Abstract
Progress in stem cell research has revolutionized the medical field for more than two decades. More recently, the discovery of induced pluripotent stem cells (iPSCs) has allowed for the development of advanced disease modeling and tissue engineering platforms. iPSCs are generated from adult somatic cells by reprogramming them into an embryonic-like state via the expression of transcription factors required for establishing pluripotency. In the context of the central nervous system (CNS), iPSCs have the potential to differentiate into a wide variety of brain cell types including neurons, astrocytes, microglial cells, endothelial cells, and oligodendrocytes. iPSCs can be used to generate brain organoids by using a constructive approach in three-dimensional (3D) culture in vitro. Recent advances in 3D brain organoid modeling have provided access to a better understanding of cell-to-cell interactions in disease progression, particularly with neurotropic viral infections. Neurotropic viral infections have been difficult to study in two-dimensional culture systems in vitro due to the lack of a multicellular composition of CNS cell networks. In recent years, 3D brain organoids have been preferred for modeling neurotropic viral diseases and have provided invaluable information for better understanding the molecular regulation of viral infection and cellular responses. Here we provide a comprehensive review of the literature on recent advances in iPSC-derived 3D brain organoid culturing and their utilization in modeling major neurotropic viral infections including HIV-1, HSV-1, JCV, ZIKV, CMV, and SARS-CoV2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Three-dimensional (3D) brain organoid cultures
The human brain is the most complex organ in our body, with many aspects of its development and human-specific pathology remaining unknown because of limited accessibility to living human brain tissue. As a result, many approaches and models have been generated in an attempt to recapitulate the human brain in an in vitro system.
Two-dimensional (2D) cell culture systems have historically been used to model various diseases and systems in vitro; however, they are quite limited when it comes to modeling complex systems such as the human brain. 2D-cell culture systems lack the cellular organization that is present in the brain, as they are grown in a monolayer format which limits cell interactions only to their periphery. This results in a lack of proper oxygen and nutrient diffusion as well as waste clearance (Antoni et al. 2015). Furthermore, 2D systems lack the tissue complexity that is present in the brain. As cells are often cultured as a single cell type, they are missing the cell-to-cell interaction between different cell types that is present in vivo. To create a 2D system that is more resemblant to cellular interactions in the human brain, co-cultures of cells can be created, such as co-culturing neurons with other neural cell types such as microglia (Haenseler et al. 2017; Vahsen et al. 2022). Despite these co-culturing techniques, the cells are still limited to peripheral contacts and lack the organization needed to recapitulate the human brain in vitro.
Various neuronal cells, such as primary neurons and neuroblastoma cells, have been used in 2D systems in neuroscience research (Liu et al. 2022). However, obtaining patient-derived brain tissue, or neural stem cells and embryonic stem cells that can differentiate into neuronal cells, is controversial and not always accessible (Gabriel and Gopalakrishnan 2017). The innovation of induced pluripotent stem cell (iPSC) technology eased this problem by opening the doors to being able to generate human neural cell types without the need to isolate cells directly from the human Central Nervous System (CNS) (Gabriel and Gopalakrishnan 2017). The somatic cell-derived iPSCs are a type of stem cell that can differentiate into other cell types in the body (Lyadova and Vasiliev 2022), which makes them an available source for researchers (Karagiannis and Kim 2021). iPSCs were developed by Takahashi and Yamanaka 2006 at Kyoto University in Japan, where they first induced pluripotency in mouse embryonic stem cells (ESCs) and then in adult human fibroblast cells a year later (Takahashi and Yamanaka 2006; Takahashi et al. 2007). Takahashi and Yamanaka generated their iPSCs from fibroblast culture by using retroviral vectors to introduce specific transcription factors such as Oct3/4, Sox2, Klf4, and c-Myc into the skin cells. They noted that their iPSCs display both the morphology and growth properties of ESCs and express ESC-specific marker genes (Takahashi and Yamanaka 2006; Takahashi et al. 2007). Researchers have created techniques for generating iPSCs without using viral vectors, like plasmid-based or episomal reprogramming, that can eliminate the dangers linked to mutations due to viral integration into the genome (Bang et al. 2018). This method involves electroporation, where the electrical shocks introduce plasmid into the genome of the cells. Additionally, CRISPR-based reprogramming allows for the precise and efficient editing of certain genes for successful reprogramming (Liu et al. 2018).
Human iPSCs (hiPSC) prove a powerful tool for easily generating human neural cell types in culture; however, the differentiated cells are still 2D and limited in the same way other 2D cell culture systems are, as listed previously. This led to the need to develop alternative models to study such systems in vitro while mimicking an in vivo environment, such as 3D culture systems. One of the first successful 3D neural systems was that of neurospheres. Neurospheres are 3D cell aggregates of multipotent neural stem cells (NSC) grown in culture, providing a good resource for studying NSCs in vitro (Soares et al. 2021). These clusters of NSCs can then be differentiated into varying cell types, such as neurons and glial cells, all within the same sphere, also known as a neural spheroid (Dingle et al. 2015; Zhou et al. 2016; Pamies et al. 2017). This further allows for a better representation of cell-to-cell interactions in vitro. Although these systems provide a better representation of the brain in vitro than traditional 2D cell culture, they lack complete cellular composition, organization, and complexity of the human brain (Reynolds et al. 1992; Pamies et al. 2017). The human brain has very specific region specificity and cellular organization that is crucial to its function, and this is lacking in neurospheres and neural spheroids as the cells do not organize (Dingle et al. 2015).
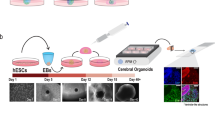
Unlike 2D cell culture systems and neurospheres, brain organoids are able to model the human brain at a cellular, structural, and developmental level, allowing researchers to model the human brain and its function in ways that were previously impossible. Brain organoids were first generated by Lancaster et al. in (2013) as a system to study microcephaly. They were able to successfully generate an iPSC-derived 3D cell system, which they dubbed “cerebral organoids” (COs), that displayed discrete brain regions, dorsal cortical organization, functional cortical neurons, and glial cell populations (Lancaster et al. 2013). The development of this system has been a major breakthrough in neural sciences research as it was the first time the human brain was able to be recapitulated in vitro with correct organization and patterning.
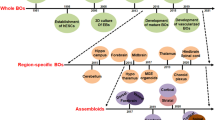
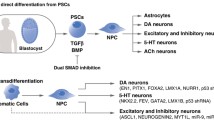
To generate organoids, specific conditions, like extracellular matrix (ECM), small molecules, and growth factors, are provided to iPSCs or tissue-derived cells (TDCs) (Zhao et al. 2022). Thus, this environment will differentiate iPSCs or TDCs into the tissue of interest, such as the lung, heart, and cerebral cortex (Zhao et al. 2022). Researchers use stem cells such as iPSCs to generate brain organoids due to their availability (Gabriel and Gopalakrishnan 2017). This method involves differentiating single-cell iPSCs into embryoid bodies (EBs) and then NSCs by using small molecules and growth factors (Hong et al. 2022). Neuroepithelium cells form during the induction phase of EBs (Hong et al. 2022). The expansion phase involves embedding the EBs in ECM such as Matrigel, which results in a budding morphology and promotes further differentiation into several cell types present in COs, such as NSCs, neurons, and glial cells (Agboola et al. 2021). The expanded EBs are cultured in suspension on an orbital shaker (Lancaster and Knoblich 2014) or in a spinning bioreactor (Qian et al. 2016) during and after the maturation phase, where they become self-organized COs.
The organoids generated using these protocols, known as “unguided organoids,” as they are allowed to freely organize themselves into forebrain, midbrain, and hindbrain regions (Lancaster et al. 2013; Qian et al. 2016). This allows for a recapitulation of the entire brain in vitro which is an extremely useful tool; however, some diseases affect specific regions of the brain. As a result, it is necessary to be able to model specifically the forebrain, midbrain, or hindbrain alone, as well as specific structures in the brain as organoids.
Many groups of researchers have worked to develop guided protocols using extrinsic factors to generate brain region-specific organoids, which contain more accurate cell populations and organization of specific brain regions and structures. A commonly used guided organoid is a cortical organoid, which is representative of the cerebral cortex. Cortical organoids have been used to study a variety of neural disorders, such as Zika virus (ZIKV) infection (Qian et al. 2016), Japanese encephalitis virus (Zhang et al. 2018), Alzheimer’s disease (AD) (Raja et al. 2016), and several other neural degenerative disorders. Beyond cortical organoids, many other brain regions and structures have successfully been generated using guide protocols. These include forebrain and midbrain organoids (Raja et al. 2016; Jo et al. 2017; Liu et al. 2019; Krenn et al. 2021). In conclusion, brain organoids possess a superior in vitro model to study ZIKV infection, allowing researchers to better understand the viral pathophysiology and utilize them as a platform to screen therapeutic interventions.
Another neurotropic virus that has been associated with microcephaly is the human Cytomegalovirus (CMV). CMV belongs to the Herpesviridae family and is a double-stranded DNA virus that can infect people of all ages. Although the majority of CMV infections are asymptomatic, newborns with congenital CMV infection may present neurologic anomalies such as lethargy and microcephaly (Boppana et al. 2013). Several groups have reported CMV infection modeling in brain organoids using unguided protocols. CMV infection in brain organoids disrupts organoid morphology, probably due to the induced cell death leading to size reduction (Sun et al. 2020). In addition, infection with CMV interferes with neurogenesis and the formation of neural rosette formation from NPCs (Sison et al. 2019). Furthermore, neural signaling, as well as networks important for neurodevelopment, has been shown to be downregulated following CMV infection in brain organoids (O’Brien et al. 2022). Moreover, brain organoids were also modeled for testing the therapeutic potential of neutralizing antibodies for the treatment of newborns with congenital CMV infection (Sun et al. 2020).
In the past three years, the COVID-19 pandemic has been a major health problem worldwide. Although the major target of SARS-CoV-2 is the respiratory system, the viral tropism involves multi-organ systems (Liu et al. 2021). Neurological manifestations in COVID-19 patients have been reported, although the neuropathology associated with SARS-CoV-2 is yet to be elucidated (Mao et al. 2020; Douaud et al. 2022; Ng et al. 2023). In recent years, several brain organoid models have been developed as a tool to gain insight into SARS-CoV-2 neuroinvasion in the CNS, using guided and unguided protocols. Yi et al. reported persistent ACE2 expression during the development of dorsal forebrain organoids from hESCs and showed susceptibility to infection using a SARS-CoV-2-pseudovirus (Yi et al. 2020). Zhang et al. also reported that ACE2, TMPRSS2, and coronavirus entry-associated proteases (cathepsin L, and furin) are readily available in hNPCs and brain organoids generated from hiPSCs and showed that the brain organoids support SARS-CoV-2 infection and replication (Zhang et al. 2020). Jacob et al. have further investigated SARS-CoV-2 infection in region-specific hiPSCs-derived brain organoids. Using cortical, hippocampal, hypothalamic, and midbrain organoids, they reported limited infection in neurons and astrocytes, but robust infection in choroid plexus (ChP) epithelial cells. Moreover, they developed ChP organoids from hiPSCS and reported productive SARS-CoV-2 infection associated with cell death (Jacob et al. 2020). Several other groups also reported SARS-CoV-2 infection modeling in dorsal cortical organoids or cortical organoids. Those studies revealed that SARS-CoV-2 can infect glial cells (McMahon et al. 2021; Andrews et al. 2022), ChP cells (McMahon et al. 2021), and neuronal cells (Zhang et al. 2020; Song et al. 2021). Mesci et al. showed that infections in neurons promote cell death with the loss of excitatory synapses. Moreover, the same group showed that the antiviral drug Sofosbuvir inhibits SARS-CoV-2 replication in a model of cortical organoids (Mesci et al. 2022). Hou et al. also utilized forebrain and midbrain organoids to study replication efficiency and neurotropism of different variants of the virus (SARS-CoV-2 WT, Delta, Omicron BA.1 and Omicron BA.2) and demonstrated a higher replication efficiency of variant Omicron BA.2 with positive viral infection in dopaminergic neurons in midbrain organoids and cortical neurons in forebrain organoids (Hou et al. 2022). Unguided brain organoids have also been developed to mainly demonstrate the virus’s cellular tropism. In line with findings using guided protocols, various neural cell types were shown to be susceptible to infection with SARS-CoV-2 (Bullen 2020; Ramani et al. 2020; Tiwari et al. 2021). Another group showed that SARS-CoV-2 infection in neurons was boosted by the presence of astrocytes in brain organoids which also led to synaptic loss and neuronal toxicity (Wang et al. 2021). Infection in astrocytes was also linked to the promotion of neuronal death (Kong et al. 2022). Pellegrini et al. reported SARS-CoV-2 infection in ChP with limited neuronal infection, probably due to the higher expression levels of ACE2 in this cell type (Pellegrini et al. 2020). Moreover, by using brain organoid models, Samudyata et al. showed SARS-CoV-2 nucleocapsid protein expression in PAX6, MAP2, GFAP, SOX10, OLIG2, and Iba1 positive cells, and revealed a role of microglia in infected organoids in increasing the engulfment of postsynaptic termini with increased phagocytosis and synapse elimination (Samudyata et al. 2022). In summary, brain organoid models have provided a superior platform to investigate neuronal susceptibility, disease mechanisms, and treatment strategies for SARS-CoV-2 infection.
Future advancements
As far as COs have come in recent years, there is still much improvement to be done. One of the biggest hindrances in CO models is the fact that they are size-restricted due to the lack of nutrient and oxygen diffusion to their centers. An improvement in this area would allow for the generation of larger, more complex, and more stable COs and organoids in general. This area is currently being explored in a variety of ways, such as bioengineering special devices to increase diffusion and organoid size (i.e., spinning bioreactors and microfluidic chips) (Lancaster et al. 2013; Kim et al. 2015; Qian et al. 2016; Karzbrun et al. 2018). Another method for improving the diffusion of nutrients and oxygen in COs is to establish vascularization within the organoids. Not only would vascularization increase nutrient diffusion throughout the CO, but it would allow for the addition of a BBB component, which is extremely important in modeling neurovirology and is lacking in brain COs (Miller et al. 2012). As mentioned previously, several attempts at vascularization in organoids have been made through various means and with various degrees of success. Methods such as endothelial cell co-culture and assembloids between vascular organoids and COs have been used with relative levels of success (Pham et al. 2018; Sun et al. 2022). However, these protocols are tedious and complicated and are not yet a reliable and feasible way to generate vascularized COs. Thus, creating reliable and reproducible methods to generate vascularized COs is an area that needs to be explored to generate more accurate models of neuroviral infections, and of the human brain modeling in general. There are also reported variations between COs due to a variety of factors. Donor cell variability due to genetic background and sex differences needs to be further characterized, as they can lead to large differences between different lines of iPSCs (Burrows et al. 2016; Volpato and Webber 2020). Additionally, there can be quite a large batch variability between COs generated from the same line of iPSCs. Batch variability from the same iPSC lines may be a result of handling, source of growth factors, media, equipment, and differences in protocols. Further improvements in protocols and techniques are needed to improve the reliability, reproducibility, and batch variations. Furthermore, new methods for measuring the electrophysiology of COs may be explored through means such as an optimized multi-electrode array. The CO models of the human brain is still a new era of modeling neurotropic viral infections and there is an endless number of possibilities that can and should be explored to improve these systems.
Conclusions
3D brain organoid models are clearly a superior in vitro platform over 2D-cell cultures with their tissue-cell complexities comparable to in vivo conditions. Human brain organoids are able to represent the human brain at the cellular, structural, and developmental levels, allowing researchers to model neurotropic viral infections in ways that were previously not possible. Brain organoids are developed by “unguided protocols,” allowing them to freely organize into the forebrain, midbrain, and hindbrain or “guided protocols” for generating organoids representing specific brain regions of interest (Fig. 1). Over the last decade, many groups of researchers have developed various types of brain organoids and modeled major neurotropic viral infections (Table 1). As we reviewed above, brain organoid models have provided invaluable knowledge towards a better understanding of molecular regulation of neurotropic viral infections and cellular responses. Although they are proven to be a significantly better platform for modeling the brain in vitro, brain organoids also possess some limitations. They are limited in size and prone to cell death at their center due to the limited diffusion of nutrients and oxygen. Improvements with vascularization attempts are in progress with promising outcomes and the biotechnology for 3D modeling of brain organoids is a fast-growing research era. Nonetheless, 3D brain organoids have shown their potential to take the in vitro culture systems to a new level and have allowed for better modeling of neurotropic viral infections.
Data Availability
The data generated during the current study are available from the corresponding author on reasonable request.
References
Abrahamson EE, Zheng W, Muralidaran V et al (2021) Modeling Aβ42 accumulation in response to herpes simplex virus 1 infection: 2D or 3D? J Virol 95:e02219–20, JVI.02219–20. https://doi.org/10.1128/JVI.02219-20
Agboola OS, Hu X, Shan Z et al (2021) Brain organoid: a 3D technology for investigating cellular composition and interactions in human neurological development and disease models in vitro. Stem Cell Res Ther 12:430. https://doi.org/10.1186/s13287-021-02369-8
Andrews MG, Mukhtar T, Eze UC et al (2022) Tropism of SARS-CoV-2 for human cortical astrocytes. Proc Natl Acad Sci USA 119:e2122236119. https://doi.org/10.1073/pnas.2122236119
Antinori A, Arendt G, Becker JT et al (2007) Updated research nosology for HIV-associated neurocognitive disorders. Neurology 69:1789–1799. https://doi.org/10.1212/01.WNL.0000287431.88658.8b
Antoni D, Burckel H, Josset E, Noel G (2015) Three-dimensional cell culture: a breakthrough in vivo. Int J Mol Sci 16:5517–5527. https://doi.org/10.3390/ijms16035517
Bagley JA, Reumann D, Bian S et al (2017) Fused cerebral organoids model interactions between brain regions. Nat Methods 14:743–751. https://doi.org/10.1038/nmeth.4304
Ballabio C, Anderle M, Gianesello M et al (2020) Modeling medulloblastoma in vivo and with human cerebellar organoids. Nat Commun 11:583. https://doi.org/10.1038/s41467-019-13989-3
Bang JS, Choi NY, Lee M et al (2018) Optimization of episomal reprogramming for generation of human induced pluripotent stem cells from fibroblasts. Animal Cells and Systems 22:132–139. https://doi.org/10.1080/19768354.2018.1451367
Baranello R, Bharani K, Padmaraju V et al (2015) Amyloid-beta protein clearance and degradation (ABCD) pathways and their role in Alzheimer’s disease. CAR 12:32–46. https://doi.org/10.2174/1567205012666141218140953
Barreras P, Pamies D, Monaco MC et al (2022) A human-derived 3D brain organoid model to study JC virus infection. J Neurovirol 28:17–26. https://doi.org/10.1007/s13365-022-01062-7
Barth H, Solis M, Kack-Kack W et al (2016) In vitro and in vivo models for the study of human polyomavirus infection. Viruses 8:292. https://doi.org/10.3390/v8100292
Boppana SB, Ross SA, Fowler KB (2013) Congenital cytomegalovirus infection: clinical outcome. Clin Infect Dis 57:S178–S181. https://doi.org/10.1093/cid/cit629
Brack-Werner R (1999) Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. AIDS 13:1–22. https://doi.org/10.1097/00002030-199901140-00003
Bradshaw MJ, Venkatesan A (2016) Herpes simplex virus-1 encephalitis in adults: pathophysiology, diagnosis, and management. Neurotherapeutics 13:493–508. https://doi.org/10.1007/s13311-016-0433-7
Broccoli V, Giannelli SG, Mazzara PG (2014) Modeling physiological and pathological human neurogenesis in the dish. Front Neurosci 8. https://doi.org/10.3389/fnins.2014.00183
Bullen CK (2020) Infectability of human brainsphere neurons suggests neurotropism of SARS-CoV-2*. ALTEX. https://doi.org/10.14573/altex.2006111
Burrows CK, Banovich NE, Pavlovic BJ et al (2016) Genetic variation, not cell type of origin, underlies the majority of identifiable regulatory differences in iPSCs. PLoS Genet 12:e1005793. https://doi.org/10.1371/journal.pgen.1005793
Cairns DM, Rouleau N, Parker RN et al (2020) A 3D human brain–like tissue model of herpes-induced Alzheimer’s disease. Sci Adv 6:eaay8828. https://doi.org/10.1126/sciadv.aay8828
Chansard A, Dubrulle N, Poujol de Molliens M et al (2021) Unveiling interindividual variability of human fibroblast innate immune response using robust cell-based protocols. Front Immunol 11:569331. https://doi.org/10.3389/fimmu.2020.569331
Clifford DB, Ances BM (2013) HIV-associated neurocognitive disorder. Lancet Infect Dis 13:976–986. https://doi.org/10.1016/S1473-3099(13)70269-X
Cortese I, Reich DS, Nath A (2021) Progressive multifocal leukoencephalopathy and the spectrum of JC virus-related disease. Nat Rev Neurol 17:37–51. https://doi.org/10.1038/s41582-020-00427-y
Cugola FR, Fernandes IR, Russo FB et al (2016) The Brazilian Zika virus strain causes birth defects in experimental models. Nature 534:267–271. https://doi.org/10.1038/nature18296
D’Aiuto L, Bloom DC, Naciri JN et al (2019) Modeling herpes simplex virus 1 infections in human central nervous system neuronal cells using two- and three-dimensional cultures derived from induced pluripotent stem cells. J Virol 93:e00111-e119. https://doi.org/10.1128/JVI.00111-19
D’Aiuto L, Caldwell JK, Wallace CT et al (2022) The impaired neurodevelopment of human neural rosettes in HSV-1-infected early brain organoids. Cells 11:3539. https://doi.org/10.3390/cells11223539
Dang J, Tiwari SK, Lichinchi G et al (2016) Zika virus depletes neural progenitors in human cerebral organoids through activation of the innate immune receptor TLR3. Cell Stem Cell 19:258–265. https://doi.org/10.1016/j.stem.2016.04.014
Dasgupta G, BenMohamed L (2011) Of mice and not humans: How reliable are animal models for evaluation of herpes CD8+-T cell-epitopes-based immunotherapeutic vaccine candidates? Vaccine 29:5824–5836. https://doi.org/10.1016/j.vaccine.2011.06.083
Deeks SG, Overbaugh J, Phillips A, Buchbinder S (2015) HIV Infection Nat Rev Dis Primers 1:15035. https://doi.org/10.1038/nrdp.2015.35
Denes CE, Everett RD, Diefenbach RJ (2020) Tour de herpes: cycling through the life and biology of HSV-1. Methods Mol Biol 2060:1–30. https://doi.org/10.1007/978-1-4939-9814-2_1
Dingle YT, Boutin ME, Chirila AM et al (2015) Three-dimensional neural spheroid culture: an in vitro model for cortical studies. Tissue Eng Part C Methods 21:1274–1283. https://doi.org/10.1089/ten.TEC.2015.0135
Dos Reis RS, Sant S, Ayyavoo V (2023) Three-dimensional human brain organoids to model HIV-1 neuropathogenesis. Methods Mol Biol 2610:167–178. https://doi.org/10.1007/978-1-0716-2895-9_14
dos Reis RS, Sant S, Keeney H et al (2020) Modeling HIV-1 neuropathogenesis using three-dimensional human brain organoids (hBORGs) with HIV-1 infected microglia. Sci Rep 10:15209. https://doi.org/10.1038/s41598-020-72214-0
Douaud G, Lee S, Alfaro-Almagro F et al (2022) SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 604:697–707. https://doi.org/10.1038/s41586-022-04569-5
Enting RH, Prins JM, Jurriaans S et al (2001) Concentrations of human immunodeficiency virus type 1 (HIV-1) RNA in cerebrospinal fluid after antiretroviral treatment initiated during primary HIV-1 infection. Clin Infect Dis 32:1095–1099. https://doi.org/10.1086/319602
Eura N, Matsui TK, Luginbühl J et al (2020) Brainstem organoids from human pluripotent stem cells. Front Neurosci 14:538. https://doi.org/10.3389/fnins.2020.00538
Ferenczy MW, Johnson KR, Marshall LJ et al (2013) Differentiation of human fetal multipotential neural progenitor cells to astrocytes reveals susceptibility factors for JC virus. J Virol 87:6221–6231. https://doi.org/10.1128/JVI.00396-13
Ferenczy MW, Marshall LJ, Nelson CDS et al (2012) Molecular biology, epidemiology, and pathogenesis of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev 25:471–506. https://doi.org/10.1128/CMR.05031-11
Gabriel E, Gopalakrishnan J (2017) Generation of iPSC-derived human brain organoids to model early neurodevelopmental disorders. JoVE 55372. https://doi.org/10.3791/55372
Gabriel E, Ramani A, Karow U et al (2017) Recent zika virus isolates induce premature differentiation of neural progenitors in human brain organoids. Cell Stem Cell 20:397-406.e5. https://doi.org/10.1016/j.stem.2016.12.005
Garcez PP, Loiola EC, Madeiro da Costa R et al (2016) Zika virus impairs growth in human neurospheres and brain organoids. Science 352:816–818. https://doi.org/10.1126/science.aaf6116
Garcia-Mesa Y, Jay TR, Checkley MA et al (2017) Immortalization of primary microglia: a new platform to study HIV regulation in the central nervous system. J Neurovirol 23:47–66. https://doi.org/10.1007/s13365-016-0499-3
Gebhardt BM, Halford WP (2005) Evidence that spontaneous reactivation of herpes virus does not occur in mice. Virol J 2:67. https://doi.org/10.1186/1743-422X-2-67
González-Scarano F, Martín-García J (2005) The neuropathogenesis of AIDS. Nat Rev Immunol 5:69–81. https://doi.org/10.1038/nri1527
Gumbs SBH, Berdenis van Berlekom A, Kübler R et al (2022) Characterization of HIV-1 infection in microglia-containing human cerebral organoids. Viruses 14:829. https://doi.org/10.3390/v14040829
Gussow AM, Giordani NV, Tran RK et al (2006) Tissue-specific splicing of the herpes simplex virus type 1 latency-associated transcript (LAT) intron in LAT transgenic mice. J Virol 80:9414–9423. https://doi.org/10.1128/JVI.00530-06
Haenseler W, Sansom SN, Buchrieser J et al (2017) A highly efficient human pluripotent stem cell microglia model displays a neuronal-co-culture-specific expression profile and inflammatory response. Stem Cell Reports 8:1727–1742. https://doi.org/10.1016/j.stemcr.2017.05.017
Hong YJ, Lee S been, Choi J et al (2022) A simple method for generating cerebral organoids from human pluripotent stem cells. IJSC 15:95–103. https://doi.org/10.15283/ijsc21195
Hou Y, Li C, Yoon C et al (2022) Enhanced replication of SARS-CoV-2 omicron BA.2 in human forebrain and midbrain organoids. Sig Transduct Target Ther 7:381. https://doi.org/10.1038/s41392-022-01241-2
Huang WK, Wong SZH, Pather SR et al (2021) Generation of hypothalamic arcuate organoids from human induced pluripotent stem cells. Cell Stem Cell 28:1657-1670.e10. https://doi.org/10.1016/j.stem.2021.04.006
Huangui **ong HT (2013) Astrocyte Dysfunctions and HIV-1 Neurotoxicity. J AIDS Clin Res 04. https://doi.org/10.4172/2155-6113.1000255
Jacob F, Pather SR, Huang W-K et al (2020) Human pluripotent stem cell-derived neural cells and brain organoids reveal SARS-CoV-2 neurotropism predominates in choroid plexus epithelium. Cell Stem Cell 27:937-950.e9. https://doi.org/10.1016/j.stem.2020.09.016
Jo J, **ao Y, Sun AX et al (2016) Midbrain-like organoids from human pluripotent stem cells contain functional dopaminergic and neuromelanin-producing neurons. Cell Stem Cell 19:248–257. https://doi.org/10.1016/j.stem.2016.07.005
Karagiannis P, Kim S-I (2021) iPSC-derived natural killer cells for cancer immunotherapy. Mol Cells 44:541–548. https://doi.org/10.14348/molcells.2021.0078
Karzbrun E, Kshirsagar A, Cohen SR et al (2018) Human brain organoids on a chip reveal the physics of folding. Nat Phys 14:515–522. https://doi.org/10.1038/s41567-018-0046-7
Kim J-Y, Fluri DA, Marchan R et al (2015) 3D spherical microtissues and microfluidic technology for multi-tissue experiments and analysis. J Biotechnol 205:24–35. https://doi.org/10.1016/j.jbiotec.2015.01.003
Kimberlin DW (2004) Neonatal herpes simplex infection. Clin Microbiol Rev 17:1–13. https://doi.org/10.1128/CMR.17.1.1-13.2004
King CA, Li X, Barbachano-Guerrero A, Bhaduri-McIntosh S (2015) STAT3 regulates lytic activation of kaposi’s sarcoma-associated herpesvirus. J Virol 89:11347–11355. https://doi.org/10.1128/JVI.02008-15
Kondo Y, Windrem MS, Zou L et al (2014) Human glial chimeric mice reveal astrocytic dependence of JC virus infection. J Clin Invest 124:5323–5336. https://doi.org/10.1172/JCI76629
Kong W, Montano M, Corley MJ et al (2022) Neuropilin-1 Mediates SARS-CoV-2 infection of astrocytes in brain organoids, inducing inflammation leading to dysfunction and death of neurons. mBio 13:e0230822. https://doi.org/10.1128/mbio.02308-22
Kovalevich J, Langford D (2012) Neuronal toxicity in HIV CNS disease. Future Virol 7:687–698. https://doi.org/10.2217/fvl.12.57
Krenn V, Bosone C, Burkard TR et al (2021) Organoid modeling of Zika and herpes simplex virus 1 infections reveals virus-specific responses leading to microcephaly. Cell Stem Cell 28:1362-1379.e7. https://doi.org/10.1016/j.stem.2021.03.004
Lafaille FG, Pessach IM, Zhang S-Y et al (2012) Impaired intrinsic immunity to HSV-1 in human iPSC-derived TLR3-deficient CNS cells. Nature 491:769–773. https://doi.org/10.1038/nature11583
Lancaster MA, Knoblich JA (2014) Generation of cerebral organoids from human pluripotent stem cells. Nat Protoc 9:2329–2340. https://doi.org/10.1038/nprot.2014.158
Lancaster MA, Renner M, Martin CA et al (2013) Cerebral organoids model human brain development and microcephaly. Nature 501:373–379. https://doi.org/10.1038/nature12517
Li Puma DD, Piacentini R, Leone L et al (2019) Herpes simplex virus type-1 infection impairs adult hippocampal neurogenesis via amyloid-β protein accumulation. Stem Cells 37:1467–1480. https://doi.org/10.1002/stem.3072
Li Z, Xu J, Lang Y et al (2020) JMX0207, a niclosamide derivative with improved pharmacokinetics, suppresses zika virus infection both in vitro and in vivo. ACS Infect Dis 6:2616–2628. https://doi.org/10.1021/acsinfecdis.0c00217
Li Z, Xu J, Lang Y et al (2022) In vitro and in vivo characterization of erythrosin B and derivatives against Zika virus. Acta Pharmaceutica Sinica B 12:1662–1670. https://doi.org/10.1016/j.apsb.2021.10.017
Linard M, Letenneur L, Garrigue I et al (2020) Interaction between APOE4 and herpes simplex virus type 1 in Alzheimer’s disease. Alzheimer’s Dement 16:200–208. https://doi.org/10.1002/alz.12008
Lindenbach BD, Rice CM (2003) Molecular biology of flaviviruses. In: Advances in Virus Research. Elsevier, pp 23–61
Liu J, Li Y, Liu Q et al (2021) SARS-CoV-2 cell tropism and multiorgan infection. Cell Discov 7:17. https://doi.org/10.1038/s41421-021-00249-2
Liu L, Chen Z, Zhang X et al (2019) Protection of ZIKV infection-induced neuropathy by abrogation of acute antiviral response in human neural progenitors. Cell Death Differ 26:2607–2621. https://doi.org/10.1038/s41418-019-0324-7
Liu P, Chen M, Liu Y et al (2018) CRISPR-based chromatin remodeling of the endogenous Oct4 or Sox2 locus enables reprogramming to pluripotency. Cell Stem Cell 22:252-261.e4. https://doi.org/10.1016/j.stem.2017.12.001
Liu R, Meng X, Yu X et al (2022) From 2D to 3D co-culture systems: a review of co-culture models to study the neural cells interaction. IJMS 23:13116. https://doi.org/10.3390/ijms232113116
Lyadova I, Vasiliev A (2022) Macrophages derived from pluripotent stem cells: prospective applications and research gaps. Cell Biosci 12:96. https://doi.org/10.1186/s13578-022-00824-4
Major EO, Miller AE, Mourrain P et al (1985) Establishment of a line of human fetal glial cells that supports JC virus multiplication. Proc Natl Acad Sci USA 82:1257–1261. https://doi.org/10.1073/pnas.82.4.1257
Major EO, Vacante DA (1989) Human fetal astrocytes in culture support the growth of the neurotropic human polyomavirus. JCV: J Neuropathol Exper Neurol 48:425–436. https://doi.org/10.1097/00005072-198907000-00004
Mallard J, Williams KC (2018) Animal models of HIV-associated disease of the central nervous system. In: Handbook of Clinical Neurology. Elsevier, pp 41–53
Mandl C, Walker DL, Frisque RJ (1987) Derivation and characterization of POJ cells, transformed human fetal glial cells that retain their permissivity for JC virus. J Virol 61:755–763. https://doi.org/10.1128/jvi.61.3.755-763.1987
Mansour AA, Gonçalves JT, Bloyd CW et al (2018) An in vivo model of functional and vascularized human brain organoids. Nat Biotechnol 36:432–441. https://doi.org/10.1038/nbt.4127
Mao L, ** H, Wang M et al (2020) Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan. China JAMA Neurol 77:683. https://doi.org/10.1001/jamaneurol.2020.1127
Marquez L, Levy ML, Munoz FM, Palazzi DL (2011) A report of three cases and review of intrauterine herpes simplex virus infection. Pediatric Infectious Disease Journal 30:153–157. https://doi.org/10.1097/INF.0b013e3181f55a5c
Matsumoto R, Suga H, Aoi T et al (2020) Congenital pituitary hypoplasia model demonstrates hypothalamic OTX2 regulation of pituitary progenitor cells. J Clin Invest 130:641–654. https://doi.org/10.1172/JCI127378
Mazzara PG, Criscuolo E, Rasponi M et al (2022) A human stem cell-derived neurosensory–epithelial circuitry on a chip to model herpes simplex virus reactivation. Biomedicines 10:2068. https://doi.org/10.3390/biomedicines10092068
McMahon CL, Staples H, Gazi M et al (2021) SARS-CoV-2 targets glial cells in human cortical organoids. Stem Cell Reports 16:1156–1164. https://doi.org/10.1016/j.stemcr.2021.01.016
Mesci P, de Souza JS, Martin-Sancho L et al (2022) SARS-CoV-2 infects human brain organoids causing cell death and loss of synapses that can be rescued by treatment with Sofosbuvir. PLoS Biol 20:e3001845. https://doi.org/10.1371/journal.pbio.3001845
Messacar K, Fischer M, Dominguez SR et al (2018) Encephalitis in US children. Infect Dis Clin North Am 32:145–162. https://doi.org/10.1016/j.idc.2017.10.007
Messam CA, Hou J, Gronostajski RM, Major EO (2003) Lineage pathway of human brain progenitor cells identified by JC virus susceptability. Ann Neurol 53:636–646. https://doi.org/10.1002/ana.10523
Miller F, Afonso PV, Gessain A, Ceccaldi P-E (2012) Blood-Brain Barrier and Retroviral Infections Virulence 3:222–229. https://doi.org/10.4161/viru.19697
Miskin DP, Koralnik IJ (2015) Novel syndromes associated with JC virus infection of neurons and meningeal cells: no longer a gray area. Curr Opin Neurol 28:288–294. https://doi.org/10.1097/WCO.0000000000000201
Miura Y, Li MY, Revah O et al (2022) Engineering brain assembloids to interrogate human neural circuits. Nat Protoc 17:15–35. https://doi.org/10.1038/s41596-021-00632-z
Miyamura T, Yoshiike K, Takemoto KK (1980) Characterization of JC papovavirus adapted to growth in human embryonic kidney cells. J Virol 35:498–504. https://doi.org/10.1128/jvi.35.2.498-504.1980
Mlakar J, Korva M, Tul N et al (2016) Zika virus associated with microcephaly. N Engl J Med 374:951–958. https://doi.org/10.1056/NEJMoa1600651
Ng J-H, Sun A, Je HS, Tan E-K (2023) Unravelling pathophysiology of neurological and psychiatric complications of COVID-19 using brain organoids. Neuroscientist 29:30–40. https://doi.org/10.1177/10738584211015136
Nilsson P, Iwata N, Muramatsu S et al (2010) Gene therapy in Alzheimer’s disease - potential for disease modification. J Cell Mol Med 14:741–757. https://doi.org/10.1111/j.1582-4934.2010.01038.x
O’Brien BS, Mokry RL, Schumacher ML et al (2022) Downregulation of neurodevelopmental gene expression in iPSC-derived cerebral organoids upon infection by human cytomegalovirus. iScience 25:104098. https://doi.org/10.1016/j.isci.2022.104098
Ormel PR, Vieira de Sá R, van Bodegraven EJ et al (2018) Microglia innately develop within cerebral organoids. Nat Commun 9:4167. https://doi.org/10.1038/s41467-018-06684-2
Padgett BL, Walker DL, ZuRhein GM et al (1971) Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet 1:1257–1260. https://doi.org/10.1016/s0140-6736(71)91777-6
Pamies D, Barreras P, Block K et al (2017) A human brain microphysiological system derived from induced pluripotent stem cells to study neurological diseases and toxicity. ALTEX 34:362–376. https://doi.org/10.14573/altex.1609122
Pellegrini L, Albecka A, Mallery DL et al (2020) SARS-CoV-2 Infects the brain choroid plexus and disrupts the blood-CSF barrier in human brain organoids. Cell Stem Cell 27:951-961.e5. https://doi.org/10.1016/j.stem.2020.10.001
Pham MT, Pollock KM, Rose MD et al (2018) Generation of human vascularized brain organoids. NeuroReport 29:588–593. https://doi.org/10.1097/WNR.0000000000001014
Pichler M, Staffler A, Bonometti N et al (2015) Premature newborns with fatal intrauterine herpes simplex virus-1 infection: first report of twins and review of the literature. J Eur Acad Dermatol Venereol 29:1216–1220. https://doi.org/10.1111/jdv.12583
Qian X, Nguyen HN, Song MM et al (2016) Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165:1238–1254. https://doi.org/10.1016/j.cell.2016.04.032
Qiao H, Guo M, Shang J et al (2020) Herpes simplex virus type 1 infection leads to neurodevelopmental disorder-associated neuropathological changes. PLoS Pathog 16:e1008899. https://doi.org/10.1371/journal.ppat.1008899
Qiao H, Zhao W, Guo M et al (2022) Cerebral organoids for modeling of HSV-1-induced-amyloid β associated neuropathology and phenotypic rescue. IJMS 23:5981. https://doi.org/10.3390/ijms23115981
Rai MA, Hammonds J, Pujato M et al (2020) Comparative analysis of human microglial models for studies of HIV replication and pathogenesis. Retrovirology 17:35. https://doi.org/10.1186/s12977-020-00544-y
Raja WK, Mungenast AE, Lin YT et al (2016) Self-organizing 3D human neural tissue derived from induced pluripotent stem cells recapitulate Alzheimer’s disease phenotypes. PLoS One 11:e0161969. https://doi.org/10.1371/journal.pone.0161969
Ramani A, Müller L, Ostermann PN et al (2020) SARS-CoV-2 targets neurons of 3D human brain organoids. EMBO J 39:e106230. https://doi.org/10.15252/embj.2020106230
Rawat P, Spector SA (2017) Development and characterization of a human microglia cell model of HIV-1 infection. J Neurovirol 23:33–46. https://doi.org/10.1007/s13365-016-0472-1
Reynolds BA, Tetzlaff W, Weiss S (1992) A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J Neurosci 12:4565–4574. https://doi.org/10.1523/JNEUROSCI.12-11-04565.1992
Salick MR, Wells MF, Eggan K, Kaykas A (2017) Modelling Zika virus infection of the develo** human brain in vitro using stem cell derived cerebral organoids. J Vis Exp 56404. https://doi.org/10.3791/56404
Samudyata OAO, Malwade S et al (2022) SARS-CoV-2 promotes microglial synapse elimination in human brain organoids. Mol Psychiatry 27:3939–3950. https://doi.org/10.1038/s41380-022-01786-2
Schaumburg C, O’Hara BA, Lane TE, Atwood WJ (2008) Human embryonic stem cell-derived oligodendrocyte progenitor cells express the serotonin receptor and are susceptible to JC virus infection. J Virol 82:8896–8899. https://doi.org/10.1128/JVI.00406-08
Sison SL, O’Brien BS, Johnson AJ et al (2019) Human cytomegalovirus disruption of calcium signaling in neural progenitor cells and organoids. J Virol 93:e00954-e1019. https://doi.org/10.1128/JVI.00954-19
Soares R, Ribeiro FF, Lourenço DM et al (2021) The neurosphere assay: an effective. Neural Regen Res 16:2229–2231. https://doi.org/10.4103/1673-5374.310678
Song E, Zhang C, Israelow B et al (2021) Neuroinvasion of SARS-CoV-2 in human and mouse brain. J Exper Med 218:e20202135. https://doi.org/10.1084/jem.20202135
Straface G, Selmin A, Zanardo V et al (2012) Herpes simplex virus infection in pregnancy. Infect Dis Obs Gynecol 1–6. https://doi.org/10.1155/2012/385697
Sun G, Chiuppesi F, Chen X et al (2020) Modeling human cytomegalovirus-induced microcephaly in human iPSC-derived brain organoids. Cell Rep Med 1:100002. https://doi.org/10.1016/j.xcrm.2020.100002
Sun XY, Ju XC, Li Y et al (2022) Generation of vascularized brain organoids to study neurovascular interactions. Elife 11. https://doi.org/10.7554/eLife.76707
Takahashi K, Tanabe K, Ohnuki M et al (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872. https://doi.org/10.1016/j.cell.2007.11.019
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676. https://doi.org/10.1016/j.cell.2006.07.024
Thompson RL, Shieh MT, Sawtell NM (2003) Analysis of herpes simplex virus ICP0 promoter function in sensory neurons during acute infection, establishment of latency, and reactivation in vivo. J Virol 77:12319–12330. https://doi.org/10.1128/JVI.77.22.12319-12330.2003
Tiwari SK, Wang S, Smith D et al (2021) Revealing tissue-specific SARS-CoV-2 infection and host responses using human stem cell-derived lung and cerebral organoids. Stem Cell Reports 16:437–445. https://doi.org/10.1016/j.stemcr.2021.02.005
Vahsen BF, Gray E, Candalija A et al (2022) Human iPSC co-culture model to investigate the interaction between microglia and motor neurons. Sci Rep 12:12606. https://doi.org/10.1038/s41598-022-16896-8
Volpato V, Webber C (2020) Addressing variability in iPSC-derived models of human disease: guidelines to promote reproducibility. Dis Model Mech 13:dmm042317. https://doi.org/10.1242/dmm.042317
Wallet C, De Rovere M, Van Assche J et al (2019) Microglial cells: the main HIV-1 reservoir in the brain. Front Cell Infect Microbiol 9:362. https://doi.org/10.3389/fcimb.2019.00362
Wang C, Zhang M, Garcia G et al (2021) ApoE-isoform-dependent SARS-CoV-2 neurotropism and cellular response. Cell Stem Cell 28:331-342.e5. https://doi.org/10.1016/j.stem.2020.12.018
Weissert R (2011) Progressive multifocal leukoencephalopathy. J Neuroimmunol 231:73–77. https://doi.org/10.1016/j.jneuroim.2010.09.021
Wells MF, Salick MR, Wiskow O et al (2016) Genetic ablation of AXL does not protect human neural progenitor cells and cerebral organoids from zika virus infection. Cell Stem Cell 19:703–708. https://doi.org/10.1016/j.stem.2016.11.011
Wiley CA, Achim CL, Christopherson C et al (1999) HIV mediates a productive infection of the brain. AIDS 13:2055–2059. https://doi.org/10.1097/00002030-199910220-00007
**ang Y, Cakir B, Park IH (2020) Generation of regionally specified human brain organoids resembling thalamus development. STAR Protoc 1. https://doi.org/10.1016/j.xpro.2019.100001
**ang Y, Tanaka Y, Cakir B et al (2019) hESC-derived thalamic organoids form reciprocal projections when fused with cortical organoids. Cell Stem Cell 24:487-497.e7. https://doi.org/10.1016/j.stem.2018.12.015
Xu M, Lee EM, Wen Z et al (2016) Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat Med 22:1101–1107. https://doi.org/10.1038/nm.4184
Xu Y-P, Qiu Y, Zhang B et al (2019) Zika virus infection induces RNAi-mediated antiviral immunity in human neural progenitors and brain organoids. Cell Res 29:265–273. https://doi.org/10.1038/s41422-019-0152-9
Yi SA, Nam KH, Yun J et al (2020) Infection of brain organoids and 2D cortical neurons with SARS-CoV-2 pseudovirus. Viruses 12:1004. https://doi.org/10.3390/v12091004
Zayyad Z, Spudich S (2015) Neuropathogenesis of HIV: from initial neuroinvasion to HIV-associated neurocognitive disorder (HAND). Curr HIV/AIDS Rep 12:16–24. https://doi.org/10.1007/s11904-014-0255-3
Zhang B, He Y, Xu Y et al (2018) Differential antiviral immunity to Japanese encephalitis virus in develo** cortical organoids. Cell Death Dis 9:719. https://doi.org/10.1038/s41419-018-0763-y
Zhang B-Z, Chu H, Han S et al (2020) SARS-CoV-2 infects human neural progenitor cells and brain organoids. Cell Res 30:928–931. https://doi.org/10.1038/s41422-020-0390-x
Zhao Z, Chen X, Dowbaj AM et al (2022) Organoids Nat Rev Methods Primers 2:94. https://doi.org/10.1038/s43586-022-00174-y
Zheng W, Benner EM, Bloom DC et al (2022) Variations in aspects of neural precursor cell neurogenesis in a human model of HSV-1 infection. Organogenesis 18:2055354. https://doi.org/10.1080/15476278.2022.2055354
Zheng W, Klammer AM, Naciri JN et al (2020) Patterns of herpes simplex virus 1 infection in neural progenitor cells. J Virol 94:e00994-e1020. https://doi.org/10.1128/JVI.00994-20
Zhou S, Szczesna K, Ochalek A et al (2016) Neurosphere based differentiation of human iPSC improves astrocyte differentiation. Stem Cells Int 2016:4937689. https://doi.org/10.1155/2016/4937689
Zhou T, Tan L, Cederquist GY et al (2017) High-content screening in hPSC-neural progenitors identifies drug candidates that inhibit zika virus infection in fetal-like organoids and adult brain. Cell Stem Cell 21:274-283.e5. https://doi.org/10.1016/j.stem.2017.06.017
Zimmer B, Ewaleifoh O, Harschnitz O et al (2018) Human iPSC-derived trigeminal neurons lack constitutive TLR3-dependent immunity that protects cortical neurons from HSV-1 infection. Proc Natl Acad Sci USA 115. https://doi.org/10.1073/pnas.1809853115
Acknowledgements
The authors wish to thank past and present members of the Department of Microbiology, Immunology and Inflammation and the Center for Neurovirology and Gene Editing for sharing reagents and ideas.
Funding
This work was supported by grants (NIH-R01 DA052284-01A1; 2 R01 MH110360-06) awarded by the NIH to IKS. This study utilized services offered by core facilities of the Comprehensive NeuroHIV Center (CNHC NIMH Grant Number P30MH092177-11).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Swingler, M., Donadoni, M., Bellizzi, A. et al. iPSC-derived three-dimensional brain organoid models and neurotropic viral infections. J. Neurovirol. 29, 121–134 (2023). https://doi.org/10.1007/s13365-023-01133-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-023-01133-3