Abstract
An exonuclease-assisted multicolor aptasensor was developed for the visual detection of ochratoxin A (OTA). It is based on the etching of gold nanorods (AuNRs) mediated by a G-quadruplex-hemin DNAzyme. A DNA sequence (AG4-OTA) was designed that comprises a hemin aptamer and an OTA aptamer. OTA binds to AG4-OTA to form an antiparallel G-quadruplex, which halts its digestion by exonuclease I (Exo I) from the 3′-end of AG4-OTA. Thus, the retained hemin aptamer can bind to hemin to form a G-quadruplex-hemin DNAzyme. This DNAzyme has peroxidase-like activity that catalyzes the oxidation of 3,3′,5,5′-tetramethylbenzidine (TMB) by H2O2 to produce its diimine derivative (TMB2+) in acidic solution. TMB2+ can etch the AuNRs by oxidizing Au(0) into Au(I). This results in the generation of rainbow-like colors and provides a multicolor platform for the visual detection of OTA. The assay is based on the use of a single isolated aptamer and possesses obvious advantages such as multi-color visual inspection, relatively high sensitivity and accuracy. It can be used to detect as little as 30 nM concentrations of OTA by visual observation and even 10 nM concentrations by spectrophotometry. The method was successfully applied to the determination of OTA in spiked beer where it gave recoveries of 101–108%, with a relative standard deviation (RSD, n = 5) of <5%.

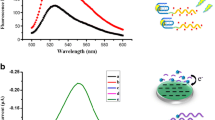
Schematic of an exonuclease-assisted multicolor bioassay based on the G-quadruplex-hemin DNAzyme-mediated etching of gold nanorods (AuNRs). It enables visual detection of ochratoxin A (OTA) with a detection limit of 30 nM.





Similar content being viewed by others
References
Amézqueta S, González-Peñas E, Murillo-Arbizu M, de Cerain AL (2009) Ochratoxin A decontamination: A review. Food Control 20:326–333
Malir F, Ostry V, Novotna E (2013) Toxicity of the mycotoxin ochratoxin A in the light of recent data. Toxin Rev 32:19–33
Sorrenti V, Giacomo CD, Acquaviva R, Barbagallo I, Bognanno M, Galvano F (2013) Toxicity of ochratoxin A and its modulation by antioxidants: a review. Toxins 5:1742–1766
Pena A, Cerejo F, Lino C, Silveira I (2005) Determination of ochratoxin A in Portuguese rice samples by high performance liquid chromatography with fluorescence detection. Anal Bioanal Chem 382:1288–1293
Solfrizzo M, Gambacorta L, Lattanzio VM, Powers S, Visconti A (2011) Simultaneous LC–MS/MS determination of aflatoxin M1, ochratoxin A, deoxynivalenol, de-epoxydeoxynivalenol, α and β-zearalenols and fumonisin B1 in urine as a multi-biomarker method to assess exposure to mycotoxins. Anal Bioanal Chem 401:2831–2841
Adányi N, Levkovets I, Rodriguez-Gil S, Ronald A, Váradi M, Szendrő I (2007) Development of immunosensor based on OWLS technique for determining Aflatoxin B1 and Ochratoxin A. Biosens Bioelectron 22:797–802
Fang X, Tan W (2010) Aptamers generated from cell-SELEX for molecular medicine: a chemical biology approach. Acc Chem Res 43:48–57
Strehlitz B, Nikolaus N, Stoltenburg R (2008) Protein detection with aptamer biosensors. Sensors 8:4296–4307
Cerchia L, De Franciscis V (2010) Targeting cancer cells with nucleic acid aptamers. Trends Biotechnol 28:517–525
Cruz-Aguado JA, Penner G (2008) Determination of ochratoxin A with a DNA aptamer. J Agric Food Chem 56:10456–10461
Bianco M, Sonato A, De Girolamo A, Pascale M, Romanato F, Rinaldi R, Arima V (2017) An aptamer-based SPR-polarization platform for high sensitive OTA detection. Sensors Actuators B Chem 241:314–320
Wang C, Qian J, Wang K, Yang X, Liu Q, Hao N, Wang C, Dong X, Huang X (2016) Colorimetric aptasensing of ochratoxin A using Au@ Fe 3 O 4 nanoparticles as signal indicator and magnetic separator. Biosens Bioelectron 77:1183–1191
Wang C, Qian J, An K, Huang X, Zhao L, Liu Q, Hao N, Wang K (2017) Magneto-controlled aptasensor for simultaneous electrochemical detection of dual mycotoxins in maize using metal sulfide quantum dots coated silica as labels. Biosens Bioelectron 89:802–809
Wang S, Zhang Y, Pang G, Zhang Y, Guo S (2017) Tuning the aggregation/disaggregation behavior of graphene quantum dots by structure-switching aptamer for high-sensitivity fluorescent Ochratoxin A sensor. Anal Chem 89:1704–1709
Wu H, Liu R, Kang X, Lian C, Lv L, Guo Z (2018) Fluorometric aptamer assay for ochratoxin A based on the use of single walled carbon nanohorns and exonuclease III-aided amplification. Microchim Acta 185:27
Zhang D, Wang W, Dong Q, Huang Y, Wen D, Mu Y, Yuan Y (2018) Colorimetric detection of genetically modified organisms based on exonuclease III -assisted target recycling and hemin/G-quadruplex DNAzyme amplification. Microchim Acta 185:75
Sang Y, Xu Y, Xu L, Cheng W, Li X, Wu J (2017) Colorimetric and visual determination of micro RNA via cycling signal amplification using T7 exonuclease. Microchim Acta 184:2465–2471
Nasir M, Nawaz MH, Latif U, Yaqub M, Hayat A, Rahim A (2017) An overview on enzyme-mimicking nanomaterials for use in electrochemical and optical assays. Microchim Acta 184:323–342
Zong C, Zhang D, Yang H, Wang S, Chu M, Li P (2017) Chemiluminescence immunoassay for cardiac troponin T by using silver nanoparticles functionalized with hemin/G-quadruplex DNAzyme on a glass chip array. Microchim Acta 184:3197–3204
Ye X, ** L, Caglayan H, Chen J, **ng G, Zheng C, Doan-Nguyen V, Kang Y, Engheta N, Kagan CR (2012) Improved size-tunable synthesis of monodisperse gold nanorods through the use of aromatic additives. ACS Nano 6:2804–2817
Lin Y, Zhao M, Guo Y, Ma X, Luo F, Guo L, Qiu B, Chen G, Lin Z (2016) Multicolor colormetric biosensor for the determination of glucose based on the etching of gold nanorods. Sci Rep 6:37879
Zhang Z, Chen Z, Cheng F, Zhang Y, Chen L (2017) Highly sensitive on-site detection of glucose in human urine with naked eye based on enzymatic-like reaction mediated etching of gold nanorods. Biosens Bioelectron 89:932–936
Chen Z, Liu R, Wang S, Qu C, Chen L, Wang Z (2013) Colorimetric sensing of copper (II) based on catalytic etching of gold nanorods. RSC Adv 3:13318–13323
Ma X, Lin Y, Guo L, Qiu B, Chen G, Yang HH, Lin Z (2017) A universal multicolor immunosensor for semiquantitative visual detection of biomarkers with the naked eyes. Biosens Bioelectron 87:122–128
Nikoobakht B, El-sayed MA (2003) Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem Mater 15:1957–1962
Orendorff CJ, Murphy CJ (2006) Quantitation of metal content in the silver-assisted growth of gold nanorods. J Phys Chem B 110:3990–3994
Beltran E, Ibáñez M, Sancho JV, Hernandez F (2009) Determination of mycotoxins in different food commodities by ultra-high-pressure liquid chromatography coupled to triple quadrupole mass spectrometry. Rapid Commun Mass Spectrom 23:1801–1809
Rodríguez-Fernández J, Pérez-Juste J, Mulvaney P, Liz-Marzán LM (2005) Spatially-directed oxidation of gold nanoparticles by Au(III)-CTAB complexes. J Phys Chem B 109:14257–14261
Cao GX, Wu XM, Dong YM, Li ZJ, Wang GL (2016) Colorimetric determination of melamine based on the reversal of the mercury(II) induced inhibition of the light-triggered oxidase-like activity of gold nanoclusters. Microchim Acta 183:441–448
Tsung CK, Kou X, Shi Q, Zhang J, Yeung MH, Wang JF, Stucky GD (2006) Selective shortening of single-crystalline gold nanorods by mild oxidation. J Am Chem Soc 128:5352–5353
Odhav B, Naicker V (2002) Mycotoxins in South African traditionally brewed beers. Food Addit Contam 19:55–61
Lin C, Zheng H, Sun M, Guo Y, Luo F, Guo L, Qiu B, Lin Z, Chen G (2018) Highly sensitive colorimetric aptasensor for ochratoxin A detection based on enzyme-encapsulated liposome. Anal Chim Acta 1002:90–96
Wang C, Dong X, Liu Q, Wang K (2015) Label-free colorimetric aptasensor for sensitive detection of ochratoxin A utilizing hybridization chain reaction. Anal Chim Acta 860:83–88
Yang C, Lates V, Prieto-Simón B, Marty JL, Yang X (2012) Aptamer-DNAzyme hairpins for biosensing of Ochratoxin A. Biosens Bioelectron 32:208–212
Yang C, Lates V, Prieto-Simón B, Marty JL, Yang X (2013) Rapid high-throughput analysis of ochratoxin A by the self-assembly of DNAzyme–aptamer conjugates in wine. Talanta 116:520–526
Acknowledgements
We gratefully acknowledge the National Key Research and Development Program of China (2017YFC1600500), NSFC (21505020, 21677034) and the Program for Changjiang Scholars and Innovative Research Team in University (No. IRT-15R11) for financial support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(PDF 561 kb)
Rights and permissions
About this article
Cite this article
Yu, X., Lin, Y., Wang, X. et al. Exonuclease-assisted multicolor aptasensor for visual detection of ochratoxin A based on G-quadruplex-hemin DNAzyme-mediated etching of gold nanorod. Microchim Acta 185, 259 (2018). https://doi.org/10.1007/s00604-018-2811-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-2811-9