Abstract
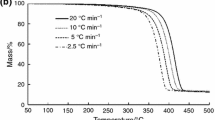
In the article, the thermal oxidative degradation kinetics of pure polypropylene/aluminum trihydroxide (PP/ATH) and PP/ATH/organo Fe-montmorillonite (Fe-OMT) nanocomposites were investigated using Kissinger, Friedman and Flynn–Wall–Ozawa methods. The results showed that thermal oxidative degradation of PP/ATH/Fe-OMT nanocomposites to PP/ATH were complex reaction: the whole process of thermal oxidative degradation were composed with the decomposition of ATH, the cracking and charring of the backbone chains of PP, and the oxidative degradation of char, which the curses of energy mutative with the process of thermal oxidative degradation. The control steps were different in each degradation stage. The activation energy was high in the original degradation stage. It was due to the molecular structure and may closely relate with onset temperature. In the intermediate process, the activation energy was low. In the last stage of the degradation, the activation energy was graveled because the carbon may be oxidized. In the whole process of thermal oxidative degradation, the activation energy of PP/ATH/Fe-OMT nanocomposite was higher than that of PP/ATH.







Similar content being viewed by others
References
Ristolainen N, Hippi U, Seppala J, Nykanen A, Ruokolainen J. Properties of polypropylene/aluminum trihydroxide composites containing nanosized organoclay. Poly Eng Sci. 2005;45(12):1568–75.
Rothon R. The emergence of magnesium hydroxide as a fire retardant additive. In: The Plastic and Rubber Institute, editor. Flame Retardants 1990 Conference. London: Elsevier; 1990.
Tang T, Chen X, Chen H, Meng X, Jiang Z, Bi W. Catalyzing carbonization of polypropylene itself by supported nickel catalyst during combustion of polypropylene/clay nanocomposite for improving fire retardancy. Chem Mater. 2005;17:2799–802.
Gilman JW, Jackson CL, Morgan AB, Jr RH, Manias E, Giannelis EP et al., Flammability properties of polymer-layered-silicate nanocomposites polypropylene and polystyrene nanocomposites. Chem Mater. 2000; 12:1866–1873.
Kong QH, Hu Y, Song L, Yi CW. Synergistic flammability and thermal stability of polypropylene/aluminum trihydroxide/Fe-montmorillonite nanocomposites. Polym Adv Technol. 2009;20:404–9.
Opfermann J. Kinetic analysis using multivariate non-linear regression. I. Basic concepts. J Therm Anal Calorim. 2000;60:641–58.
Mamleev V, Bourbigot S, Le Bras M, Duquesne S, Sestak J. Modelling of nonisothermal kinetics in thermogravimetry. Phys Chem Chem Phys. 2000;2(20):4708–16.
Mamleev V, Bourbigot S, Le Bras M, Lefevbre J. Three model-free methods for calculation of activation energy in TG. J Therm Anal Calorim. 2004;78(3):1009–27.
Opfermann JR, Kaisersberger E, Flammersheim HJ. Model-free analysis of thermoanalytical data-advantages and limitations. Thermochim Acta. 2002;391(1–2):117–27.
Rose N, Le Bras M, Bourbigot S, Delobel R, Costes B. Comprehensive study of the oxidative degradation of an epoxy resin using the degradation front model. Polym Degrad Stab. 1996;54(2–3):355–60.
Flynn JH. Polymer degradation. Handbook of thermal analysis and calorimetry, Amsterdam: Elsevier; 2002; 3: 587–651.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Lomakin SM, Dubnikova IL, Shchegolikhin AN, Zaikov GE, Kozlowski R, Kim GM, Michler GH. Thermal degradation and combustion behavior of the polyethylene/clay nanocomposite prepared by melt intercalation. J Therm Anal Calorim. 2008;94(3):719–26.
Abate L, Blanco I, Bottino FA, Di Pasquale G, Fabbri E, Orestano A, Pollicino A. Kinetic study of the thermal degradation of PS/MMT nanocomposites prepared with imidazolium surfactants. J Therm Anal Calorim. 2008;91(3):681–6.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Japan. 1965;38(1):1881–6.
Friedman HL. Kinetics of thermal degradation of char-forming plastics from thermogravimetry: application to phenolic plastic. J Polym Sci Part C. 1964;6:183–95.
Kong QH, Hu Y, Song L, Wang YL, Chen ZY, Wan WC. Influence of Fe-MMT on crosslinking and thermal degradation in silicone rubber/clay nanocomposites. Polym Adv Technol. 2006; 17(6): 463–467.
Zhu J, Uhl FM, Morgan AB, Wilkie CA. Studies on the mechanism by which the formation of nanocomposites enhances thermal stability. Chem Mater. 2001; 13(12): 4649–4654.
Goodarzi V, Jafari SH, Khonakdar HA, Monemian SA, Mortazavi M. An assessment of the role of morphology in thermal/thermo-oxidative degradation mechanism of PP/EVA/clay nanocomposites. Polym Degrad Stab. 2010;95(5):859–69.
Wang DY, Wang YZ, Wang JS, Chen DQ, Zhou Q, Yang B, Li WY. Thermal oxidative degradation behaviours of flame-retardant copolyesters containing phosphorous linked pendent group/montmorillonite nanocomposites. Polym Degrad Stab. 2005;87:171–6.
Vyazovkin S, Dranca I, Fan XW, Advincula R. Kinetics of the thermal and thermo-oxidative degradation of a polystyrene/clay nanocomposite. Macromol Rapid Comm. 2004;25(3):498–503.
Acknowledgements
The study was financially supported by the Foundation of State Key Laboratory of Fire Science (No. HZ2010-KF03), Natural Science fund of University in Jiangsu (No. 09KJD620001) and the joint fund of NSFC and CAAC (No. 61079015).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kong, Q., Hu, Y., Song, L. et al. Kinetics of thermo-oxidative degradation of polypropylene/aluminum trihydroxide/organo Fe-montmorillonite nanocomposites. J Therm Anal Calorim 104, 1145–1151 (2011). https://doi.org/10.1007/s10973-011-1346-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1346-7