Abstract
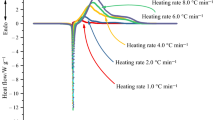
The thermal stability of HMT under dynamic, isothermal and adiabatic conditions was investigated using differential scanning calorimeter (DSC) and accelerating rate calorimeter (ARC), respectively. It is found from the dynamic DSC results that the exothermic decomposition reaction appears immediately after endothermic peak, a coupling phenomenon of heat absorption and generation, and the endothermic peak and exothermic peak were indentified at about 277–289 and 279–296 °C (Tpeak) with the heating rates 1, 2, 4 and 8 °C min−1. The ARC results reveal that the initial decomposition temperature of HMT is about 236.55 °C, and the total gas production in decomposition process is 6.9 mol kg−1. Based on the isothermal DSC and ARC data, some kinetic parameters have been determined using thermal safety software. The simulation results show that the exothermic decomposition process of HMT can be expressed by an autocatalytic reaction mechanism. There is also a good agreement between the kinetic model and kinetic parameters simulated based on the isothermal DSC and ARC data. Thermal hazards of HMT can be evaluated by carrying out thermal explosion simulations, which were based on kinetic models (Isothermal DSC and ARC) to predict several thermal hazard indicators, such as TD24, TD8, TCL, SADT, ET and CT so that we can optimize the conditions of transportation and storage for chemical, also minimizing industrial disasters.










Similar content being viewed by others
Abbreviations
- β/°C min−1 :
-
Heating rate
- T onset/°C:
-
Onset temperature of decomposition
- T peak/°C:
-
Peak temperature of decomposition
- ΔH d/J g−1 :
-
Enthalpy of decomposition
- q max/W g−1 :
-
Maximum specific heat release rate
- θ/s:
-
Isothermal induction period
- t/s:
-
Reaction time
- T/K:
-
Temperature
- α :
-
Reaction progress, the extent of conversion
- E α/J mol−1 :
-
Activation energy
- A/s−1 :
-
Pre-exponential factor
- f (α):
-
Differential form of the reaction model
- P/bar:
-
Pressure
- V/m3 :
-
Volume
- n/mol:
-
Amount of substance
- C p/J g−1 K−1 :
-
Specific heat capacity
- ρ/g−1 cm−3 :
-
Density
- λ/W m−1 K−1 :
-
Heat conductivity
- U/W−1 m−2 Km−1 :
-
Heat transfer coefficient
References
Ortiz GMH. Comparative hydrothermal synthesis of hydroxyapatite by using cetyltrimethylammonium bromide and hexamethylenetetramine as additives. Ceram Int. 2018;44(4):3658–63.
Levashova VI, Yangirova IV. Synthesis and inhibitory properties of compositions based on boric acid, mixture of individual polyethylene polyamines and hexamethylenetetramine. J Therm Anal Calorim. 2016;56:73–6.
Wang S, Pa R, Zhang Z. Determination of formaldehyde in pharm aceutical product of urotropine. Chin J Pharm Anal. 2010;30(9):1774–6.
Li QL. Study on the technology of synthesis of HMX. Tai yuan: North university of China; 2007.
Li WM. Study on process of HMX by acetic anhydride method. Tai yuan: North University of China; 2009.
Gao HS, Chen WH, Chen LP, et al. On the thermal stability of BPO under isothermal conditions. J Saf Environ. 2014;14(1):109–12.
Gusev EA, Dalidovich SV, Krasovskaya LI. Investigation of urotropine thermal decomposition reaction in self-generated atmosphere by means of thermal analysis method. Thermochim Acta. 1985;93:21–4.
Singh G, Baranwal BP, Kapoor IPS, et al. Preparation, X-ray crystallography, and thermal decomposition of some transition metal perchlorate complexes of hexamethylenetetramine. J Phys Chem A. 2007;111(50):12972–6.
Peng HL, Chen LP, Chen WH, et al. Thermal decomposition kinetics of urotropin. Chin J Energ Mater. 2016;24(5):497–502.
Kossoy AA, Akhmetshin YG. Identification of kinetic models for the assessment of reaction hazards. Process Saf Prog. 2007;26(3):209–20.
Kossoy AA, Sheinman I. Evaluating thermal explosion hazard by using kinetics-based simulation approach. Process Saf Environ Protect. 2004;82(6):421–30.
Cheng YF, Liu SH, Shu CM, et al. Energy estimation and modeling solid thermal explosion containment on reactor for three organic peroxides by calorimetric technique. J Therm Anal Calorim. 2017;130:1201–11.
Hechu Kateryna, Slater Carl, Santillana Begoña, et al. A novel approach for interpreting the solidification behaviour of peritectic steels by combining CSLM and DSC. Mater Charact. 2017;133:25–32.
Singh G, Pandey DK. Thermal studies on energetic compounds. J Therm Anal Calorim. 2004;76(2):507–19.
Tenchov Boris, Abarova Silviya, Koynova Rumiana. A new approach for investigating neurodegenerative disorders in mice based on DSC. J Therm Anal Calorim. 2017;127:483–6.
Material Safety Data Sheet. https://baike.baidu.com
Chen JR, Cheng SY, Yuan MH, Kossoy AA, Shu CM. Hierarchical kinetic simulation for autocatalytic decomposition of cumene hydroperoxide at low temperatures. J Therm Anal Calorim. 2009;96(3):751–8.
Li XR, Koseki H. SADT prediction of autocatalytic material using isothermal calorimetty analysis. Thermochim Acta. 2005;431(1/2):113–6.
Vyazovkin S, Chrissafis K, Lorenzo MLD, Koga N, Pijolat M, Roduit B, Sbirrazzuoli N. Sun˜ol JJ. ICTAC kinetics committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim Acta. 2014;590:1–23.
Tseng JM, Wu TC, Hsieh TF, Huang PJ, Lin CP. The thermal hazard evaluation of 1,1-di(tert-butylperoxy) cyclohexane by DSC using non-isothermal and isothermal kinetic simulation. J Loss Prev Process Ind. 2012;25(4):703–8.
Thermal Safety Software (TSS) (2009) ChemInform Saint Petersburg (CISP), Ltd., St. Petersburg
Thermal Safety Software (TSS) ChemInform Saint-Petersburg (CISP) Ltd., St. Petersburg, http://www.cisp.spb.ru
Kossoy AA, Benin A, Akhmetshin Y. An advanced approach to reactivity rating. J Hazard Mater. 2005;118:9–17.
Wang SY, Kossoy AA, Yao YD, Chen LP, Chen WH. Kinetics-based simulation approach to evaluate thermal hazards of benzaldehyde oxime by DSC tests. Thermochim Acta. 2017;655:319–25.
Stoessel F. Thermal safety of chemical processes. Weinheim: Wiley; 2008. p. 270–82.
Zhang CY, ** SH, Ji JW, et al. Thermal hazard assessment of TNT and DNAN under adiabatic condition by using accelerating rate calorimeter (ARC). J Therm Anal Calorim. 2018;131:89–93.
Frank-Kamenetskii DA. Diffusion and heat exchange in chemical kinetics. 2nd ed. New York: Plenum Press; 1969.
Bowes PC. Self-heating: evaluating and controlling the hazards. New York: Elsevier; 1984.
Kosoy AA, Sheinman I. Comparative analysis of the methods for SADT determination. J Hazard Mater. 2007;142:26–38.
Zhao Y, ** SW, Tao ZH, et al. Crystal and molecular structures of four organic acid-base adducts from hexamethylenetetramine, N, N, N, N-Tetramethylethylenediamine, and organic acids. J Chem Crystallogr. 2016;46:188–202.
Acknowledgements
This investigation was financed by the National key R&D Program of China. The authors thank this support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rao, G., Feng, W., Zhang, J. et al. Simulation approach to decomposition kinetics and thermal hazards of hexamethylenetetramine. J Therm Anal Calorim 135, 2447–2456 (2019). https://doi.org/10.1007/s10973-018-7359-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7359-8