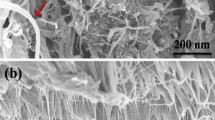
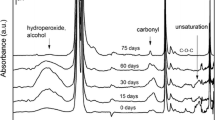
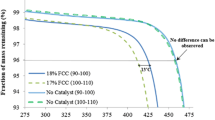
Abstract
The influence of fullerene (C60) on the thermal and thermal-oxidative degradation of high-density polyethylene (HDPE) was studied using non-isothermal thermogravimetric analysis under nitrogen (N2) and air atmosphere. Kinetic parameters of the degradation were evaluated using the Flynn–Wall–Ozawa method, which does not require the knowledge of the reaction mechanism. The results showed that the addition of C60 enhanced the thermal stability of HDPE and increased the activation energy both in N2 and air atmosphere and especially affected the initial stage of degradation. In N2, C60-trapped carbon-centered radical originated from the degradation of HDPE to improve the thermal stability and increase the activation energy. While in air, C60 trapped the alkyl radicals and alkyl peroxide radicals to inhibit the hydrogen abstraction (especially the initial stage of thermo-oxidative degradation) and form more stable species, which improved the thermal stability and increased the activation energy during the thermal degradation of HDPE. Comparing with that of pure HDPE, the changes of activation energy for HDPE/C60 nanocomposites were higher in air than in N2, especially in the initial stage.





Similar content being viewed by others
References
Ball ZT, Sivula K, Frechet JMJ. Well-defined fullerene-containing homopolymers and diblock copolymers with high fullerene content and their use for solution-phase and bulk organization. Macromolecules. 2006;39:70–2.
Imahori H. Creation of fullerene-base artificial photosynthetic systems. Bull Chem Soc Jpn. 2007;80:621–36.
Ohsava S, Maeda K, Yashima E. Synthesis and chiroptical properties of optically active helical poly(phenylacetylene)s bearing fullerenes pendants. Macromolecules. 2007;40:9244–51.
Chubarova EV, Melenevskaya EYu, Sudareva NN, Andreeva OA, Malachova II, Ratnikova OV. Degradation of macromolecular chains in fullerene C60–polystyrene composites. J Macromol Sci Part B Phys. 2005;44:455–69.
Troitskii BB, Troitskaya LS, Dmitriev AA, Yakhnov AS. Inhibition of thermo-oxidative degradation of poly(methyl methacrylate) and polystyrene by C60. Eur Polym J. 2000;36:1073–84.
Song PA, Zhu Y, Tong LF, Fang ZP. C60 reduces the flammability of polypropylene nanocomposites by in situ forming a gelled-ball network. Nanotechnology. 2008;19:225707–16.
Pozdnyakov AO, Handge UA, Konchits AA, Altstadt V. Thermal stability of PMMA–C60 nanocomposite: a spectroscopic study. Tech Phys Lett. 2010;36:960–3.
Ouyang J, Pan Y, Zhou S, Goh SH. Suparmolecular assembled C60-containing carboxylated poly(dimethylsiloxane) composites. Polymer. 2006;47:6140–8.
Zeinalov EB, Koßmehl G. Fullerene C60 as an antioxidant for polymers. Polym Degrad Stab. 2001;71:197–202.
Zuev VV, Bertini F, Audisio G. Fullerene C60 as stabilizer for acrylic polymers. Polym Degrad Stab. 2005;90:28–33.
Kong Q, Hu Y, Song L, Tang Y. Kinetics of thermo-oxidative degradation of polypropylene/aluminum trihydroxide/organo Fe–montmorillonite nanocomposites. J Therm Anal Calorim. 2011;104:1145–51.
Regnier N, Guibe C. Methodology for multistage degradation of polymide polymer. Polym Degrad Stab. 1997;55:165–72.
Day M, Cooney JD, Wiles DM. The thermal stability of poly(aryl-ether–ether–ketone) as assessed by thermogravimetry. J Appl Polym Sci. 1989;38:323–37.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Criado JM, Sanchez-Jimenez PE, Perez-Maqueda LE. Critical study of the isoconversional methods of kinetic analysis. J Therm Anal Calorim. 2008;92:199–203.
Fernandes VJ Jr, Araujo AS, Fernandes GJT. Catalytic degradation of polyethylene evaluated by TG. J Therm Anal Calorim. 1997;49:255–60.
Gao ZM, Amasaki I, Nakada M. A thermogravimetric study on thermal degradation of polyethylene. J Anal Appl Pyrolysis. 2003;67:1–9.
Dorigato A, Pegoretti A, Frache A. Thermal stability of high density polyethylene-fumed silica nanocomposites. J Therm Anal Calorim. 2012;109:863–73.
Zhao LP, Song PA, Cao ZH, Fang ZP, Guo ZH. Thermal stability and rheological behaviors of high-density polyethylene/fullerene nanocomposites. J Nanomater. 2012. doi:10.1155/2012/340962.
Krusic PJ, Wasserman E, Keizer PN, Morton JR, Preston KF. Radical reactions of C60. Science. 1991;54:1183–5.
Gao ZM, Amasaki I, Kaneko T, Nakada M. Calculation of acitivation energy from fraction of bonds broken for thermal degradation of polyethylene. Polym Degrad Stab. 2003;81:125–30.
Zanetti M, Camino G, Reichert P, Mulhaupt R. Thermal behavior of poly(propylene) layered silicate nanocomposites. Macromol Rapid Commun. 2001;22:176–80.
Peterson JD, Vyazovkin S, Wight CA. Kinetics of the thermal and thermo-oxidative degradation of polystyrene, polyethylene and polypropylene. Macromol Chem Phys. 2001;202:775–84.
Holmstrom A, Sorvik EM. Thermal degradation of polyethylene in nitrogen atmosphere of low oxygen content. III. Structural changes occurring in low-density polyethylene at oxygen contents below 1.2 %. J Appl Polym Sci. 1974;18:779–804.
Hirsch A, Grosser T, Skiebe A, Soi A. Synthesis of isomerically pure organodihydrofullerenes. Chem Ber. 1993;126:1061–7.
Bingel C. Cyclopropanierung von fullerene. Chem Ber. 1993;126:1957–9.
Gan LB, Zhou DJ, Luo CP, Tan HS, Huang CH, Lu MJ, Pan JQ, Wu Y. Synthesis of fullerene amino acid derivatives by direct interaction of amino acid ester with C60. J Org Chem. 1996;61:1954–61.
Krusic PJ, Wasserman E, Parkinson BA, Malone B, Holler ER Jr, Keizer PN, Morton JR, Preston KF. Electron spin resonance study of the radical reactivity of C60. J Am Chem Soc. 1991;113:6274–5.
Morton JR, Preston KF, Krusic PJ, Hill SA, Wasserman E. ESR studies of the reaction of alkyl radicals with fullerene (C60). J Phys Chem. 1992;96:3576–8.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 51073140) and the Natural Science Foundation of Ningbo (No. 2011A610124).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, L., Cao, Z., Fang, Z. et al. Influence of fullerene on the kinetics of thermal and thermo-oxidative degradation of high-density polyethylene by capturing free radicals. J Therm Anal Calorim 114, 1287–1294 (2013). https://doi.org/10.1007/s10973-013-3158-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3158-4