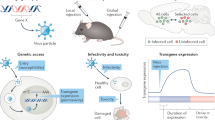
Abstract
Neural circuits provide an anatomical basis for functional networks. Therefore, dissecting the structure of neural circuits is essential to understanding how the brain works. Recombinant neurotropic viruses are important tools for neural circuit tracing with many advantages over non-viral tracers: they allow for anterograde, retrograde, and trans-synaptic delivery of tracers in a cell type-specific, circuit-selective manner. In this review, we summarize the recent developments in the viral tools for neural circuit tracing, discuss the key principles of using viral tools in neuroscience research, and highlight innovations for develo** and optimizing viral tools for neural circuit tracing across diverse animal species, including nonhuman primates.

Similar content being viewed by others
References
Nassi JJ, Cepko CL, Born RT, Beier KT. Neuroanatomy goes viral! Front Neuroanat 2015, 9: 80.
Xu X, Holmes TC, Luo MH, Beier KT, Horwitz GD, Zhao F. Viral vectors for neural circuit map** and recent advances in trans-synaptic anterograde tracers. Neuron 2020, 107: 1029–1047.
Lin K, Zhong X, Ying M, Li L, Tao S, Zhu X, et al. A mutant vesicular stomatitis virus with reduced cytotoxicity and enhanced anterograde trans-synaptic efficiency. Mol Brain 2020, 13: 45.
Shi XW, Jia F, Lyu P, Xu FQ. A new anterograde trans-synaptic tracer based on Sindbis virus. Neural Regen Res 2022, 17: 2761–2764.
Wang D, Tai PWL, Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat Rev Drug Discov 2019, 18: 358–378.
Kondratov O, Kondratova L, Mandel RJ, Coleman K, Savage MA, Gray-Edwards HL, et al. A comprehensive study of a 29-capsid AAV library in a non-human primate central nervous system. Mol Ther 2021, 29: 2806–2820.
Nectow AR, Nestler EJ. Viral tools for neuroscience. Nat Rev Neurosci 2020, 21: 669–681.
Parr-Brownlie LC, Bosch-Bouju C, Schoderboeck L, Sizemore RJ, Abraham WC, Hughes SM. Lentiviral vectors as tools to understand central nervous system biology in mammalian model organisms. Front Mol Neurosci 2015, 8: 14.
Jia F, Zhu X, Lv P, Hu L, Liu Q, ** S, et al. Rapid and sparse labeling of neurons based on the mutant virus-like particle of semliki forest virus. Neurosci Bull 2019, 35: 378–388.
Jia F, Miao H, Zhu X, Xu F. Pseudo-typed Semliki Forest virus delivers EGFP into neurons. J Neurovirol 2017, 23: 205–215.
Soudais C, Laplace-Builhe C, Kissa K, Kremer EJ. Preferential transduction of neurons by canine adenovirus vectors and their efficient retrograde transport in vivo. FASEB J 2001, 15: 2283–2285.
Tervo DGR, Hwang BY, Viswanathan S, Gaj T, Lavzin M, Ritola KD, et al. A designer AAV variant permits efficient retrograde access to projection neurons. Neuron 2016, 92: 372–382.
Chan KY, Jang MJ, Yoo BB, Greenbaum A, Ravi N, Wu WL, et al. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat Neurosci 2017, 20: 1172–1179.
Lin K, Zhong X, Li L, Ying M, Yang T, Zhang Z, et al. AAV9-Retro mediates efficient transduction with axon terminal absorption and blood-brain barrier transportation. Mol Brain 2020, 13: 138.
Ayuso E, Mingozzi F, Bosch F. Production, purification and characterization of adeno-associated vectors. Curr Gene Ther 2010, 10: 423–436.
Srivastava A. In vivo tissue-tropism of adeno-associated viral vectors. Curr Opin Virol 2016, 21: 75–80.
Grimm D, Zolotukhin S. E pluribus unum: 50 years of research, millions of viruses, and one goal—Tailored acceleration of AAV evolution. Mol Ther 2015, 23: 1819–1831.
Li C, Samulski RJ. Engineering adeno-associated virus vectors for gene therapy. Nat Rev Genet 2020, 21: 255–272.
Wu Z, Kan SBJ, Lewis RD, Wittmann BJ, Arnold FH. Machine learning-assisted directed protein evolution with combinatorial libraries. Proc Natl Acad Sci U S A 2019, 116: 8852–8858.
Bryant DH, Bashir A, Sinai S, Jain NK, Ogden PJ, Riley PF, et al. Deep diversification of an AAV capsid protein by machine learning. Nat Biotechnol 2021, 39: 691–696.
Schmit PF, Pacouret S, Zinn E, Telford E, Nicolaou F, Broucque F, et al. Cross-packaging and capsid mosaic formation in multiplexed AAV libraries. Mol Ther Methods Clin Dev 2020, 17: 107–121.
Ravindra Kumar S, Miles TF, Chen X, Brown D, Dobreva T, Huang Q, et al. Multiplexed Cre-dependent selection yields systemic AAVs for targeting distinct brain cell types. Nat Methods 2020, 17: 541–550.
Nonnenmacher M, Wang W, Child MA, Ren XQ, Huang C, Ren AZ, et al. Rapid evolution of blood-brain-barrier-penetrating AAV capsids by RNA-driven biopanning. Mol Ther Methods Clin Dev 2021, 20: 366–378.
**ao X, Li J, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol 1998, 72: 2224–2232.
Urabe M, Ding C, Kotin RM. Insect cells as a factory to produce adeno-associated virus type 2 vectors. Hum Gene Ther 2002, 13: 1935–1943.
Wu Y, Jiang L, Geng H, Yang T, Han Z, He X, et al. A recombinant baculovirus efficiently generates recombinant adeno-associated virus vectors in cultured insect cells and larvae. Mol Ther Methods Clin Dev 2018, 10: 38–47.
Wu Y, Han Z, Duan M, Jiang L, Tian T, ** D, et al. Popularizing recombinant baculovirus-derived OneBac system for laboratory production of all recombinant adeno-associated virus vector serotypes. Curr Gene Ther 2021, 21: 167–176.
Watakabe A, Ohtsuka M, Kinoshita M, Takaji M, Isa K, Mizukami H, et al. Comparative analyses of adeno-associated viral vector serotypes 1, 2, 5, 8 and 9 in marmoset, mouse and macaque cerebral cortex. Neurosci Res 2015, 93: 144–157.
Castle MJ, Turunen HT, Vandenberghe LH, Wolfe JH. Controlling AAV tropism in the nervous system with natural and engineered capsids. Methods Mol Biol 2016, 1382: 133–149.
Salegio EA, Samaranch L, Kells AP, Mittermeyer G, San Sebastian W, Zhou S, et al. Axonal transport of adeno-associated viral vectors is serotype-dependent. Gene Ther 2013, 20: 348–352.
Zingg B, Chou XL, Zhang ZG, Mesik L, Liang F, Tao HW, et al. AAV-mediated anterograde transsynaptic tagging: Map** corticocollicular input-defined neural pathways for defense behaviors. Neuron 2017, 93: 33–47.
Ito T, Ono M, Matsui R, Watanabe D, Ohmori H. Avian adeno-associated virus as an anterograde transsynaptic vector. J Neurosci Methods 2021, 359: 109221.
Deverman BE, Pravdo PL, Simpson BP, Kumar SR, Chan KY, Banerjee A, et al. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat Biotechnol 2016, 34: 204–209.
Hanlon KS, Meltzer JC, Buzhdygan T, Cheng MJ, Sena-Esteves M, Bennett RE, et al. Selection of an efficient AAV vector for robust CNS transgene expression. Mol Ther Methods Clin Dev 2019, 15: 320–332.
Goertsen D, Flytzanis NC, Goeden N, Chuapoco MR, Cummins A, Chen Y, et al. AAV capsid variants with brain-wide transgene expression and decreased liver targeting after intravenous delivery in mouse and marmoset. Nat Neurosci 2022, 25: 106–115.
Haggerty DL, Grecco GG, Reeves KC, Atwood B. Adeno-associated viral vectors in neuroscience research. Mol Ther Methods Clin Dev 2020, 17: 69–82.
Liu Q, Wang X, **e C, Ding S, Yang H, Guo S, et al. A novel human acute encephalitis caused by pseudorabies virus variant strain. Clin Infect Dis 2021, 73: e3690–e3700.
Jia F, Lv P, Miao H, Shi X, Mei H, Li L, et al. Optimization of the fluorescent protein expression level based on pseudorabies virus bartha strain for neural circuit tracing. Front Neuroanat 2019, 13: 63.
DeFalco J, Tomishima M, Liu H, Zhao C, Cai X, Marth JD, et al. Virus-assisted map** of neural inputs to a feeding center in the hypothalamus. Science 2001, 291: 2608–2613.
Enquist LW, Card JP. Recent advances in the use of neurotropic viruses for circuit analysis. Curr Opin Neurobiol 2003, 13: 603–606.
Papazoglou I, Lee JH, Cui Z, Li C, Fulgenzi G, Bahn YJ, et al. A distinct hypothalamus-to-β cell circuit modulates insulin secretion. Cell Metab 2022, 34: 285-298.e7.
Oyibo HK, Znamenskiy P, Oviedo HV, Enquist LW, Zador AM. Long-term Cre-mediated retrograde tagging of neurons using a novel recombinant pseudorabies virus. Front Neuroanat 2014, 8: 86.
Wickersham IR, Finke S, Conzelmann KK, Callaway EM. Retrograde neuronal tracing with a deletion-mutant rabies virus. Nat Methods 2007, 4: 47–49.
Tang Y, Li L, Sun L, Yu J, Hu Z, Lian K, et al. In vivo two-photon calcium imaging in dendrites of rabies virus-labeled V1 corticothalamic neurons. Neurosci Bull 2020, 36: 545–553.
Wickersham IR, Lyon DC, Barnard RJO, Mori T, Finke S, Conzelmann KK, et al. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron 2007, 53: 639–647.
Reardon TR, Murray AJ, Turi GF, Wirblich C, Croce KR, Schnell MJ, et al. Rabies virus CVS-N2c(ΔG) strain enhances retrograde synaptic transfer and neuronal viability. Neuron 2016, 89: 711–724.
Zhu X, Lin K, Liu Q, Yue X, Mi H, Huang X, et al. Rabies virus pseudotyped with CVS-N2C glycoprotein as a powerful tool for retrograde neuronal network tracing. Neurosci Bull 2020, 36: 202–216.
Kim EJ, Jacobs MW, Ito-Cole T, Callaway EM. Improved monosynaptic neural circuit tracing using engineered rabies virus glycoproteins. Cell Rep 2016, 15: 692–699.
Ciabatti E, González-Rueda A, Mariotti L, Morgese F, Tripodi M. Life-long genetic and functional access to neural circuits using self-inactivating rabies virus. Cell 2017, 170: 382-392.e14.
Chatterjee S, Sullivan HA, MacLennan BJ, Xu R, Hou Y, Lavin TK, et al. Nontoxic, double-deletion-mutant rabies viral vectors for retrograde targeting of projection neurons. Nat Neurosci 2018, 21: 638–646.
Sun P, ** S, Tao S, Wang J, Li A, Li N, et al. Highly efficient and super-bright neurocircuit tracing using vector mixing-based virus cocktail. bioRxiv, 2020: 705772. https://doi.org/10.1101/705772.
Jia F, Li L, Liu H, Lv P, Shi X, Wu Y, et al. Development of a rabies virus-based retrograde tracer with high trans-monosynaptic efficiency by reshuffling glycoprotein. Mol Brain 2021, 14: 109.
Sun L, Tang Y, Yan K, Yu J, Zou Y, Xu W, et al. Differences in neurotropism and neurotoxicity among retrograde viral tracers. Mol Neurodegener 2019, 14: 8.
Zemanick MC, Strick PL, Dix RD. Direction of transneuronal transport of herpes simplex virus 1 in the primate motor system is strain-dependent. Proc Natl Acad Sci U S A 1991, 88: 8048–8051.
Dum RP, Levinthal DJ, Strick PL. The spinothalamic system targets motor and sensory areas in the cerebral cortex of monkeys. J Neurosci 2009, 29: 14223–14235.
Zeng WB, Jiang HF, Gang YD, Song YG, Shen ZZ, Yang H, et al. Anterograde monosynaptic transneuronal tracers derived from herpes simplex virus 1 strain H129. Mol Neurodegener 2017, 12: 38.
Su P, Ying M, Han Z, **a J, ** S, Li Y, et al. High-brightness anterograde transneuronal HSV1 H129 tracer modified using a Trojan horse-like strategy. Mol Brain 2020, 13: 5.
Su P, Ying M, **a J, Li Y, Wu Y, Wang H, et al. Rigorous anterograde trans-monosynaptic tracing of genetic defined neurons with retargeted HSV1 H129. bioRxiv 2020, https://doi.org/10.1101/2020.12.01.407312.
Beier KT, Saunders A, Oldenburg IA, Miyamichi K, Akhtar N, Luo L, et al. Anterograde or retrograde transsynaptic labeling of CNS neurons with vesicular stomatitis virus vectors. Proc Natl Acad Sci U S A 2011, 108: 15414–15419.
Mundell NA, Beier KT, Pan YA, Lapan SW, Göz Aytürk D, Berezovskii VK, et al. Vesicular stomatitis virus enables gene transfer and transsynaptic tracing in a wide range of organisms. J Comp Neurol 2015, 523: 1639–1663.
Kler S, Ma M, Narayan S, Ahrens MB, Pan YA. Cre-dependent anterograde transsynaptic labeling and functional imaging in zebrafish using VSV with reduced cytotoxicity. Front Neuroanat 2021, 15: 758350.
Azzouz M, Ralph GS, Storkebaum E, Walmsley LE, Mitrophanous KA, Kingsman SM, et al. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature 2004, 429: 413–417.
Mazarakis ND, Azzouz M, Rohll JB, Ellard FM, Wilkes FJ, Olsen AL, et al. Rabies virus glycoprotein pseudoty** of lentiviral vectors enables retrograde axonal transport and access to the nervous system after peripheral delivery. Hum Mol Genet 2001, 10: 2109–2121.
Kobayashi K, Kato S, Kobayashi K. Genetic manipulation of specific neural circuits by use of a viral vector system. J Neural Transm 2018, 125: 67–75.
Wehbi A, Kremer EJ, Dopeso-Reyes IG. Location of the cell adhesion molecule coxsackievirus and adenovirus receptor in the adult mouse brain. Front Neuroanat 2020, 14: 28.
Carter ME, Soden ME, Zweifel LS, Palmiter RD. Genetic identification of a neural circuit that suppresses appetite. Nature 2013, 503: 111–114.
Lavoie A, Liu BH. Canine adenovirus 2: A natural choice for brain circuit dissection. Front Mol Neurosci 2020, 13: 9.
Bredenbeek PJ, Frolov I, Rice CM, Schlesinger S. Sindbis virus expression vectors: Packaging of RNA replicons by using defective helper RNAs. J Virol 1993, 67: 6439–6446.
Kebschull JM, Garcia da Silva P, Zador AM. A New Defective Helper RNA to Produce Recombinant Sindbis Virus that Infects Neurons but does not Propagate. Front Neuroanat 2016, 10: 56.
Furuta T, Tomioka R, Taki K, Nakamura K, Tamamaki N, Kaneko T. In vivo transduction of central neurons using recombinant Sindbis virus: Golgi-like labeling of dendrites and axons with membrane-targeted fluorescent proteins. J Histochem Cytochem 2001, 49: 1497–1508.
Passoni G, Langevin C, Palha N, Mounce BC, Briolat V, Affaticati P, et al. Imaging of viral neuroinvasion in the zebrafish reveals that Sindbis and chikungunya viruses favour different entry routes. Dis Model Mech 2017, 10: 847–857.
Kuramoto E. Method for labeling and reconstruction of single neurons using Sindbis virus vectors. J Chem Neuroanat 2019, 100: 101648.
Ehrengruber MU, Renggli M, Raineteau O, Hennou S, Vähä-Koskela MJV, Hinkkanen AE, et al. Semliki Forest virus A7(74) transduces hippocampal neurons and glial cells in a temperature-dependent dual manner. J Neurovirology 2003, 9: 16–28.
Ehrengruber MU, Schlesinger S, Lundstrom K. Alphaviruses: Semliki forest virus and Sindbis virus vectors for gene transfer into neurons. Curr Protoc Neurosci 2011, Chapter 4: Unit 4.22.
Guo B, Chen J, Chen Q, Ren K, Feng D, Mao H, et al. Anterior cingulate cortex dysfunction underlies social deficits in Shank3 mutant mice. Nat Neurosci 2019, 22: 1223–1234.
Kim J, Dittgen T, Nimmerjahn A, Waters J, Pawlak V, Helmchen F, et al. Sindbis vector SINrep(nsP2S726): A tool for rapid heterologous expression with attenuated cytotoxicity in neurons. J Neurosci Methods 2004, 133: 81–90.
Lundstrom K, Abenavoli A, Malgaroli A, Ehrengruber MU. Novel semliki forest virus vectors with reduced cytotoxicity and temperature sensitivity for long-term enhancement of transgene expression. Mol Ther 2003, 7: 202–209.
El-Shamayleh Y, Ni AM, Horwitz GD. Strategies for targeting primate neural circuits with viral vectors. J Neurophysiol 2016, 116: 122–134.
Zhou C, Yang X, Wu S, Zhong Q, Luo T, Li A, et al. Continuous subcellular resolution three-dimensional imaging on intact macaque brain. Sci Bull 2022, 67: 85–96.
An H, Cho DW, Lee SE, Yang YS, Han SC, Lee CJ. Differential cellular tropism of lentivirus and adeno-associated virus in the brain of cynomolgus monkey. Exp Neurobiol 2016, 25: 48–54.
Han X, Qian X, Bernstein JG, Zhou HH, Franzesi GT, Stern P, et al. Millisecond-timescale optical control of neural dynamics in the nonhuman primate brain. Neuron 2009, 62: 191–198.
Xu F, Shen Y, Ding L, Yang CY, Tan H, Wang H, et al. High-throughput map** of a whole rhesus monkey brain at micrometer resolution. Nat Biotechnol 2021, 39: 1521–1528.
Nhan HL, Callaway EM. Morphology of superior colliculus- and middle temporal area-projecting neurons in primate primary visual cortex. J Comp Neurol 2012, 520: 52–80.
Nassi JJ, Callaway EM. Multiple circuits relaying primate parallel visual pathways to the middle temporal area. J Neurosci 2006, 26: 12789–12798.
Nathanson JL, Yanagawa Y, Obata K, Callaway EM. Preferential labeling of inhibitory and excitatory cortical neurons by endogenous tropism of adeno-associated virus and lentivirus vectors. Neuroscience 2009, 161: 441–450.
Han Z, Shi X, Ying M, Zheng N, Li M, Zhang Z, et al. Tools for neural circuit tracing based on neurotropic viruses. Chinese J Anal Chem 2019, 47: 1639–1650.
Chen H. Intron splicing-mediated expression of AAV Rep and Cap genes and production of AAV vectors in insect cells. Mol Ther 2008, 16: 924–930.
Mietzsch M, Grasse S, Zurawski C, Weger S, Bennett A, Agbandje-McKenna M, et al. OneBac: platform for scalable and high-titer production of adeno-associated virus serotype 1–12 vectors for gene therapy. Hum Gene Ther 2014, 25: 212–222.
Wu Y, Mei T, Jiang L, Han Z, Dong R, Yang T, et al. Development of versatile and flexible Sf9 packaging cell line-dependent OneBac system for large-scale recombinant adeno-associated virus production. Hum Gene Ther Methods 2019, 30: 172–183.
Acknowledgments
This review was supported by the National Science and Technology Innovation 2030 (2021ZD0201003), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB32030200), the National Natural Science Foundation of China (31830035), and the Shenzhen Key Laboratory of Viral Vectors for Biomedicine (ZDSYS20200811142401005).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors claim that there are no conflicts of interest.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Q., Wu, Y., Wang, H. et al. Viral Tools for Neural Circuit Tracing. Neurosci. Bull. 38, 1508–1518 (2022). https://doi.org/10.1007/s12264-022-00949-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-022-00949-z