Abstract
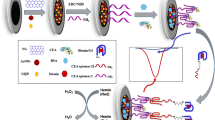
A reliable fluorometric assay is described for the determination carcinoembryonic antigen (CEA) using exonuclease III (Exo III) and a 2-aminopurine binding aptamer. In the absence of CEA, dsDNA is degraded by Exo III, and free 2-AP (which has a blue fluorescence with excitation/emission maxima of 310/365 nm) is released. Strong fluorescence is generated after addition of graphene oxide (GO) to the solution. However, the 2-AP modified DNA (T2) cannot be degraded in the presence of CEA by Exo III due to the interaction between CEA and aptamer T1. Hence, only weak fluorescence can be detected after addition of GO. In this system, CEA can be quantified in the 0.05 - 2 ng·mL-1 concentration range with a detection limit of 30 pg·mL-1 (at S/N = 3). The method was successfully applied to analyze serum samples for CEA.

An exonuclease III-assisted fluorometric aptasensor has been developed for the detection of carcinoembryonic antigen using graphene oxide and 2-aminopurine.




Similar content being viewed by others
References
Tang Z, Chen H, He H, Ma C (2019) Assays for alkaline phosphatase activity: progress and prospects. Trends Anal Chem 113:32–43. https://doi.org/10.1016/j.trac.2019.01.019
Wang K, He MQ, Zhai FH, He RH, Yu YL (2017) A label-free and enzyme-free ratiometric fluorescence biosensor for sensitive detection of carcinoembryonic antigen based on target-aptamer complex recycling amplification. Sensors Actuators B Chem 253:893–899. https://doi.org/10.1016/j.snb.2017.07.047
Wang QL, Cui HF, Song XJ, Fan SF, Chen LL, Li MM, Li ZY (2018) A label-free and lectin-based sandwich aptasensor for detection of carcinoembryonic antigen. Sensors Actuators B Chem 260:48–54. https://doi.org/10.1016/j.snb.2017.12.105
Khang H, Cho K, Chong S, Lee JH (2017) All-in-one dual-aptasensor capable of rapidly quantifying carcinoembryonic antigen. Biosens Bioelectron 90:46–52. https://doi.org/10.1016/j.bios.2016.11.043
Feng DX, Lu XC, Dong X, Ling YY, Zhang YZ (2013) Label-free electrochemical immunosensor for the carcinoembryonic antigen using a glassy carbon electrode modified with electrodeposited Prussian Blue, a graphene and carbon nanotube assembly and an antibody immobilized on gold nanoparticles. Microchim Acta 180:767–774. https://doi.org/10.1007/s00604-013-0985-8
Liu FM, Zhang HL, Wu ZH, Dong HD, Zhou L, Yang DW, Ge YQ, Jia CP, Liu HY, ** QH, Zhao JL, Zhang QQ, Mao HG (2016) Highly sensitive and selective lateral flow immunoassay based on magnetic nanoparticles for quantitative detection of carcinoembryonic antigen. Talanta 161:205–210. https://doi.org/10.1016/j.talanta.2016.08.048
Luo C, Wen W, Lin FG, Zhang XH, Gu HS, Wang SF (2015) Simplified aptamer-based colorimetric method using unmodified gold nanoparticles for the detection of carcinoma embryonic antigen. RSC Adv 5:10994–10999. https://doi.org/10.1039/c4ra14833a
Shan J, Ma ZF (2017) A review on amperometric immunoassays for tumor markers based on the use of hybrid materials consisting of conducting polymers and noble metal nanomaterials. Microchim Acta 184:969–979. https://doi.org/10.1007/s00604-017-2146-y
Jiang J, Zhao SL, Huang Y, Qin GX, Ye FG (2013) Highly sensitive immunoassay of carcinoembryonic antigen by capillary electrophoresis with gold nanoparticles amplified chemiluminescence detection. J Chromatogr A 1282:161–166. https://doi.org/10.1016/j.chroma.2013.01.066
Martínez-Mancera FD, García-López P, Hernández-López JL (2015) Pre-clinical validation study of a miniaturized electrochemical immunoassay based on square wave voltammetry for early detection of carcinoembryonic antigen in human serum. Clin Chim Acta 444:199–205. https://doi.org/10.1016/j.cca.2015.02.017
Zhao LJ, Cheng M, Liu GN, Lu HY, Gao Y, Yan X, Liu FM, Sun P, Lu GY (2018) A fluorescent biosensor based on molybdenum disulfide nanosheets and protein aptamer for sensitive detection of carcinoembryonic antigen. Sens Actuators B Chemi 273:185–190. https://doi.org/10.1016/j.snb.2018.06.004
Khusbu F, Zhou X, Chen H, Ma C, Wang K (2018) Thioflavin T as a fluorescence probe for biosensing applications. Trends Anal Chem 109:1–18. https://doi.org/10.1016/j.trac.2018.09.013
Yu XH, Lin YH, Wang XS, Xu LJ, Wang ZE, Fu FF (2018) Exonuclease-assisted multicolor aptasensor for visual detection of ochratoxin A based on G-quadruplex-hemin DNAzyme-mediated etching of gold nanorod. Microchim Acta 185:259. https://doi.org/10.1007/s00604-018-2811-9
Zhao H, **ang X, Chen M, Ma C (2019) Aptamer-based fluorometric ochratoxin A assay based on photoinduced electron transfer. Toxins 11:65. https://doi.org/10.3390/toxins11020065
Xu QJ, Wang GX, Zhang MM, Xu GY, Lin JH, Luo XL (2018) Aptamer based label free thrombin assay based on the use of silver nanoparticles incorporated into self-polymerized dopamine. Microchim Acta 185:253. https://doi.org/10.1007/s00604-018-2787-5
Zhou XT, Wang LM, Shen GQ, Zhang DW, **e JL, Mamut A, Huang WW, Zhou SS (2018) Colorimetric determination of ofloxacin using unmodified aptamers and the aggregation of gold nanoparticles. Microchim Acta 185:355. https://doi.org/10.1007/s00604-018-2895-2
Ma CB, Wu KF, Zhao H, Liu HS, Wang KM, **a K (2018) Fluorometric aptamer-based determination of ochratoxin A based on the use of graphene oxide and RNase H-aided amplification. Microchim Acta 185:347. https://doi.org/10.1007/s00604-018-2885-4
Wu K, Ma C, Zhao H, He H, Chen H (2018) Label-free G-quadruplex aptamer fluorescence assay for ochratoxin A using a thioflavin T probe. Toxins 10:198. https://doi.org/10.3390/toxins10050198
Wen DX, He MH, Ma KF, Cui Y, Kong JM, Yang HX, Liu QY (2018) Highly sensitive fluorometric determination of thrombin by on-chip signal amplification initiated by terminal deoxynucleotidyl transferase. Microchim Acta 185:380. https://doi.org/10.1007/s00604-018-2903-6
Wu K, Ma C, Zhao H, Chen M, Deng Z (2019) Sensitive aptamer-based fluorescene assay for ochratoxin A based on RNase H signal amplification. Food Chem 277:273–278. https://doi.org/10.1016/j.foodchem.2018.10.130
Li MK, Hu LY, Niu CG, Huang DW, Zeng GM (2018) A fluorescent DNA based probe for Hg(II) based on thymine-Hg(II)-thymine interaction and enrichment via magnetized graphene oxide. Microchim Acta 185:207. https://doi.org/10.1007/s00604-018-2689-6
Kim JH, Park SJ, Min DH (2017) Emerging approaches for graphene oxide biosensor. Anal Chem 89:232–248. https://doi.org/10.1021/acs.analchem.6b04248
Zhang H, Zhang HL, Aldalbahi A, Zuo XL, Fan CH, Mi XQ (2017) Fluorescent biosensors enabled by graphene and graphene oxide. Biosens Bioelectron 89:96–106. https://doi.org/10.1016/j.bios.2016.07.030
Chen M, Li W, Ma C, Wu K, He H, Wang K (2019) Fluorometric determination of the activity of uracil-DNA glycosylase by using graphene oxide and exonuclease I assisted signal amplification. Microchim Acta 186:110. https://doi.org/10.1007/s00604-019-3247-6
Liu DK, Lu X, Yang YW, Zhai YY, Zhang J, Li L (2018) A novel fluorescent aptasensor for the highly sensitive and selective detection of cardiac troponin I based on a graphene oxide platform. Anal Bioanal Chem 410:4285–4291. https://doi.org/10.1007/s00216-018-1076-9
Meng FB, Xu H, Yao X, Qin X, Jiang TT, Gao SM, Zhang YH, Yang D, Liu X (2017) Mercury detection based on label-free and isothermal enzyme-free amplified fluorescence platform. Talanta 162:368–373. https://doi.org/10.1016/j.talanta.2016.10.001
**ao KY, Liu J, Chen H, Zhang S, Kong JL (2018) A label-free and high-efficient GO-based aptasensor for cancer cells based on cyclic enzymatic signal amplification. Biosens Bioelectron 91:76–81. https://doi.org/10.1016/j.bios.2016.11.057
Chen J, Ge J, Zhang L, Li ZH, Li JJ, Sun YJ, Qu LB (2016) Reduced graphene oxide nanosheets functionalized with poly (styrene sulfonate) as a peroxidase mimetic in a colorimetric assay for ascorbic acid. Microchim Acta 183:1847–1853. https://doi.org/10.1007/s00604-016-1826-3
Lee CY, Park KS, Park HG (2015) A fluorescent G-quadruplex probe for the assay of base excision repair enzyme activity. Chem Commun 51:13744–13747. https://doi.org/10.1039/c5cc05010c
Liu C, Lv SF, Gong H, Chen CY, Chen XM, Cai CQ (2017) 2-aminopurine probe in combination with catalyzed hairpin assembly signal amplification for simple and sensitive detection of microRNA. Talanta 174:336–340. https://doi.org/10.1016/j.talanta.2017.06.028
Liao R, He K, Chen CY, Chen XM, Cai CQ (2016) Double-strand displacement biosensor and quencher-free fluorescence strategy for rapid detection of microRNA. Anal Chem 88:4254–4258. https://doi.org/10.1021/acs.analchem.5b04154
Liu HY, Bai YF, Qin J, Wang HY, Wang YZ, Chen ZZ, Feng F (2018) Exonuclease I assisted fluorometric aptasensor for adenosine detection using 2-AP modified DNA. Sensors Actuators B Chem 256:413–419. https://doi.org/10.1016/j.snb.2017.10.117
Ma CB, Liu HS, Wu KF, Chen MJ, Zheng LY, Wang J (2017) An Exonuclease I-Based Quencher-Free Fluorescent Method Using DNA Hairpin Probes for Rapid Detection of MicroRNA. Sensors 17:760. https://doi.org/10.3390/s17040760
Acknowledgments
This work was supported by State Key Laboratory of Chemo/ Biosensing and Chemometrics, Hunan University (2017006), The Research Innovation Program for Graduates of Central South University (2018zzts384, 2018zzts399).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 271 kb)
Rights and permissions
About this article
Cite this article
Chen, M., Ma, C., Zhao, H. et al. Exonuclease III-assisted fluorometric aptasensor for the carcinoembryonic antigen using graphene oxide and 2-aminopurine. Microchim Acta 186, 500 (2019). https://doi.org/10.1007/s00604-019-3621-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3621-4