has its drawbacks, particularly in terms of lower temporal precision. The effects of drugs persist for several hours, during which neurons remain in an abnormal state. Cognitive processes in the brain often change rapidly, with multiple cognitive events occurring within seconds or even one second. Chemogenetics may struggle to separate these transient and sequentially occurring cognitive processes (Raper and Galvan 2022).
In Vitro Models
In response to the growing sophistication of tools for the exploration of neural circuits in epilepsy, there is an emergent need for more advanced in vitro models. These models could serve to address current limitations and bolster biomedical research, with a particular focus on the discovery of antiepileptic drugs (Quadrato et al. 2017; Elder et al. 2022). Traditional murine models have demonstrated efficacy in elucidating the impact of epilepsy-associated genes on neural cells and circuits. However, they are unsuitable for high-throughput screening procedures and exhibit dissimilarities in brain development compared to their human counterparts (Silbereis et al. 2016; Jiang et al. 2018b). In efforts to overcome these challenges, human stem cell-derived models have been introduced. These models include neurons derived from iPSCs and organoids cultured from patient cells, both of which offer a patient-specific genetic background and human-specific cell types.
iPSC-Derived Neurons
Over recent years, iPSCs have taken a prominent role in epilepsy research, given their inherent pluripotency which lends itself well to modeling neurological disorders such as epilepsy (Shi et al. 2012). It has been found that iPSCs could reproduce the genetic mutations and background of the affected brain cells, thus making them suitable for modeling these disorders (Bhargava et al. 2022). Following the initial success in generating iPSCs from mouse fibroblasts, significant advancements have facilitated their application in epilepsy pathogenesis studies (Karagiannis et al. 2019).
A variety of genetic mutations, especially in different ion channels, play a role in the development of epilepsy. These mutations can affect the excitatory/inhibitory (E/I) balance and the stability of neural circuits. Key channels involved include voltage-gated sodium, potassium, and calcium channels, along with ligand-gated glutamatergic and GABAergic receptors (Oyrer et al. 2018). However, few of these mutations have been examined in humanized in vitro models. With the application of epilepsy patient-derived iPSCs to generate both excitatory and inhibitory neurons, voltage-gated sodium channels (Nav), including Nav1.1, Nav1.2, and Nav1.6 (encoded by SCN1A, SCN2A, and SCN8A, respectively), which have been associated with epilepsy, have been examined for their essential role in the depolarizing phase of the action potential (Child and Benarroch 2014). While most studies are focusing primarily on SCN1A and only investigating Nav1.1 in vitro, Tidball et al. have developed iPSC lines from epilepsy patients with SCN2A and SCN8A LOF mutations for prospective studies (Kim et al. 2018; Tidball et al. 2017). Mutations in voltage-gated potassium channels, such as Kv7.2 and KNa1.1, have also been modeled in vitro (Simkin and Kiskinis 2018).
It is understood that the hippocampus' homeostatic set point is regulated by dihydroorotate dehydrogenase. However, maladaptive changes can readily disrupt this homeostatic plasticity. Mutations in ion channels and components of the mTOR pathway have surfaced as a primary area of focus for neural circuits research in epilepsy (LaSarge et al. 2021; Weng et al. 2022). The dynamic interactions between these components warrant further investigation through humanized in vitro systems. These systems offer a promising platform to identify homeostatic alterations within neural circuits, which could contribute to epileptogenesis.
There are also several limitations of iPSCs technology. For complex, multigenic epilepsy types, iPSCs technology is difficult to model effectively. In addition, the overall low reprogramming efficiency and the high cost used to produce and characterize each cell line discourage their application in experiments (Huangfu et al. 2008; Sahlgren Bendtsen and Hall 2023). More basic research is still needed to develop this technology.
Organoids
2D cultures of iPSC-derived neurons can offer a valuable understanding of molecular mechanisms at the individual neuron level (Heider et al. 2021). However, they fall short in enabling investigation into neurogenesis and neuronal circuits. Moreover, they lack the 3D architecture characteristic of the brain and exhibit immature electrophysiological properties (Malik and Rao 2013). Organoid models effectively address these constraints, particularly in diseases associated with dysfunctional neural circuits, such as epilepsy (Andrews and Kriegstein 2022). Advances in human iPSC techniques have facilitated the development of 3D brain models, including brain organoids and cerebral organoids (Swingler et al. 2023). These models have often been employed for in vitro neuro-developmental research, but their potential in the context of epilepsy, especially for studying neural circuits, has been relatively underexplored (Pranty et al. 2022).
Birey et al. employed brain cortical spheroids (a specific type of cerebral organoid) to examine the developmental features of neurodevelopmental disorders, including epilepsy (Birey et al. 2017). Another study conducted by Blair et al. created cortical organoids from genetically engineered stem cell lines to investigate tuberous sclerosis (TS) complex (Blair et al. 2018). Additionally, Litwa further discussed the ability of brain organoids to reveal TS pathology, unravel disease mechanisms, and illuminate potential contributions of neurodevelopmental alterations to subsequent age-related neurodegeneration (Litwa 2022). Besides, Sun et al. developed 3D cortical organoids, which followed a normal developmental trajectory, to investigate whether functional deficits are relevant to the progression of Angelman syndrome and model epilepsy susceptibility in this syndrome (Sun et al. 2019a). Variations in neuronal network dynamics in numerous cells were monitored in both wild-type and knockout cortical organoids. In a study by Samarasinghe et al., calcium sensor imaging and extracellular recording techniques were used to analyze the functions of brain organoids at the network-level (Samarasinghe et al. 2021). Their research indicated that the fusion organoid system is amenable to detailed cellular and circuit analyses, providing insights into modeling human neurological disorders. Given the absence of in vivo connectivity, which hinders the integration with other circuits controlling behaviors, Revah et al. transplanted human stem cell-derived cortical organoids into the somatosensory cortex of newborn athymic rats (Revah et al. 2022). Their results demonstrated that these organoids were able to develop mature cell types and integrate into sensory and motivation-related circuits.
Although organoids have developed rapidly in recent years, for brain organoids, their development has not been smooth due to the lack of an in vivo brain tissue microenvironment, neuronal circuits, blood vessels, and immune system (Cakir et al. 2020). This collection of techniques facilitated a thorough understanding of the MS-hippocampus cholinergic circuit's role in TLE. The incorporation of imaging, neuronal manipulation, and circuit tracing allows researchers to examine pathological alterations, manipulate-specific neurons, and trace circuit connections, ultimately resulting in solid conclusions regarding the circuit's functionality and its prospective therapeutic significance in epilepsy. In a similar vein, Sere et al. investigated the role of the LH in absence epilepsy through the utilization of a blend of viral tract tracing, optogenetics, and both in vitro and in vivo electrophysiology (Sere et al. 2021). These techniques facilitated the characterization of neuronal activity within the LH and its relationship with the brainstem dorsal raphe nucleus, a region associated with the serotonergic system. The researchers discovered that LH neurons project to the DRN and their activity correlates with spike-wave discharges during absence seizures. This finding suggests that the LH, an area of the brain involved in autonomic regulation and heavily innervating the DRN, has a role in absence seizures and related comorbidities. It supports the hypothesis of a long-range subcortical regulation of serotonergic neuromodulation during such seizures.
However, the more combined techniques there are, the inevitably higher costs and more complex integration schemes will be faced. In summary, as the neural circuits studied become increasingly complex, the problems inherent in single techniques cannot be ignored, and the combination of multiple techniques is inevitably becoming a mainstream trend.
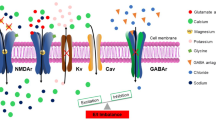
Drug Therapy Targeting Neural Circuits in Epilepsy
GABA deficiency, excessive serotonin activity, hyperactive dopamine, and glutamate excitotoxicity could enhance the occurrence of epilepsy. Neurons containing these neurotransmitters constitute the main neural circuits of epilepsy (Werner and Coveñas 2019). In recent years, more and more drugs targeting neural circuits have been developed for the treatment of epilepsy patients (Fig. 3). These drugs usually work by acting on specific neuronal cells, either exciting or inhibiting the release of synaptic vesicles, preventing excessive excitation or inhibition of nerve cells, and ultimately regulating the relevant axis to suppress epileptic seizures (Huang et al. 2023; Gunn and Baram 2017; Mao et al. 2022). Additionally, the latest research, such as investigating neuronal connection patterns through AI algorithms and using them as the basis for functional operation, was not discussed separately due to limited literature (Gao et al. 2023; Cao et al. 2023; Zhang et al. 2021b). Undoubtedly, these technologies are also very significant for the study of epilepsy. In conclusion, the array of methods utilized to explore neural circuitry in epilepsy has resulted in significant insights into the disorder's pathophysiology. As we continue to refine and expand these techniques, the prospect of identifying new therapeutic targets and devising more effective, individualized treatment for epilepsy patients becomes ever more attainable. Through continued interdisciplinary collaboration and technological innovation, we anticipate substantial progress in the treatment and clinical management of epilepsy.
Data Availability
Not applicable.
Code Availability
Not applicable.
Abbreviations
- DREADDs:
-
Designer receptors exclusively activated by designer drugs
- DGC:
-
Dentate gyrus cell
- SST:
-
Somatostatin
- PV- INs:
-
Parvalbumin inhibitory interneurons
- S286L-TG:
-
S286L-mutant Chrna4
- iPSCs:
-
Induced pluripotent stem cells
- LH:
-
Lateral hypothalamus
- TLE:
-
Temporal lobe epilepsy
- DS:
-
Dravet syndrome
- ChR2:
-
Channelrhodopsin-2
- PV:
-
Parvalbumin
- DG:
-
Dentate gyrus
- MS:
-
Medial septum
- PIL:
-
Posterior intralaminar thalamic nucleus
- CR:
-
Calretinin
- DRN:
-
Dorsal raphé nucleus
- mTLE:
-
Mesial temporal lobe epilepsy
References
Ait Ouares K, Jaafari N, Kuczewski N, Canepari M (2020) Imaging native calcium currents in brain slices. Adv Exp Med Biol 1131:73–91. https://doi.org/10.1007/978-3-030-12457-1_4
Akyuz E, Polat AK, Eroglu E, Kullu I, Angelopoulou E, Paudel YN (2021) Revisiting the role of neurotransmitters in epilepsy: an updated review. Life Sci 265:118826. https://doi.org/10.1016/j.lfs.2020.118826
Ali R, Connolly ID, Feroze AH, Awad AJ, Choudhri OA, Grant GA (2016) Epilepsy: a disruptive force in history. World Neurosurg 90:685–690. https://doi.org/10.1016/j.wneu.2015.11.060
Andrade-Machado R, Benjumea Cuartas V, Muhammad IK (2021) Recognition of interictal and ictal discharges on EEG. Focal vs generalized epilepsy. Epilepsy Behav 117:107830. https://doi.org/10.1016/j.yebeh.2021.107830
Andrews MG, Kriegstein AR (2022) Challenges of Organoid Research. Annu Rev Neurosci 45:23–39. https://doi.org/10.1146/annurev-neuro-111020-090812
Aravanis AM, Wang LP, Zhang F, Meltzer LA, Mogri MZ, Schneider MB, Deisseroth K (2007) An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J Neural Eng 4(3):S143-156. https://doi.org/10.1088/1741-2560/4/3/s02
Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL (2007) Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci USA 104(12):5163–5168. https://doi.org/10.1073/pnas.0700293104
Arnold EC, McMurray C, Gray R, Johnston D (2019) Epilepsy-Induced Reduction in HCN Channel Expression Contributes to an Increased Excitability in Dorsal, But Not Ventral, Hippocampal CA1 Neurons. eNeuro 6 (2). doi:https://doi.org/10.1523/eneuro.0036-19.2019
Assenza G, Lanzone J, Dubbioso R, Coppola A, Boscarino M, Ricci L, Insola A, Bilo L, Tombini M, Di Lazzaro V (2020) Thalamic and cortical hyperexcitability in juvenile myoclonic epilepsy. Clin Neurophysiol 131(8):2041–2046. https://doi.org/10.1016/j.clinph.2020.04.164
Beghi E (2020) The epidemiology of epilepsy. Neuroepidemiology 54(2):185–191. https://doi.org/10.1159/000503831
Beghi E, Giussani G, Sander JW (2015) The natural history and prognosis of epilepsy. Epileptic Disord 17(3):243–253. https://doi.org/10.1684/epd.2015.0751
Beier KT, Borghuis BG, El-Danaf RN, Huberman AD, Demb JB, Cepko CL (2013) Transsynaptic tracing with vesicular stomatitis virus reveals novel retinal circuitry. J Neurosci 33(1):35–51. https://doi.org/10.1523/jneurosci.0245-12.2013
Beier KT, Saunders A, Oldenburg IA, Miyamichi K, Akhtar N, Luo L, Whelan SP, Sabatini B, Cepko CL (2011) Anterograde or retrograde transsynaptic labeling of CNS neurons with vesicular stomatitis virus vectors. Proc Natl Acad Sci USA 108(37):15414–15419. https://doi.org/10.1073/pnas.1110854108
Berglind F, Andersson M, Kokaia M (2018) Dynamic interaction of local and transhemispheric networks is necessary for progressive intensification of hippocampal seizures. Sci Rep 8(1):5669. https://doi.org/10.1038/s41598-018-23659-x
Berndt A, Lee SY, Ramakrishnan C, Deisseroth K (2014) Structure-guided transformation of channelrhodopsin into a light-activated chloride channel. Science 344(6182):420–424. https://doi.org/10.1126/science.1252367
Berndt A, Yizhar O, Gunaydin LA, Hegemann P, Deisseroth K (2009) Bi-stable neural state switches. Nat Neurosci 12(2):229–234. https://doi.org/10.1038/nn.2247
Bhargava A, Sandoval Castellanos AM, Shah S, Ning K (2022) An insight into the iPSCs-derived two-dimensional culture and three-dimensional organoid models for neurodegenerative disorders. Interface Focus 12(5):20220040. https://doi.org/10.1098/rsfs.2022.0040
Birey F, Andersen J, Makinson CD, Islam S, Wei W, Huber N, Fan HC, Metzler KRC, Panagiotakos G, Thom N, O’Rourke NA, Steinmetz LM, Bernstein JA, Hallmayer J, Huguenard JR, Paşca SP (2017) Assembly of functionally integrated human forebrain spheroids. Nature 545(7652):54–59. https://doi.org/10.1038/nature22330
Bjorkli C, Louet C, Flo TH, Hemler M, Sandvig A, Sandvig I (2021) In vivo microdialysis in mice captures changes in Alzheimer’s disease cerebrospinal fluid biomarkers consistent with develo** pathology. J Alzheimers Dis 84(4):1781–1794. https://doi.org/10.3233/jad-210715
Blair JD, Hockemeyer D, Bateup HS (2018) Genetically engineered human cortical spheroid models of tuberous sclerosis. Nat Med 24(10):1568–1578. https://doi.org/10.1038/s41591-018-0139-y
Bonnin EA, Golmohammadi A, Rehm R, Tetzlaff C, Rizzoli SO (2024) High-resolution analysis of bound Ca(2+) in neurons and synapses. Life Sci Alliance. https://doi.org/10.26508/lsa.202302030
Borst A, Leibold C (2023) Connecting connectomes to physiology. J Neurosci 43(20):3599–3610. https://doi.org/10.1523/jneurosci.2208-22.2023
Botterill JJ, Lu YL, LaFrancois JJ, Bernstein HL, Alcantara-Gonzalez D, Jain S, Leary P, Scharfman HE (2019) An excitatory and epileptogenic effect of dentate gyrus mossy cells in a mouse model of epilepsy. Cell Rep 29(9):2875-2889.e2876. https://doi.org/10.1016/j.celrep.2019.10.100
Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K (2005) Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci 8(9):1263–1268. https://doi.org/10.1038/nn1525
Breathnach CS, Moynihan JB (2014) Joseph Erlanger (1874–1965): the cardiovascular investigator who won a Nobel Prize in neurophysiology. J Med Biogr 22(4):228–232. https://doi.org/10.1177/0967772013506680
Buckingham SD, Mann HJ, Hearnden OK, Sattelle DB (2020) Turning a drug target into a drug candidate: a new paradigm for neurological drug discovery? BioEssays 42(9):e2000011. https://doi.org/10.1002/bies.202000011
Butler CR, Westbrook GL, Schnell E (2022) Adaptive mossy cell circuit plasticity after status epilepticus. J Neurosci 42(14):3025–3036. https://doi.org/10.1523/jneurosci.1008-21.2022
Cai W, Hu M, Li C, Wu R, Lu D, **e C, Zhang W, Li T, Shen S, Huang H, Qiu W, Liu Q, Lu Y, Lu Z (2023) FOXP3+ macrophage represses acute ischemic stroke-induced neural inflammation. Autophagy 19(4):1144–1163. https://doi.org/10.1080/15548627.2022.2116833
Cakir B, **ang Y, Tanaka Y, Kural MH, Parent M, Kang YJ, Chapeton K, Patterson B, Yuan Y, He CS, Raredon MSB, Dengelegi J, Kim KY, Sun P, Zhong M, Lee S, Patra P, Hyder F, Niklason LE, Lee SH, Yoon YS, Park IH (2019) Engineering of human brain organoids with a functional vascular-like system. Nat Methods 16(11):1169–1175. https://doi.org/10.1038/s41592-019-0586-5
Cǎlin A, Stancu M, Zagrean AM, Jefferys JGR, Ilie AS, Akerman CJ (2018) Chemogenetic recruitment of specific interneurons suppresses seizure activity. Front Cell Neurosci 12:293. https://doi.org/10.3389/fncel.2018.00293
Cao Y, Sun C, Huang J, Sun P, Wang L, He S, Liao J, Lu Z, Lu Y, Zhong C (2022) Dysfunction of the hippocampal-lateral septal circuit impairs risk assessment in epileptic mice. Front Mol Neurosci 15:828891. https://doi.org/10.3389/fnmol.2022.828891
Cao Z, Sun B, Zhou G, Mao S, Zhu S, Zhang J, Ke C, Zhao Y, Shao J (2023) Memristor-based neural networks: a bridge from device to artificial intelligence. Nanoscale Horiz 8(6):716–745. https://doi.org/10.1039/d2nh00536k
Chang WC, Ng JK, Nguyen T, Pellissier L, Claeysen S, Hsiao EC, Conklin BR (2007) Modifying ligand-induced and constitutive signaling of the human 5-HT4 receptor. PLoS ONE 2(12):e1317. https://doi.org/10.1371/journal.pone.0001317
Chen B, Xu C, Wang Y, Lin W, Wang Y, Chen L, Cheng H, Xu L, Hu T, Zhao J, Dong P, Guo Y, Zhang S, Wang S, Zhou Y, Hu W, Duan S, Chen Z (2020) A disinhibitory nigra-parafascicular pathway amplifies seizure in temporal lobe epilepsy. Nat Commun 11(1):923. https://doi.org/10.1038/s41467-020-14648-8
Chen KD, Hall AM, Garcia-Curran MM, Sanchez GA, Daglian J, Luo R, Baram TZ (2021) Augmented seizure susceptibility and hippocampal epileptogenesis in a translational mouse model of febrile status epilepticus. Epilepsia 62(3):647–658. https://doi.org/10.1111/epi.16814
Chen TS, Huang TH, Lai MC, Huang CW (2023) The role of glutamate receptors in epilepsy. Biomedicines. https://doi.org/10.3390/biomedicines11030783
Chen Y, Wu XL, Hu HB, Yang SN, Zhang ZY, Fu GL, Zhang CT, Li ZM, Wu F, Si KW, Ma YB, Ji SF, Zhou JS, Ren XY, **ao XL, Liu JX (2023b) Neuronal MeCP2 in the dentate gyrus regulates mossy fiber sprouting of mice with temporal lobe epilepsy. Neurobiol Dis 188:106346. https://doi.org/10.1016/j.nbd.2023.106346
Chen Z, Wang Y, Avoli M (2023c) Preface to the special issue neural circuit mechanisms in epilepsy and targeted therapeutics. Neurobiol Dis 185:106256. https://doi.org/10.1016/j.nbd.2023.106256
Chilcott E, Díaz JA, Bertram C, Berti M, Karda R (2022) Genetic therapeutic advancements for Dravet Syndrome. Epilepsy Behav 132:108741. https://doi.org/10.1016/j.yebeh.2022.108741
Child ND, Benarroch EE (2014) Differential distribution of voltage-gated ion channels in cortical neurons: implications for epilepsy. Neurology 82(11):989–999. https://doi.org/10.1212/wnl.0000000000000228
Chou N, Shin H, Kim K, Chae U, Jang M, Jeong UJ, Hwang KS, Yi B, Lee SE, Woo J, Cho Y, Lee C, Baker BJ, Oh SJ, Nam MH, Choi N, Cho IJ (2022) A multimodal multi-shank fluorescence neural probe for cell-type-specific electrophysiology in multiple regions across a neural circuit. Adv Sci (Weinh) 9(2):e2103564. https://doi.org/10.1002/advs.202103564
Chow BY, Han X, Dobry AS, Qian X, Chuong AS, Li M, Henninger MA, Belfort GM, Lin Y, Monahan PE, Boyden ES (2010) High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature 463(7277):98–102. https://doi.org/10.1038/nature08652
Choy M, Dadgar-Kiani E, Cron GO, Duffy BA, Schmid F, Edelman BJ, Asaad M, Chan RW, Vahdat S, Lee JH (2022) Repeated hippocampal seizures lead to brain-wide reorganization of circuits and seizure propagation pathways. Neuron 110(2):221-236.e224. https://doi.org/10.1016/j.neuron.2021.10.010
Christenson Wick Z, Leintz CH, Xamonthiene C, Huang BH, Krook-Magnuson E (2017) Axonal sprouting in commissurally projecting parvalbumin-expressing interneurons. J Neurosci Res 95(12):2336–2344. https://doi.org/10.1002/jnr.24011
Collard R, Aziz MC, Rapp K, Cutshall C, Duyvesteyn E, Metcalf CS (2022) Galanin analogs prevent mortality from seizure-induced respiratory arrest in mice. Front Neural Circuits 16:901334. https://doi.org/10.3389/fncir.2022.901334
Coward P, Wada HG, Falk MS, Chan SD, Meng F, Akil H, Conklin BR (1998) Controlling signaling with a specifically designed Gi-coupled receptor. Proc Natl Acad Sci USA 95(1):352–357. https://doi.org/10.1073/pnas.95.1.352
Cross H (2015) Epilepsy: behavioural, psychological, and ketogenic diet treatments. BMJ Clin Evid 2015
Cui G, Jun SB, ** X, Luo G, Pham MD, Lovinger DM, Vogel SS, Costa RM (2014) Deep brain optical measurements of cell type-specific neural activity in behaving mice. Nat Protoc 9(6):1213–1228. https://doi.org/10.1038/nprot.2014.080
Deisseroth K (2011) Optogenetics. Nat Methods 8(1):26–29. https://doi.org/10.1038/nmeth.f.324
Deisseroth K, Feng G, Majewska AK, Miesenböck G, Ting A, Schnitzer MJ (2006) Next-generation optical technologies for illuminating genetically targeted brain circuits. J Neurosci 26(41):10380–10386. https://doi.org/10.1523/jneurosci.3863-06.2006
Deng L, Ravenscraft B, Xu XM (2022) Exploring propriospinal neuron-mediated neural circuit plasticity using recombinant viruses after spinal cord injury. Exp Neurol 349:113962. https://doi.org/10.1016/j.expneurol.2021.113962
Desloovere J, Boon P, Larsen LE, Goossens MG, Delbeke J, Carrette E, Wadman W, Vonck K, Raedt R (2022) Chemogenetic seizure control with clozapine and the novel ligand JHU37160 outperforms the effects of levetiracetam in the intrahippocampal kainic acid mouse model. Neurotherapeutics 19(1):342–351. https://doi.org/10.1007/s13311-021-01160-0
Desloovere J, Boon P, Larsen LE, Merckx C, Goossens MG, Van den Haute C, Baekelandt V, De Bundel D, Carrette E, Delbeke J, Meurs A, Vonck K, Wadman W, Raedt R (2019) Long-term chemogenetic suppression of spontaneous seizures in a mouse model for temporal lobe epilepsy. Epilepsia 60(11):2314–2324. https://doi.org/10.1111/epi.16368
Dhaher R, Gruenbaum SE, Sandhu MRS, Ottestad-Hansen S, Tu N, Wang Y, Lee TW, Deshpande K, Spencer DD, Danbolt NC, Zaveri HP, Eid T (2021) Network-related changes in neurotransmitters and seizure propagation during rodent epileptogenesis. Neurology 96(18):e2261–e2271. https://doi.org/10.1212/wnl.0000000000011846
Di Giovanni G, Esposito E, Di Matteo V (2009) In vivo microdialysis in Parkinson’s research. J Neural Transm Suppl 73:223–243. https://doi.org/10.1007/978-3-211-92660-4_18
Diaz Verdugo C, Myren-Svelstad S, Aydin E, Van Hoeymissen E, Deneubourg C, Vanderhaeghe S, Vancraeynest J, Pelgrims R, Cosacak MI, Muto A, Kizil C, Kawakami K, Jurisch-Yaksi N, Yaksi E (2019) Glia-neuron interactions underlie state transitions to generalized seizures. Nat Commun 10(1):3830. https://doi.org/10.1038/s41467-019-11739-z
Dijkstra KK, Cattaneo CM, Weeber F, Chalabi M, van de Haar J, Fanchi LF, Slagter M, van der Velden DL, Kaing S, Kelderman S, van Rooij N, van Leerdam ME, Depla A, Smit EF, Hartemink KJ, de Groot R, Wolkers MC, Sachs N, Snaebjornsson P, Monkhorst K, Haanen J, Clevers H, Schumacher TN, Voest EE (2018) Generation of tumor-reactive T cells by co-culture of peripheral blood lymphocytes and tumor organoids. Cell 174(6):1586-1598.e1512. https://doi.org/10.1016/j.cell.2018.07.009
Drew PJ, Winder AT, Zhang Q (2019) Twitches, blinks, and fidgets: important generators of ongoing neural activity. Neuroscientist 25(4):298–313. https://doi.org/10.1177/1073858418805427
Du X, Zhang H, Parent JM (2017) Rabies tracing of birthdated dentate granule cells in rat temporal lobe epilepsy. Ann Neurol 81(6):790–803. https://doi.org/10.1002/ana.24946
Duebel J, Marazova K, Sahel JA (2015) Optogenetics. Curr Opin Ophthalmol 26(3):226–232. https://doi.org/10.1097/icu.0000000000000140
Eapen PM, Rao CM, Nampoothiri M (2019) Crosstalk between neurokinin receptor signaling and neuroinflammation in neurological disorders. Rev Neurosci 30(3):233–243. https://doi.org/10.1515/revneuro-2018-0021
Eelkman Rooda OHJ, Kros L, Faneyte SJ, Holland PJ, Gornati SV, Poelman HJ, Jansen NA, Tolner EA, van den Maagdenberg A, De Zeeuw CI, Hoebeek FE (2021) Single-pulse stimulation of cerebellar nuclei stops epileptic thalamic activity. Brain Stimul 14(4):861–872. https://doi.org/10.1016/j.brs.2021.05.002
Elder N, Fattahi F, McDevitt TC, Zholudeva LV (2022) Diseased, differentiated and difficult: strategies for improved engineering of in vitro neurological systems. Front Cell Neurosci 16:962103. https://doi.org/10.3389/fncel.2022.962103
Enquist LW (2002) Exploiting circuit-specific spread of pseudorabies virus in the central nervous system: insights to pathogenesis and circuit tracers. J Infect Dis 186(Suppl 2):S209-214. https://doi.org/10.1086/344278
Fenno L, Yizhar O, Deisseroth K (2011) The development and application of optogenetics. Annu Rev Neurosci 34:389–412. https://doi.org/10.1146/annurev-neuro-061010-113817
Fenton GE, Nath K, Malkemper EP (2022) Electrophysiology and the magnetic sense: a guide to best practice. J Comp Physiol A 208(1):185–195. https://doi.org/10.1007/s00359-021-01517-y
Fiolka R (2021) Light-sheet microscopy at high resolution. Nat Biotechnol 39(11):1345–1346. https://doi.org/10.1038/s41587-021-01101-4
Fukuyama K, Fukuzawa M, Okada M (2020) Upregulated and hyperactivated thalamic connexin 43 plays important roles in pathomechanisms of cognitive impairment and seizure of autosomal dominant sleep-related hypermotor epilepsy with S284L-mutant α4 subunit of nicotinic ACh receptor. Pharmaceuticals (Basel) 13(5):99. https://doi.org/10.3390/ph13050099
Fukuyama K, Fukuzawa M, Shiroyama T, Okada M (2020b) Pathogenesis and pathophysiology of autosomal dominant sleep-related hypermotor epilepsy with S284L-mutant α4 subunit of nicotinic ACh receptor. Br J Pharmacol 177(9):2143–2162. https://doi.org/10.1111/bph.14974
Gao D, Shenoy R, Yi S, Lee J, Xu M, Rong Z, Deo A, Nathan D, Zheng JG, Williams RS, Chen Y (2023) Synaptic resistor circuits based on Al oxide and Ti silicide for concurrent learning and signal processing in artificial intelligence systems. Adv Mater 35(15):e2210484. https://doi.org/10.1002/adma.202210484
Gerrard LB, Tantirigama MLS, Bekkers JM (2018) Pre- and Postsynaptic Activation of GABA(B) Receptors Modulates Principal Cell Excitation in the Piriform Cortex. Front Cell Neurosci 12:28. https://doi.org/10.3389/fncel.2018.00028
Goldenberg AM, Schmidt S, Mitelman R, Levy DR, Prigge M, Katz Y, Yizhar O, Beck H, Lampl I (2023) Localized chemogenetic silencing of inhibitory neurons: a novel mouse model of focal cortical epileptic activity. Cereb Cortex 33(6):2838–2856. https://doi.org/10.1093/cercor/bhac245
Goossens MG, Boon P, Wadman W, Van den Haute C, Baekelandt V, Verstraete AG, Vonck K, Larsen LE, Sprengers M, Carrette E, Desloovere J, Meurs A, Delbeke J, Vanhove C, Raedt R (2021) Long-term chemogenetic suppression of seizures in a multifocal rat model of temporal lobe epilepsy. Epilepsia 62(3):659–670. https://doi.org/10.1111/epi.16840
Gu F, Hazra A, Aulakh A, Žiburkus J (2014) Purinergic control of hippocampal circuit hyperexcitability in Dravet syndrome. Epilepsia 55(2):245–255. https://doi.org/10.1111/epi.12487
Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, Lammel S, Mirzabekov JJ, Airan RD, Zalocusky KA, Tye KM, Anikeeva P, Malenka RC, Deisseroth K (2014) Natural neural projection dynamics underlying social behavior. Cell 157(7):1535–1551. https://doi.org/10.1016/j.cell.2014.05.017
Gunn BG, Baram TZ (2017) Stress and seizures: space, time and hippocampal circuits. Trends Neurosci 40(11):667–679. https://doi.org/10.1016/j.tins.2017.08.004
Guo B, Zhang M, Hao W, Wang Y, Zhang T, Liu C (2023) Neuroinflammation mechanisms of neuromodulation therapies for anxiety and depression. Transl Psychiatry 13(1):5. https://doi.org/10.1038/s41398-022-02297-y
Hatcher A, Yu K, Meyer J, Aiba I, Deneen B, Noebels JL (2020) Pathogenesis of peritumoral hyperexcitability in an immunocompetent CRISPR-based glioblastoma model. J Clin Invest 130(5):2286–2300. https://doi.org/10.1172/jci133316
Haut SR, Gursky JM, Privitera M (2019) Behavioral interventions in epilepsy. Curr Opin Neurol 32(2):227–236. https://doi.org/10.1097/wco.0000000000000661
Heider J, Vogel S, Volkmer H, Breitmeyer R (2021) Human iPSC-derived glia as a tool for neuropsychiatric research and drug development. Int J Mol Sci 22(19):10254. https://doi.org/10.3390/ijms221910254
Hersh AM, Weber-Levine C, Jiang K, Young L, Kerensky M, Routkevitch D, Tsehay Y, Perdomo-Pantoja A, Judy BF, Lubelski D, Theodore N, Manbachi A (2022) Applications of elastography in operative neurosurgery: a systematic review. J Clin Neurosci 104:18–28. https://doi.org/10.1016/j.jocn.2022.07.019
Heuzeroth H, Wawra M, Fidzinski P, Dag R, Holtkamp M (2019) The 4-aminopyridine model of acute seizures in vitro elucidates efficacy of new antiepileptic drugs. Front Neurosci 13:677. https://doi.org/10.3389/fnins.2019.00677
Hillman EMC, Voleti V, Li W, Yu H (2019) Light-sheet microscopy in neuroscience. Annu Rev Neurosci 42:295–313. https://doi.org/10.1146/annurev-neuro-070918-050357
Hoffman SM, Tang AY, Avalos JL (2022) Optogenetics Illuminates Applications in Microbial Engineering. Annu Rev Chem Biomol Eng 13:373–403. https://doi.org/10.1146/annurev-chembioeng-092120-092340
Holmgaard R, Nielsen JB, Benfeldt E (2010) Microdialysis sampling for investigations of bioavailability and bioequivalence of topically administered drugs: current state and future perspectives. Skin Pharmacol Physiol 23(5):225–243. https://doi.org/10.1159/000314698
Hristova K, Martinez-Gonzalez C, Watson TC, Codadu NK, Hashemi K, Kind PC, Nolan MF, Gonzalez-Sulser A (2021) Medial septal GABAergic neurons reduce seizure duration upon optogenetic closed-loop stimulation. Brain 144(5):1576–1589. https://doi.org/10.1093/brain/awab042
Hu M, Zhu K, Chen XL, Zhang YJ, Zhang JS, **ao XL, Liu JX, Liu Y (2015) Newly generated neurons at 2 months post-status epilepticus are functionally integrated into neuronal circuitry in mouse hippocampus. Exp Neurol 273:273–287. https://doi.org/10.1016/j.expneurol.2015.09.007
Huang L, **ao W, Wang Y, Li J, Gong J, Tu E, Long L, **ao B, Yan X, Wan L (2024) Metabotropic glutamate receptors (mGluRs) in epileptogenesis: an update on abnormal mGluRs signaling and its therapeutic implications. Neural Regen Res 19(2):360–368. https://doi.org/10.4103/1673-5374.379018
Huangfu D, Osafune K, Maehr R, Guo W, Eijkelenboom A, Chen S, Muhlestein W, Melton DA (2008) Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol 26(11):1269–1275. https://doi.org/10.1038/nbt.1502
Huisken J, Swoger J, Del Bene F, Wittbrodt J, Stelzer EH (2004) Optical sectioning deep inside live embryos by selective plane illumination microscopy. Science 305(5686):1007–1009. https://doi.org/10.1126/science.1100035
Islam MT, Rumpf F, Tsuno Y, Kodani S, Sakurai T, Matsui A, Maejima T, Mieda M (2022) Vasopressin neurons in the paraventricular hypothalamus promote wakefulness via lateral hypothalamic orexin neurons. Curr Biol 32(18):3871-3885.e3874. https://doi.org/10.1016/j.cub.2022.07.020
Jamieson BB, Piet R (2022) Kisspeptin neuron electrophysiology: Intrinsic properties, hormonal modulation, and regulation of homeostatic circuits. Front Neuroendocrinol 66:101006. https://doi.org/10.1016/j.yfrne.2022.101006
Jia F, Lv P, Miao H, Shi X, Mei H, Li L, Xu X, Tao S, Xu F (2019) Optimization of the fluorescent protein expression level based on pseudorabies virus bartha strain for neural circuit tracing. Front Neuroanat 13:63. https://doi.org/10.3389/fnana.2019.00063
Jiang G, Pu T, Li Z, Zhang X, Zhou R, Cao X, Yu J, Wang X (2018a) Lithium affects rat hippocampal electrophysiology and epileptic seizures in a dose dependent manner. Epilepsy Res 146:112–120. https://doi.org/10.1016/j.eplepsyres.2018.07.021
Jiang X, Lupien-Meilleur A, Tazerart S, Lachance M, Samarova E, Araya R, Lacaille JC, Rossignol E (2018b) Remodeled cortical inhibition prevents motor seizures in generalized epilepsy. Ann Neurol 84(3):436–451. https://doi.org/10.1002/ana.25301
Juhász C, John F (2020) Utility of MRI, PET, and ictal SPECT in presurgical evaluation of non-lesional pediatric epilepsy. Seizure 77:15–28. https://doi.org/10.1016/j.seizure.2019.05.008
Kahn JB, Port RG, Yue C, Takano H, Coulter DA (2019) Circuit-based interventions in the dentate gyrus rescue epilepsy-associated cognitive dysfunction. Brain 142(9):2705–2721. https://doi.org/10.1093/brain/awz209
Karagiannis P, Takahashi K, Saito M, Yoshida Y, Okita K, Watanabe A, Inoue H, Yamashita JK, Todani M, Nakagawa M, Osawa M, Yashiro Y, Yamanaka S, Osafune K (2019) Induced pluripotent stem cells and their use in human models of disease and development. Physiol Rev 99(1):79–114. https://doi.org/10.1152/physrev.00039.2017
Kato S, Kobayashi K (2020) Pseudotyped lentiviral vectors for tract-targeting and application for the functional control of selective neural circuits. J Neurosci Methods 344:108854. https://doi.org/10.1016/j.jneumeth.2020.108854
Kennedy RT (2013) Emerging trends in in vivo neurochemical monitoring by microdialysis. Curr Opin Chem Biol 17(5):860–867. https://doi.org/10.1016/j.cbpa.2013.06.012
Kim HW, Quan Z, Kim YB, Cheong E, Kim HD, Cho M, Jang J, Yoo YR, Lee JS, Kim JH, Kim YI, Kim DS, Kang HC (2018) Differential effects on sodium current impairments by distinct SCN1A mutations in GABAergic neurons derived from Dravet syndrome patients. Brain Dev 40(4):287–298. https://doi.org/10.1016/j.braindev.2017.12.002
Korgaonkar AA, Li Y, Sekhar D, Subramanian D, Guevarra J, Swietek B, Pallottie A, Singh S, Kella K, Elkabes S, Santhakumar V (2020) Toll-like receptor 4 signaling in neurons enhances calcium-permeable α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor currents and drives post-traumatic epileptogenesis. Ann Neurol 87(4):497–515. https://doi.org/10.1002/ana.25698
Krebs-Kraft DL, Frantz KJ, Parent MB (2007) In vivo microdialysis: a method for sampling extracellular fluid in discrete brain regions. In: Lajtha A, Baker G, Dunn S, Holt A (eds) Handbook of neurochemistry and molecular neurobiology: practical neurochemistry methods. Springer, New York, pp 219–256. https://doi.org/10.1007/978-0-387-30401-4_9
Kulbida R, Wang Y, Mandelkow EM, Schoch S, Becker AJ, van Loo KM (2015) Molecular imaging reveals epileptogenic Ca2+-channel promoter activation in hippocampi of living mice. Brain Struct Funct 220(5):3067–3073. https://doi.org/10.1007/s00429-014-0801-1
Kullmann DM, Schorge S, Walker MC, Wykes RC (2014) Gene therapy in epilepsy-is it time for clinical trials? Nat Rev Neurol 10(5):300–304. https://doi.org/10.1038/nrneurol.2014.43
Kuypers HG, Ugolini G (1990) Viruses as transneuronal tracers. Trends Neurosci 13(2):71–75. https://doi.org/10.1016/0166-2236(90)90071-h
LaSarge CL, Pun RYK, Gu Z, Riccetti MR, Namboodiri DV, Tiwari D, Gross C, Danzer SC (2021) mTOR-driven neural circuit changes initiate an epileptogenic cascade. Prog Neurobiol 200:101974. https://doi.org/10.1016/j.pneurobio.2020.101974
Lau LA, Staley KJ, Lillis KP (2022) In vitro ictogenesis is stochastic at the single neuron level. Brain 145(2):531–541. https://doi.org/10.1093/brain/awab312
Laxpati NG, Kasoff WS, Gross RE (2014) Deep brain stimulation for the treatment of epilepsy: circuits, targets, and trials. Neurotherapeutics 11(3):508–526. https://doi.org/10.1007/s13311-014-0279-9
Lee D, Krishnan B, Zhang H, Park HR, Ro EJ, Jung YN, Suh H (2019) Activity of hippocampal adult-born neurons regulates alcohol withdrawal seizures. JCI Insight 4(19):128770. https://doi.org/10.1172/jci.insight.128770
Lee SH, Zhang Y, Park J, Kim B, Kim Y, Lee SH, Kim GH, Huh YH, Lee B, Kim Y, Lee Y, Kim JY, Kang H, Choi SY, Jang S, Li Y, Kim S, ** C, Pang K, Kim E, Lee Y, Kim H, Kim E, Choi JH, Kim J, Lee KJ, Choi SY, Han K (2020) Haploinsufficiency of Cyfip2 causes lithium-responsive prefrontal dysfunction. Ann Neurol 88(3):526–543. https://doi.org/10.1002/ana.25827
Legaria AA, Matikainen-Ankney BA, Yang B, Ahanonu B, Licholai JA, Parker JG, Kravitz AV (2022) Fiber photometry in striatum reflects primarily nonsomatic changes in calcium. Nat Neurosci 25(9):1124–1128. https://doi.org/10.1038/s41593-022-01152-z
Lerchner W, **ao C, Nashmi R, Slimko EM, van Trigt L, Lester HA, Anderson DJ (2007) Reversible silencing of neuronal excitability in behaving mice by a genetically targeted, ivermectin-gated Cl- channel. Neuron 54(1):35–49. https://doi.org/10.1016/j.neuron.2007.02.030
Lerner TN, Ye L, Deisseroth K (2016) Communication in neural circuits: tools, opportunities, and challenges. Cell 164(6):1136–1150. https://doi.org/10.1016/j.cell.2016.02.027
Li D, Yang H, **ong F, Xu X, Zeng WB, Zhao F, Luo MH (2020) Anterograde neuronal circuit tracers derived from herpes simplex virus 1: development, application, and perspectives. Int J Mol Sci 21(16):5937. https://doi.org/10.3390/ijms21165937
Liang X, Luo H (2021) Optical tissue clearing: illuminating brain function and dysfunction. Theranostics 11(7):3035–3051. https://doi.org/10.7150/thno.53979
Liao M, Kundap U, Rosch RE, Burrows DRW, Meyer MP, Ouled Amar Bencheikh B, Cossette P, Samarut É (2019) Targeted knockout of GABA-A receptor gamma 2 subunit provokes transient light-induced reflex seizures in zebrafish larvae. Dis Model Mech 12(11):dmm040782. https://doi.org/10.1242/dmm.040782
Litwa K (2022) Shared mechanisms of neural circuit disruption in tuberous sclerosis across lifespan: Bridging neurodevelopmental and neurodegenerative pathology. Front Genet 13:997461. https://doi.org/10.3389/fgene.2022.997461
Liu H, Qu D, Cao Y, Li H, Wu X, Zhu Y, Tao J, Li Y, Cao C (2024) TAT-modified martentoxin displays intravenous antiseizure activities. ACS Chem Neurosci 15(1):205–214. https://doi.org/10.1021/acschemneuro.3c00744
Liu J, Baraban SC (2019) Network properties revealed during multi-scale calcium imaging of seizure activity in Zebrafish. eNeuro. https://doi.org/10.1523/eneuro.0041-19.2019
Liu J, Salvati KA, Baraban SC (2021) In vivo calcium imaging reveals disordered interictal network dynamics in epileptic stxbp1b zebrafish. iScience 24(6):102558. https://doi.org/10.1016/j.isci.2021.102558
Liu Q, Wu Y, Wang H, Jia F, Xu F (2022) Viral tools for neural circuit tracing. Neurosci Bull 38(12):1508–1518. https://doi.org/10.1007/s12264-022-00949-z
Liu X, Richardson AG (2021) Edge deep learning for neural implants: a case study of seizure detection and prediction. J Neural Eng. https://doi.org/10.1088/1741-2552/abf473
Loewy AD (1998) Viruses as transneuronal tracers for defining neural circuits. Neurosci Biobehav Rev 22(6):679–684. https://doi.org/10.1016/s0149-7634(98)00006-2
Lovett-Barron M, Turi GF, Kaifosh P, Lee PH, Bolze F, Sun XH, Nicoud JF, Zemelman BV, Sternson SM, Losonczy A (2012) Regulation of neuronal input transformations by tunable dendritic inhibition. Nat Neurosci 15(3):423–430, s421–423. https://doi.org/10.1038/nn.3024
Luna-Munguia H, Zestos AG, Gliske SV, Kennedy RT, Stacey WC (2019) Chemical biomarkers of epileptogenesis and ictogenesis in experimental epilepsy. Neurobiol Dis 121:177–186. https://doi.org/10.1016/j.nbd.2018.10.005
Lv QY, Chen MM, Li Y, Yu Y, Liao H (2022) Brain circuit dysfunction in specific symptoms of depression. Eur J Neurosci 55(9–10):2393–2403. https://doi.org/10.1111/ejn.15221
Magnus CJ, Lee PH, Bonaventura J, Zemla R, Gomez JL, Ramirez MH, Hu X, Galvan A, Basu J, Michaelides M, Sternson SM (2019) Ultrapotent chemogenetics for research and potential clinical applications. Science 364(6436):eaav5282. https://doi.org/10.1126/science.aav5282
Malezieux M, Klein AS, Gogolla N (2023) Neural circuits for emotion. Annu Rev Neurosci 46:211–231. https://doi.org/10.1146/annurev-neuro-111020-103314
Malik N, Rao MS (2013) A review of the methods for human iPSC derivation. Methods Mol Biol 997:23–33. https://doi.org/10.1007/978-1-62703-348-0_3
Mao L, Wang K, Zhang Q, Wang J, Zhao Y, Peng W, Ding J (2022) Felt stigma and its underlying contributors in epilepsy patients. Front Public Health 10:879895. https://doi.org/10.3389/fpubh.2022.879895
Martin X, Dolivo M (1983) Neuronal and transneuronal tracing in the trigeminal system of the rat using the herpes virus suis. Brain Res 273(2):253–276. https://doi.org/10.1016/0006-8993(83)90850-8
Mattis J, Somarowthu A, Goff KM, Jiang E, Yom J, Sotuyo N, McGarry LM, Feng H, Kaneko K, Goldberg EM (2022) Corticohippocampal circuit dysfunction in a mouse model of Dravet syndrome. Elife. https://doi.org/10.7554/eLife.69293
McAdoo DJ, Wu P (2008) Microdialysis in central nervous system disorders and their treatment. Pharmacol Biochem Behav 90(2):282–296. https://doi.org/10.1016/j.pbb.2008.03.001
Melonakos ED, Moody OA, Nikolaeva K, Kato R, Nehs CJ, Solt K (2020) Manipulating neural circuits in anesthesia research. Anesthesiology 133(1):19–30. https://doi.org/10.1097/aln.0000000000003279
Merlin S, Vidyasagar T (2023) Optogenetics in primate cortical networks. Front Neuroanat 17:1193949. https://doi.org/10.3389/fnana.2023.1193949
Miles R, Tóth K, Gulyás AI, Hájos N, Freund TF (1996) Differences between somatic and dendritic inhibition in the hippocampus. Neuron 16(4):815–823. https://doi.org/10.1016/s0896-6273(00)80101-4
Mineur YS, Picciotto MR (2023) How can I measure brain acetylcholine levels in vivo? Advantages and caveats of commonly used approaches. J Neurochem 167(1):3–15. https://doi.org/10.1111/jnc.15943
Miziak B, Chrościńska-Krawczyk M, Błaszczyk B, Radzik I, Czuczwar SJ (2013) Novel approaches to anticonvulsant drug discovery. Expert Opin Drug Discov 8(11):1415–1427. https://doi.org/10.1517/17460441.2013.837047
Mokhothu TM, Tanaka KZ (2021) Characterizing hippocampal oscillatory signatures underlying seizures in temporal lobe epilepsy. Front Behav Neurosci 15:785328. https://doi.org/10.3389/fnbeh.2021.785328
Mulcahey PJ, Chen Y, Driscoll N, Murphy BB, Dickens OO, Johnson ATC, Vitale F, Takano H (2022) Multimodal, multiscale insights into hippocampal seizures enabled by transparent, graphene-based microelectrode. Arrays. Neuro 9(3):386–21. https://doi.org/10.1523/eneuro.0386-21.2022
Muldoon SF, Villette V, Tressard T, Malvache A, Reichinnek S, Bartolomei F, Cossart R (2015) GABAergic inhibition shapes interictal dynamics in awake epileptic mice. Brain 138(Pt 10):2875–2890. https://doi.org/10.1093/brain/awv227
Murphy KR, Farrell JS, Gomez JL, Stedman QG, Li N, Leung SA, Good CH, Qiu Z, Firouzi K, Butts Pauly K, Khuri-Yakub BPT, Michaelides M, Soltesz I, de Lecea L (2022) A tool for monitoring cell type-specific focused ultrasound neuromodulation and control of chronic epilepsy. Proc Natl Acad Sci USA 119(46):e2206828119. https://doi.org/10.1073/pnas.2206828119
Myren-Svelstad S, Jamali A, Ophus SS, D’Gama PP, Ostenrath AM, Mutlu AK, Hoffshagen HH, Hotz AL, Neuhauss SCF, Jurisch-Yaksi N, Yaksi E (2022) Elevated photic response is followed by a rapid decay and depressed state in ictogenic networks. Epilepsia 63(10):2543–2560. https://doi.org/10.1111/epi.17380
Nietz AK, Popa LS, Streng ML, Carter RE, Kodandaramaiah SB, Ebner TJ (2022) Wide-field calcium imaging of neuronal network dynamics in vivo. Biology (Basel) 11(11):601. https://doi.org/10.3390/biology11111601
Nowotny T, van Albada SJ, Fellous JM, Haas JS, Jolivet RB, Metzner C, Sharpee T (2021) Editorial: advances in computational neuroscience. Front Comput Neurosci 15:824899. https://doi.org/10.3389/fncom.2021.824899
Oesterhelt D, Stoeckenius W (1971) Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol 233(39):149–152. https://doi.org/10.1038/newbio233149a0
Oyrer J, Maljevic S, Scheffer IE, Berkovic SF, Petrou S, Reid CA (2018) Ion channels in genetic epilepsy: from genes and mechanisms to disease-targeted therapies. Pharmacol Rev 70(1):142–173. https://doi.org/10.1124/pr.117.014456
Panthi S, Leitch B (2021) Chemogenetic activation of feed-forward inhibitory parvalbumin-expressing interneurons in the cortico-thalamocortical network during absence seizures. Front Cell Neurosci 15:688905. https://doi.org/10.3389/fncel.2021.688905
Parrot S, Denoroy L, Renaud B, Benetollo C (2015) Why Optogenetics Needs in Vivo Neurochemistry. ACS Chem Neurosci 6(7):948–950. https://doi.org/10.1021/acschemneuro.5b00003
Paschen E, Elgueta C, Heining K, Vieira DM, Kleis P, Orcinha C, Häussler U, Bartos M, Egert U, Janz P, Haas CA (2020) Hippocampal low-frequency stimulation prevents seizure generation in a mouse model of mesial temporal lobe epilepsy. Elife 9:e545148. https://doi.org/10.7554/eLife.54518
Paz JT, Huguenard JR (2015) Microcircuits and their interactions in epilepsy: is the focus out of focus? Nat Neurosci 18(3):351–359. https://doi.org/10.1038/nn.3950
Peralvárez-Marín A, Garriga P (2016) Optogenetics comes of age: novel inhibitory light-gated anionic channels allow efficient silencing of neural function. ChemBioChem 17(3):204–206. https://doi.org/10.1002/cbic.201500608
Poth KM, Texakalidis P, Boulis NM (2021) Chemogenetics: beyond lesions and electrodes. Neurosurgery 89(2):185–195. https://doi.org/10.1093/neuros/nyab147
Pranty AI, Shumka S, Adjaye J (2022) Bilirubin-induced neurological damage: current and emerging iPSC-derived brain organoid models. Cells 11(17):2647. https://doi.org/10.3390/cells11172647
Qi Y, Cheng H, Lou Q, Wang X, Lai N, Gao C, Wu S, Xu C, Ruan Y, Chen Z, Wang Y (2022) Paradoxical effects of posterior intralaminar thalamic calretinin neurons on hippocampal seizure via distinct downstream circuits. iScience 25(5):104218. https://doi.org/10.1016/j.isci.2022.104218
Quadrato G, Nguyen T, Macosko EZ, Sherwood JL, Min Yang S, Berger DR, Maria N, Scholvin J, Goldman M, Kinney JP, Boyden ES, Lichtman JW, Williams ZM, McCarroll SA, Arlotta P (2017) Cell diversity and network dynamics in photosensitive human brain organoids. Nature 545(7652):48–53. https://doi.org/10.1038/nature22047
Raper J, Galvan A (2022) Applications of chemogenetics in non-human primates. Curr Opin Pharmacol 64:102204. https://doi.org/10.1016/j.coph.2022.102204
Revah O, Gore F, Kelley KW, Andersen J, Sakai N, Chen X, Li MY, Birey F, Yang X, Saw NL, Baker SW, Amin ND, Kulkarni S, Mudipalli R, Cui B, Nishino S, Grant GA, Knowles JK, Shamloo M, Huguenard JR, Deisseroth K, Pașca SP (2022) Maturation and circuit integration of transplanted human cortical organoids. Nature 610(7931):319–326. https://doi.org/10.1038/s41586-022-05277-w
Robbins M, Christensen CN, Kaminski CF, Zlatic M (2021) Calcium imaging analysis—how far have we come? F1000Res 10:258. https://doi.org/10.12688/f1000research.51755.2
Rosch RE, Hunter PR, Baldeweg T, Friston KJ, Meyer MP (2018) Calcium imaging and dynamic causal modelling reveal brain-wide changes in effective connectivity and synaptic dynamics during epileptic seizures. PLoS Comput Biol 14(8):e1006375. https://doi.org/10.1371/journal.pcbi.1006375
Rubinger L, Hazrati LN, Ahmed R, Rutka J, Snead C, Widjaja E (2017) Microscopic and macroscopic infarct complicating pediatric epilepsy surgery. Epilepsia 58(3):393–401. https://doi.org/10.1111/epi.13667
Russo EB (2017) Cannabis and epilepsy: An ancient treatment returns to the fore. Epilepsy Behav 70(Pt B):292–297. https://doi.org/10.1016/j.yebeh.2016.09.040
Sahlgren Bendtsen KM, Hall VJ (2023) The breakthroughs and caveats of using human pluripotent stem cells in modeling Alzheimer’s disease. Cells. https://doi.org/10.3390/cells12030420
Samarasinghe RA, Miranda OA, Buth JE, Mitchell S, Ferando I, Watanabe M, Allison TF, Kurdian A, Fotion NN, Gandal MJ, Golshani P, Plath K, Lowry WE, Parent JM, Mody I, Novitch BG (2021) Identification of neural oscillations and epileptiform changes in human brain organoids. Nat Neurosci 24(10):1488–1500. https://doi.org/10.1038/s41593-021-00906-5
Santos RP, Nardi AE, Gomes MDM (2022) Emil du Bois-Reymond and Dom Pedro II: Research development on bioelectricity and electric fish. Front Physiol 13:985473. https://doi.org/10.3389/fphys.2022.985473
Sere P, Zsigri N, Raffai T, Furdan S, Győri F, Crunelli V, Lőrincz ML (2021) Activity of the lateral hypothalamus during genetically determined absence seizures. Int J Mol Sci. https://doi.org/10.3390/ijms22179466
Serrat R, Oliveira-Pinto A, Marsicano G, Pouvreau S (2022) Imaging mitochondrial calcium dynamics in the central nervous system. J Neurosci Methods 373:109560. https://doi.org/10.1016/j.jneumeth.2022.109560
Shafran R, Bennett S, Coughtrey A, Welch A, Walji F, Cross JH, Heyman I, Sibelli A, Smith J, Ross J, Dalrymple E, Varadkar S, Moss-Morris R (2020) Optimising evidence-based psychological treatment for the mental health needs of children with epilepsy: principles and methods. Clin Child Fam Psychol Rev 23(2):284–295. https://doi.org/10.1007/s10567-019-00310-3
Sharpee TO, Destexhe A, Kawato M, Sekulić V, Skinner FK, Wójcik DK, Chintaluri C, Cserpán D, Somogyvári Z, Kim JK, Kilpatrick ZP, Bennett MR, Josić K, Elices I, Arroyo D, Levi R, Rodriguez FB, Varona P, Hwang E, Kim B, Han HB, Kim T, McKenna JT, Brown RE, McCarley RW, Choi JH, Rankin J, Popp PO, Rinzel J, Tabas A, Rupp A, Balaguer-Ballester E, Maturana MI, Grayden DB, Cloherty SL, Kameneva T, Ibbotson MR, Meffin H, Koren V, Lochmann T, Dragoi V, Obermayer K, Psarrou M, Schilstra M, Davey N, Torben-Nielsen B, Steuber V, Ju H, Yu J, Hines ML, Chen L, Yu Y, Kim J, Leahy W, Shlizerman E, Birgiolas J, Gerkin RC, Crook SM, Viriyopase A, Memmesheimer RM, Gielen S, Dabaghian Y, DeVito J, Perotti L, Kim AJ, Fenk LM, Cheng C, Maimon G, Zhao C, Widmer Y, Sprecher S, Senn W, Halnes G, Mäki-Marttunen T, Keller D, Pettersen KH, Andreassen OA, Einevoll GT, Yamada Y, Steyn-Ross ML, Alistair Steyn-Ross D, Mejias JF, Murray JD, Kennedy H, Wang XJ, Kruscha A, Grewe J, Benda J, Lindner B, Badel L, Ohta K, Tsuchimoto Y, Kazama H, Kahng B, Tam ND, Pollonini L, Zouridakis G, Soh J, Kim D, Yoo M, Palmer SE, Culmone V, Bojak I, Ferrario A, Merrison-Hort R, Borisyuk R, Kim CS, Tezuka T, Joo P, Rho YA, Burton SD, Bard Ermentrout G, Jeong J, Urban NN, Marsalek P, Kim HH, Moon SH, Lee DW, Lee SB, Lee JY, Molkov YI, Hamade K, Teka W, Barnett WH, Kim T, Markin S, Rybak IA, Forro C, Dermutz H, Demkó L, Vörös J, Babichev A, Huang H, Verduzco-Flores S, Dos Santos F, Andras P, Metzner C, Schweikard A, Zurowski B, Roach JP, Sander LM, Zochowski MR, Skilling QM, Ognjanovski N, Aton SJ, Zochowski M, Wang SJ, Ouyang G, Guang J, Zhang M, Michael Wong KY, Zhou C, Robinson PA, Sanz-Leon P, Drysdale PM, Fung F, Abeysuriya RG, Rennie CJ, Zhao X, Choe Y, Yang HF, Mi Y, Lin X, Wu S, Liedtke J, Schottdorf M, Wolf F, Yamamura Y, Wickens JR, Rumbell T, Ramsey J, Reyes A, Draguljić D, Hof PR, Luebke J, Weaver CM, He H, Yang X, Ma H, Xu Z, Wang Y, Baek K, Morris LS, Kundu P, Voon V, Agnes EJ, Vogels TP, Podlaski WF, Giese M, Kuravi P, Vogels R, Seeholzer A, Podlaski W, Ranjan R, Vogels T, Torres JJ, Baroni F, Latorre R, Gips B, Lowet E, Roberts MJ, de Weerd P, Jensen O, van der Eerden J, Goodarzinick A, Niry MD, Valizadeh A, Pariz A, Parsi SS, Warburton JM, Marucci L, Tamagnini F, Brown J, Tsaneva-Atanasova K, Kleberg FI, Triesch J, Moezzi B, Iannella N, Schaworonkow N, Plogmacher L, Goldsworthy MR, Hordacre B, McDonnell MD, Ridding MC, Zapotocky M, Smit D, Fouquet C, Trembleau A, Dasgupta S, Nishikawa I, Aihara K, Toyoizumi T, Robb DT, Mellen N, Toporikova N, Tang R, Tang YY, Liang G, Kiser SA, Howard JH, Jr., Goncharenko J, Voronenko SO, Ahamed T, Stephens G, Yger P, Lefebvre B, Spampinato GLB, Esposito E, et Olivier Marre MS, Choi H, Song MH, Chung S, Lee DD, Sompolinsky H, Phillips RS, Smith J, Chatzikalymniou AP, Ferguson K, Alex Cayco Gajic N, Clopath C, Angus Silver R, Gleeson P, Marin B, Sadeh S, Quintana A, Cantarelli M, Dura-Bernal S, Lytton WW, Davison A, Li L, Zhang W, Wang D, Song Y, Park S, Choi I, Shin HS, Choi H, Pasupathy A, Shea-Brown E, Huh D, Sejnowski TJ, Vogt SM, Kumar A, Schmidt R, Van Wert S, Schiff SJ, Veale R, Scheutz M, Lee SW, Gallinaro J, Rotter S, Rubchinsky LL, Cheung CC, Ratnadurai-Giridharan S, Shomali SR, Ahmadabadi MN, Shimazaki H, Nader Rasuli S, Zhao X, Rasch MJ, Wilting J, Priesemann V, Levina A, Rudelt L, Lizier JT, Spinney RE, Rubinov M, Wibral M, Bak JH, Pillow J, Zaho Y, Park IM, Kang J, Park HJ, Jang J, Paik SB, Choi W, Lee C, Song M, Lee H, Park Y, Yilmaz E, Baysal V, Ozer M, Saska D, Nowotny T, Chan HK, Diamond A, Herrmann CS, Murray MM, Ionta S, Hutt A, Lefebvre J, Weidel P, Duarte R, Morrison A, Lee JH, Iyer R, Mihalas S, Koch C, Petrovici MA, Leng L, Breitwieser O, Stöckel D, Bytschok I, Martel R, Bill J, Schemmel J, Meier K, Esler TB, Burkitt AN, Kerr RR, Tahayori B, Nolte M, Reimann MW, Muller E, Markram H, Parziale A, Senatore R, Marcelli A, Skiker K, Maouene M, Neymotin SA, Seidenstein A, Lakatos P, Sanger TD, Menzies RJ, McLauchlan C, van Albada SJ, Kedziora DJ, Neymotin S, Kerr CC, Suter BA, Shepherd GMG, Ryu J, Lee SH, Lee J, Lee HJ, Lim D, Wang J, Lee H, Jung N, Anh Quang L, Maeng SE, Lee TH, Lee JW, Park CH, Ahn S, Moon J, Choi YS, Kim J, Jun SB, Lee S, Lee HW, Jo S, Jun E, Yu S, Goetze F, Lai PY, Kim S, Kwag J, Jang HJ, Filipović M, Reig R, Aertsen A, Silberberg G, Bachmann C, Buttler S, Jacobs H, Dillen K, Fink GR, Kukolja J, Kepple D, Giaffar H, Rinberg D, Shea S, Koulakov A, Bahuguna J, Tetzlaff T, Kotaleski JH, Kunze T, Peterson A, Knösche T, Kim M, Kim H, Park JS, Yeon JW, Kim SP, Kang JH, Lee C, Spiegler A, Petkoski S, Palva MJ, Jirsa VK, Saggio ML, Siep SF, Stacey WC, Bernar C, Choung OH, Jeong Y, Lee YI, Kim SH, Jeong M, Lee J, Kwon J, Kralik JD, Jahng J, Hwang DU, Kwon JH, Park SM, Kim S, Kim H, Kim PS, Yoon S, Lim S, Park C, Miller T, Clements K, Ahn S, Ji EH, Issa FA, Baek J, Oba S, Yoshimoto J, Doya K, Ishii S, Mosqueiro TS, Strube-Bloss MF, Smith B, Huerta R, Hadrava M, Hlinka J, Bos H, Helias M, Welzig CM, Harper ZJ, Kim WS, Shin IS, Baek HM, Han SK, Richter R, Vitay J, Beuth F, Hamker FH, Toppin K, Guo Y, Graham BP, Kale PJ, Gollo LL, Stern M, Abbott LF, Fedorov LA, Giese MA, Ardestani MH, Faraji MJ, Preuschoff K, Gerstner W, van Gendt MJ, Briaire JJ, Kalkman RK, Frijns JHM, Lee WH, Frangou S, Fulcher BD, Tran PHP, Fornito A, Gliske SV, Lim E, Holman KA, Fink CG, Kim JS, Mu S, Briggman KL, Sebastian Seung H, Wegener D, Bohnenkamp L, Ernst UA, Devor A, Dale AM, Lines GT, Edwards A, Tveito A, Hagen E, Senk J, Diesmann M, Schmidt M, Bakker R, Shen K, Bezgin G, Hilgetag CC, van Albada SJ, Sun H, Sourina O, Huang GB, Klanner F, Denk C, Glomb K, Ponce-Alvarez A, Gilson M, Ritter P, Deco G, Witek MAG, Clarke EF, Hansen M, Wallentin M, Kringelbach ML, Vuust P, Klingbeil G, De Schutter E, Chen W, Zang Y, Hong S, Takashima A, Zamora C, Gallimore AR, Goldschmidt D, Manoonpong P, Karoly PJ, Freestone DR, Soundry D, Kuhlmann L, Paninski L, Cook M, Lee J, Fishman YI, Cohen YE, Roberts JA, Cocchi L, Sweeney Y, Lee S, Jung WS, Kim Y, Jung Y, Song YK, Chavane F, Soman K, Muralidharan V, Srinivasa Chakravarthy V, Shivkumar S, Mandali A, Pragathi Priyadharsini B, Mehta H, Davey CE, Brinkman BAW, Kekona T, Rieke F, Buice M, De Pittà M, Berry H, Brunel N, Breakspear M, Marsat G, Drew J, Chapman PD, Daly KC, Bradle SP, Seo SB, Su J, Kavalali ET, Blackwell J, Shiau L, Buhry L, Basnayake K, Lee SH, Levy BA, Baker CI, Leleu T, Philips RT, Chhabria K (2016) 25th Annual Computational Neuroscience Meeting: CNS-2016. BMC Neurosci 17 Suppl 1 (Suppl 1):54. https://doi.org/10.1186/s12868-016-0283-6
Shi Y, Kirwan P, Livesey FJ (2012) Directed differentiation of human pluripotent stem cells to cerebral cortex neurons and neural networks. Nat Protoc 7(10):1836–1846. https://doi.org/10.1038/nprot.2012.116
Shimoda Y, Beppu K, Ikoma Y, Morizawa YM, Zuguchi S, Hino U, Yano R, Sugiura Y, Moritoh S, Fukazawa Y, Suematsu M, Mushiake H, Nakasato N, Iwasaki M, Tanaka KF, Tominaga T, Matsui K (2022) Optogenetic stimulus-triggered acquisition of seizure resistance. Neurobiol Dis 163:105602. https://doi.org/10.1016/j.nbd.2021.105602
Silbereis JC, Pochareddy S, Zhu Y, Li M, Sestan N (2016) The cellular and molecular landscapes of the develo** human central nervous system. Neuron 89(2):248–268. https://doi.org/10.1016/j.neuron.2015.12.008
Simkin D, Kiskinis E (2018) Modeling pediatric epilepsy through iPSC-based technologies. Epilepsy Curr 18(4):240–245. https://doi.org/10.5698/1535-7597.18.4.240
Singh K, Dua A (2023) Anesthesia for Awake Craniotomy. In: StatPearls. StatPearls Publishing
Copyright © 2023, StatPearls Publishing LLC., Treasure Island (FL),
Sivaramakrishnan S, Lynch WP (2017) Rebound from inhibition: self-correction against neurodegeneration? J Clin Cell Immunol. https://doi.org/10.4172/2155-9899.1000492
Somarowthu A, Goff KM, Goldberg EM (2021) Two-photon calcium imaging of seizures in awake, head-fixed mice. Cell Calcium 96:102380. https://doi.org/10.1016/j.ceca.2021.102380
Song J, Patel RV, Sharif M, Ashokan A, Michaelides M (2022) Chemogenetics as a neuromodulatory approach to treating neuropsychiatric diseases and disorders. Mol Ther 30(3):990–1005. https://doi.org/10.1016/j.ymthe.2021.11.019
Sorokin JM, Davidson TJ, Frechette E, Abramian AM, Deisseroth K, Huguenard JR, Paz JT (2017) Bidirectional control of generalized epilepsy networks via rapid real-time switching of firing mode. Neuron 93(1):194–210. https://doi.org/10.1016/j.neuron.2016.11.026
Strader CD, Gaffney T, Sugg EE, Candelore MR, Keys R, Patchett AA, Dixon RA (1991) Allele-specific activation of genetically engineered receptors. J Biol Chem 266(1):5–8
Studer F, Jarre G, Pouyatos B, Nemoz C, Brauer-Krisch E, Muzelle C, Serduc R, Heinrich C, Depaulis A (2022) Aberrant neuronal connectivity in the cortex drives generation of seizures in rat absence epilepsy. Brain 145(6):1978–1991. https://doi.org/10.1093/brain/awab438
Sugaya Y, Kano M (2021) Endocannabinoid-mediated control of neural circuit excitability and epileptic seizures. Front Neural Circuits 15:781113. https://doi.org/10.3389/fncir.2021.781113
Sun AX, Yuan Q, Fukuda M, Yu W, Yan H, Lim GGY, Nai MH, D’Agostino GA, Tran HD, Itahana Y, Wang D, Lokman H, Itahana K, Lim SWL, Tang J, Chang YY, Zhang M, Cook SA, Rackham OJL, Lim CT, Tan EK, Ng HH, Lim KL, Jiang YH, Je HS (2019a) Potassium channel dysfunction in human neuronal models of Angelman syndrome. Science 366(6472):1486–1492. https://doi.org/10.1126/science.aav5386
Sun L, Tang Y, Yan K, Yu J, Zou Y, Xu W, **ao K, Zhang Z, Li W, Wu B, Hu Z, Chen K, Fu ZF, Dai J, Cao G (2019b) Differences in neurotropism and neurotoxicity among retrograde viral tracers. Mol Neurodegener 14(1):8. https://doi.org/10.1186/s13024-019-0308-6
Sun Y, ** S, Lin X, Chen L, Qiao X, Jiang L, Zhou P, Johnston KG, Golshani P, Nie Q, Holmes TC, Nitz DA, Xu X (2019c) CA1-projecting subiculum neurons facilitate object-place learning. Nat Neurosci 22(11):1857–1870. https://doi.org/10.1038/s41593-019-0496-y
Suzuki T, Morimoto N, Akaike A, Osakada F (2019) Multiplex neural circuit tracing with G-deleted rabies viral vectors. Front Neural Circuits 13:77. https://doi.org/10.3389/fncir.2019.00077
Swanson JL, Chin PS, Romero JM, Srivastava S, Ortiz-Guzman J, Hunt PJ, Arenkiel BR (2022) Advancements in the quest to map, monitor, and manipulate neural circuitry. Front Neural Circuits 16:886302. https://doi.org/10.3389/fncir.2022.886302
Swingler M, Donadoni M, Bellizzi A, Cakir S, Sariyer IK (2023) iPSC-derived three-dimensional brain organoid models and neurotropic viral infections. J Neurovirol 29(2):121–134. https://doi.org/10.1007/s13365-023-01133-3
Tan C, Robbins EM, Wu B, Cui XT (2021) Recent advances in in vivo neurochemical monitoring. Micromachines (Basel). https://doi.org/10.3390/mi12020208
Tao T, Deng P, Wang Y, Zhang X, Guo Y, Chen W, Qin J (2022) Microengineered multi-organoid system from hiPSCs to recapitulate human liver-islet axis in normal and type 2 diabetes. Adv Sci (Weinh) 9(5):e2103495. https://doi.org/10.1002/advs.202103495
Testa G, Olimpico F, Pancrazi L, Borello U, Cattaneo A, Caleo M, Costa M, Mainardi M (2019) Cortical seizures in FoxG1(+/-) mice are accompanied by Akt/S6 overactivation, excitation/inhibition imbalance and impaired synaptic transmission. Int J Mol Sci. https://doi.org/10.3390/ijms20174127
Thijs RD, Surges R, O’Brien TJ, Sander JW (2019) Epilepsy in adults. Lancet 393(10172):689–701. https://doi.org/10.1016/s0140-6736(18)32596-0
Tidball AM, Dang LT, Glenn TW, Kilbane EG, Klarr DJ, Margolis JL, Uhler MD, Parent JM (2017) Rapid generation of human genetic loss-of-function iPSC lines by simultaneous reprogramming and gene editing. Stem Cell Rep 9(3):725–731. https://doi.org/10.1016/j.stemcr.2017.07.003
Tipton AE, Cruz Del Angel Y, Hixson K, Carlsen J, Strode D, Busquet N, Mesches MH, Gonzalez MI, Napoli E, Russek SJ, Brooks-Kayal AR (2023) Selective neuronal knockout of STAT3 function inhibits epilepsy progression, improves cognition, and restores dysregulated gene networks in a temporal lobe epilepsy model. Ann Neurol 94(1):106–122. https://doi.org/10.1002/ana.26644
Todd KG, Butterworth RF (2001) In vivo microdialysis in an animal model of neurological disease: thiamine deficiency (Wernicke) encephalopathy. Methods 23(1):55–61. https://doi.org/10.1006/meth.2000.1105
Tomasello DL, Wlodkowic D (2022) Noninvasive electrophysiology: emerging prospects in aquatic neurotoxicity testing. Environ Sci Technol 56(8):4788–4794. https://doi.org/10.1021/acs.est.1c08471
Tran CH, Vaiana M, Nakuci J, Somarowthu A, Goff KM, Goldstein N, Murthy P, Muldoon SF, Goldberg EM (2020) Interneuron desynchronization precedes seizures in a mouse model of Dravet syndrome. J Neurosci 40(13):2764–2775. https://doi.org/10.1523/jneurosci.2370-19.2020
Uff C, Frith D, Harrison C, Powell M, Kitchen N (2011) Sir Victor Horsley’s 19th century operations at the National Hospital for Neurology and Neurosurgery. Queen Square J Neurosurg 114(2):534–542. https://doi.org/10.3171/2010.9.Jns09731
van den Pol AN, Ozduman K, Wollmann G, Ho WS, Simon I, Yao Y, Rose JK, Ghosh P (2009) Viral strategies for studying the brain, including a replication-restricted self-amplifying delta-G vesicular stomatis virus that rapidly expresses transgenes in brain and can generate a multicolor golgi-like expression. J Comput Neurol 516(6):456–481. https://doi.org/10.1002/cne.22131
Vardy E, Robinson JE, Li C, Olsen RHJ, DiBerto JF, Giguere PM, Sassano FM, Huang XP, Zhu H, Urban DJ, White KL, Rittiner JE, Crowley NA, Pleil KE, Mazzone CM, Mosier PD, Song J, Kash TL, Malanga CJ, Krashes MJ, Roth BL (2015) A new DREADD facilitates the multiplexed chemogenetic interrogation of behavior. Neuron 86(4):936–946. https://doi.org/10.1016/j.neuron.2015.03.065
Vezzani A, Fu**ami RS, White HS, Preux PM, Blümcke I, Sander JW, Löscher W (2016) Infections, inflammation and epilepsy. Acta Neuropathol 131(2):211–234. https://doi.org/10.1007/s00401-015-1481-5
Villasana-Salazar B, Vezzani A (2023) Neuroinflammation microenvironment sharpens seizure circuit. Neurobiol Dis 178:106027. https://doi.org/10.1016/j.nbd.2023.106027
Vogt N (2021) Calcium imaging in the NIR. Nat Methods 18(1):32. https://doi.org/10.1038/s41592-020-01044-9
Walker MC, Kullmann DM (2020) Optogenetic and chemogenetic therapies for epilepsy. Neuropharmacology 168:107751. https://doi.org/10.1016/j.neuropharm.2019.107751
Wan Y, McDole K, Keller PJ (2019) Light-sheet microscopy and its potential for understanding developmental processes. Annu Rev Cell Dev Biol 35:655–681. https://doi.org/10.1146/annurev-cellbio-100818-125311
Wang Y, Chen Z (2019) An update for epilepsy research and antiepileptic drug development: toward precise circuit therapy. Pharmacol Ther 201:77–93. https://doi.org/10.1016/j.pharmthera.2019.05.010
Wang Y, Wang Y, Xu C, Wang S, Tan N, Chen C, Chen L, Wu X, Fei F, Cheng H, Lin W, Qi Y, Chen B, Liang J, Zhao J, Xu Z, Guo Y, Zhang S, Li X, Zhou Y, Duan S, Chen Z (2020) Direct septum-hippocampus cholinergic circuit attenuates seizure through driving somatostatin inhibition. Biol Psychiatry 87(9):843–856. https://doi.org/10.1016/j.biopsych.2019.11.014
Weng OY, Li Y, Wang LY (2022) Modeling epilepsy using human induced pluripotent stem cells-derived neuronal cultures carrying mutations in ion channels and the mechanistic target of rapamycin pathway. Front Mol Neurosci 15:810081. https://doi.org/10.3389/fnmol.2022.810081
Wengert ER, Miralles RM, Wedgwood KCA, Wagley PK, Strohm SM, Panchal PS, Idrissi AM, Wenker IC, Thompson JA, Gaykema RP, Patel MK (2021) Somatostatin-positive interneurons contribute to seizures in SCN8A epileptic encephalopathy. J Neurosci 41(44):9257–9273. https://doi.org/10.1523/jneurosci.0718-21.2021
Wenzel M, Hamm JP, Peterka DS, Yuste R (2017) Reliable and elastic propagation of cortical seizures in vivo. Cell Rep 19(13):2681–2693. https://doi.org/10.1016/j.celrep.2017.05.090
Wenzel M, Hamm JP, Peterka DS, Yuste R (2019) Acute focal seizures start as local synchronizations of neuronal ensembles. J Neurosci 39(43):8562–8575. https://doi.org/10.1523/jneurosci.3176-18.2019
Werner FM, Coveñas R (2019) Neural networks in generalized epilepsy and novel antiepileptic drugs. Curr Pharm Des 25(4):396–400. https://doi.org/10.2174/1381612825666190319121505
Whitebirch AC, LaFrancois JJ, Jain S, Leary P, Santoro B, Siegelbaum SA, Scharfman HE (2022) Enhanced excitability of the hippocampal CA2 region and its contribution to seizure activity in a mouse model of temporal lobe epilepsy. Neuron 110(19):3121-3138.e3128. https://doi.org/10.1016/j.neuron.2022.07.020
Wicker E, Hyder SK, Forcelli PA (2022) Pathway-specific inhibition of critical projections from the mediodorsal thalamus to the frontal cortex controls kindled seizures. Prog Neurobiol 214:102286. https://doi.org/10.1016/j.pneurobio.2022.102286
Wolf S, Supatto W, Debrégeas G, Mahou P, Kruglik SG, Sintes JM, Beaurepaire E, Candelier R (2015) Whole-brain functional imaging with two-photon light-sheet microscopy. Nat Methods 12(5):379–380. https://doi.org/10.1038/nmeth.3371
Wong RO (1998) Calcium imaging and multielectrode recordings of global patterns of activity in the develo** nervous system. Histochem J 30(3):217–229. https://doi.org/10.1023/a:1003251504594
**ao W, Jiao ZL, Senol E, Yao J, Zhao M, Zhao ZD, Chen X, Cao P, Fu Y, Gao Z, Shen WL, Xu XH (2022) Neural circuit control of innate behaviors. Sci China Life Sci 65(3):466–499. https://doi.org/10.1007/s11427-021-2043-2
Xu C, Wang Y, Zhang S, Nao J, Liu Y, Wang Y, Ding F, Zhong K, Chen L, Ying X, Wang S, Zhou Y, Duan S, Chen Z (2019) Subicular pyramidal neurons gate drug resistance in temporal lobe epilepsy. Ann Neurol 86(4):626–640. https://doi.org/10.1002/ana.25554
Xu L, Liu MZ, Yang YY, Wang Y, Hua XX, Du LX, Zhu JY, Shen Y, Wang YQ, Zhang L, Mi WL, Mu D (2022) Geraniol enhances inhibitory inputs to the paraventricular thalamic nucleus and induces sedation in mice. Phytomedicine 98:153965. https://doi.org/10.1016/j.phymed.2022.153965
Yang H, Shan W, Fan J, Deng J, Luan G, Wang Q, Zhang Y, You H (2022) Map** the neural circuits responding to deep brain stimulation of the anterior nucleus of the thalamus in the rat brain. Epilepsy Res 187:107027. https://doi.org/10.1016/j.eplepsyres.2022.107027
Yang W, Williams A, Sun QQ (2021) Circuit mechanisms underlying epileptogenesis in a mouse model of focal cortical malformation. Curr Biol 31(2):334-345.e334. https://doi.org/10.1016/j.cub.2020.10.029
Young JC, Vaughan DN, Nasser HM, Jackson GD (2019) Anatomical imaging of the piriform cortex in epilepsy. Exp Neurol 320:113013. https://doi.org/10.1016/j.expneurol.2019.113013
Zestos AG, Luna-Munguia H, Stacey WC, Kennedy RT (2019) Use and future prospects of in vivo microdialysis for epilepsy studies. ACS Chem Neurosci 10(4):1875–1883. https://doi.org/10.1021/acschemneuro.8b00271
Zhang CQ, McMahon B, Dong H, Warner T, Shen W, Gallagher M, Macdonald RL, Kang JQ (2019a) Molecular basis for and chemogenetic modulation of comorbidities in GABRG2-deficient epilepsies. Epilepsia 60(6):1137–1149. https://doi.org/10.1111/epi.15160
Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, Deisseroth K (2007) Multimodal fast optical interrogation of neural circuitry. Nature 446(7136):633–639. https://doi.org/10.1038/nature05744
Zhang L, Wang Y (2021) Gene therapy in epilepsy. Biomed Pharmacother 143:112075. https://doi.org/10.1016/j.biopha.2021.112075
Zhang LW, Bi AL, Li Q, Bi HS (2021a) Application of fiber photometry in neuroscience research. Sheng Li Xue Bao 73(2):306–314
Zhang M, Eichhorn SW, Zingg B, Yao Z, Cotter K, Zeng H, Dong H, Zhuang X (2021b) Spatially resolved cell atlas of the mouse primary motor cortex by MERFISH. Nature 598(7879):137–143. https://doi.org/10.1038/s41586-021-03705-x
Zhang X, Qiao Z, Liu N, Gao L, Wei L, Liu A, Ma Z, Wang F, Hou S, Li J, Shen H (2019b) Stereotypical patterns of epileptiform calcium signal in hippocampal CA1, CA3, dentate gyrus and entorhinal cortex in freely moving mice. Sci Rep 9(1):4518. https://doi.org/10.1038/s41598-019-41241-x
Zhou QG, Nemes AD, Lee D, Ro EJ, Zhang J, Nowacki AS, Dymecki SM, Najm IM, Suh H (2019) Chemogenetic silencing of hippocampal neurons suppresses epileptic neural circuits. J Clin Invest 129(1):310–323. https://doi.org/10.1172/jci95731
Zhu J, Lee KY, Jewett KA, Man HY, Chung HJ, Tsai NP (2017) Epilepsy-associated gene Nedd4-2 mediates neuronal activity and seizure susceptibility through AMPA receptors. PLoS Genet 13(2):e1006634. https://doi.org/10.1371/journal.pgen.1006634
Zou W, Guo Z, Suo L, Zhu J, He H, Li X, Wang K, Chen R (2022) Nucleus accumbens shell modulates seizure propagation in a mouse temporal lobe epilepsy model. Front Cell Dev Biol 10:1031872. https://doi.org/10.3389/fcell.2022.1031872
Acknowledgements
This study was supported by the Hunan Provincial Science and Technology Foundation (#2022JJ40817), the Scientific Research Project of Hunan Provincial Health Commission (#20200475), the Key research and development program of Hunan Province of China (#2022SK2042), the National Multidisciplinary Cooperative Diagnosis and Treatment Capacity Project for Major Diseases of **angya Hospital, Central South University(z027001).
Funding
No specific source of funding is associated with this work.
Author information
Authors and Affiliations
Contributions
WX and PL managed the data and drafted the manuscript; FK and JK drew the figures; JG and LW designed the outline and revised the manuscript; LW and XY proof-edited and finalized the manuscript; LL, GG, BX, and LW provided financial support. We confirm that the manuscript had been read and approved by all named authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare there are no conflicts of interest.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
**ao, W., Li, P., Kong, F. et al. Unraveling the Neural Circuits: Techniques, Opportunities and Challenges in Epilepsy Research. Cell Mol Neurobiol 44, 27 (2024). https://doi.org/10.1007/s10571-024-01458-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10571-024-01458-5