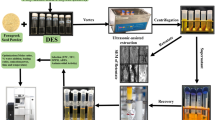
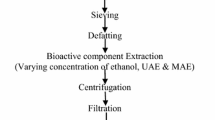
Abstract
The effects of germination pretreatments on fenugreek seeds were analyzed by employing an ultrasound-assisted deep eutectic solvent (UAE-DES) extraction process. The germinated and UAE-DES extracted fenugreek sample of day 3 (GFE3) was found to be the most effective based on the results of flavonoid contents, antioxidant properties, and antibacterial potential. The results of HPLC-DAD analysis also suggested GFE3 as an effective pretreatment with the highest extraction yields of kaempferol (5.33 ± 0.26 mg/gm d.w.), rutin (9.06 ± 0.44 mg/gm d.w.) and quercetin (25.69 ± 0.44 mg/gm d.w.) among all treatments. The results of simulated digestion showed that a major portion of flavonoid contents were lost during gastric digestion due to poor stability values and a maximum TFC release of 60.23% was recorded after intestinal digestion for GFE3. Subsequently, GFE3 exhibited good inhibition properties against α-amylase (86.53 ± 4.24%), α-glucosidase (95.67 ± 4.68%) and pancreatic lipase enzymes (82.73 ± 4.05%). Hence, GFE3 can be used as a potential pretreatment for enhanced antioxidant activity and improved glycemic, lipid and digestive responses, which can be further used in food, nutraceutical and pharmaceutical industries.







Similar content being viewed by others
Data availability
All the data related to this study has been presented in the article.
References
S.A. Wani et al., Sensory, functional characteristics and in vitro digestibility of snacks supplemented with non-traditional ingredient raw and processed fenugreek. Int. J. Food Sci. Technol. 57(8), 4716–4725 (2022). https://doi.org/10.1111/ijfs.15441
W. Ashraf et al., Optimization of extraction process and estimation of flavonoids from Fenugreek using Green extracting deep Eutectic solvents coupled with Ultrasonication. Food Bioprocess. Technol. (2023). https://doi.org/10.1007/s11947-023-03170-6
T. Herrera et al., Acid hydrolysis of saponin-rich extracts of quinoa, lentil, fenugreek and soybean to yield sapogenin-rich extracts and other bioactive compounds. J. Sci. Food Agric. 99(6), 3157–3167 (2019). https://doi.org/10.1002/jsfa.9531
P.S. Chaubey et al., Evaluation of debittered and germinated fenugreek (Trigonella Foenum Graecum L.) seed flour on the chemical characteristics, biological activities, and sensory profile of fortified bread. J. Food Process. Preserv. 42(1), e13395 (2018). https://doi.org/10.1111/jfpp.13395
R. Niknam, Extraction, Detection, and Characterization of Various Chemical Components of Trigonella foenum-graecum L. (Fenugreek) Known as a Valuable Seed in Agriculture, in Fenugreek: Biology and Applications, M. Naeem, T. Aftab, M.M.A. Khan et al., Editors. 2021, Springer Singapore: Singapore. pp. 189–217. https://doi.org/10.1007/978-981-16-1197-1_9
O. Laila et al., Enhancement of nutraceutical and anti-diabetic potential of fenugreek (Trigonella foenum-graecum). Sprouts with natural elicitors. Saudi Pharma J. 31(1), 1–13 (2023). https://doi.org/10.1016/j.jsps.2022.11.001
L. Wang et al., Effect of ultrasound combined with exogenous GABA treatment on polyphenolic metabolites and antioxidant activity of mung bean during germination. Ultrason. Sonochem. 94, 106311 (2023). https://doi.org/10.1016/j.ultsonch.2023.106311
P. Pająk et al., Antioxidant properties, phenolic and mineral composition of germinated Chia, golden flax, evening primrose, phacelia and fenugreek. Food Chem. 275, 69–76 (2019). https://doi.org/10.1016/j.foodchem.2018.09.081
A. Nogueira et al., Seed germination and seedling development assisted by ultrasound: gaps and future research directions. J. Sci. Food Agric. (2023). https://doi.org/10.1002/jsfa.12994
W. Ashraf et al., Techno.logical Advancement in the Processing of Lycopene: a review. Food Rev. Int. 38(5), 857–883 (2022). https://doi.org/10.1080/87559129.2020.1749653
J. Lei et al., Natural green deep eutectic solvents-based eco-friendly and efficient extraction of flavonoids from Selaginella moellendorffii: process optimization, composition identification and biological activity. Separ Purif. Technol. 283, 120203 (2022). https://doi.org/10.1016/j.seppur.2021.120203
J.O. Airouyuwa et al., Natural deep Eutectic solvents and microwave-assisted green extraction for efficient recovery of Bioactive compounds from By-Products of date fruit (Phoenix dactylifera L.) Processing: modeling, optimization, and Phenolic characterization. Food Bioprocess. Technol. 16(4), 824–843 (2023). https://doi.org/10.1007/s11947-022-02960-8
P. Arya, P. Kumar, Comparison of ultrasound and microwave assisted extraction of diosgenin from Trigonella foenum graceum seed. Ultrason. Sonochem. 74, 105572 (2021). https://doi.org/10.1016/j.ultsonch.2021.105572
S.A. Wani, S. Bishnoi, P. Kumar, Ultrasound and microwave assisted extraction of diosgenin from fenugreek seed and fenugreek-supplemented cookies. J. Food Measure. 10(3), 527–532 (2016). https://doi.org/10.1007/s11694-016-9331-2
S. Shakuntala et al., Characterisation of germinated fenugreek (Trigonella foenum-graecum L.) seed fractions. Int. J. Food Sci. Technol. 46(11), 2337–2343 (2011). https://doi.org/10.1111/j.1365-2621.2011.02754.x
J.B. Johnson et al., Phytochemical composition and biological activity of native Australian ginger (Alpinia caerulea). J. Food Measure. (2024). https://doi.org/10.1007/s11694-023-02326-4
L. Wu et al., Investigation of biological activity of Alpinia platychilus extracts and its use as a natural preservative in fruits. J. Food Measure. (2023). https://doi.org/10.1007/s11694-023-02285-w
H. Gouja et al., Optimization of the rapid effective extraction, antioxidant, antiproliferative and alpha-amylase activities in Plantago ovata seed non-adherent and adherent mucilage by RSM. J. Food Measure. (2024). https://doi.org/10.1007/s11694-024-02363-7
K. Ali et al., Preparation and structural characterization of pullulan and whey protein isolate-based electrospun nanofiber. Food Biosci. 56, 103218 (2023). https://doi.org/10.1016/j.fbio.2023.103218
A. Saini et al., Conventional and non-conventional approaches for the extraction of rosehip phytocompounds and its bioactive, structural and antimicrobial characterization. J. Food Measure 2024 https://doi.org/10.1007/s11694-024-02361-9
T. Meher, A. Jayadeep, Effect of bioprocessing through germination and hydrothermal treatment on nutrients, nutraceuticals, and antioxidant properties in red and black rice. J. Food Measure. (2024). https://doi.org/10.1007/s11694-024-02372-6
W. Ashraf et al., Influence of selected hydrocolloids on the rheological, functional, and textural properties of wheat-pumpkin flour bread. J. Food Process. Preserv. 44(10), e14777 (2020). https://doi.org/10.1111/jfpp.14777
X.-. Meng, C. Tan, Y. Feng, Solvent extraction and in vitro simulated gastrointestinal digestion of phenolic compounds from purple sweet potato. Int. J. Food Sci. Technol. 54(10), 2887–2896 (2019). https://doi.org/10.1111/ijfs.14153
I. Gupta, M. Serva Peddha, Characterization of polyphenols profile, antioxidant, and in vitro activities of Nigella sativa L. (black cumin) seed oleoresin. J. Food Measure. (2024). https://doi.org/10.1007/s11694-023-02311-x
E. Shawky et al., Comparative metabolomics analysis of bioactive constituents of the leaves of different Trigonella species: correlation study to α-amylase and α-glycosidase inhibitory effects. Ind. Crops Prod. 182, 114947 (2022). https://doi.org/10.1016/j.indcrop.2022.114947
K. Ali et al., Improving the functional characteristics of thymol-loaded pullulan and whey protein isolate-based electrospun nanofiber. Food Biosci. 57, 103620 (2024). https://doi.org/10.1016/j.fbio.2024.103620
A. khosravi, S.H. Razavi, A.M. Fadda, Advanced assessments on innovative methods to improve the bioaccessibility of polyphenols in wheat. Process. Biochem. 88, 1–14 (2020). https://doi.org/10.1016/j.procbio.2019.09.005
G. Zhang et al., Effects of Germination on the Nutritional Properties, phenolic profiles, and antioxidant activities of Buckwheat. J. Food Sci. 80(5) (2015). https://doi.org/10.1111/1750-3841.12830. p. H1111-H1119
N. Singh et al., Ethnopharmacological, phytochemical and clinical studies on Fenugreek (Trigonella foenum-graecum L). Food Biosci. 46, 101546 (2022). https://doi.org/10.1016/j.fbio.2022.101546
A. Manzoor et al., Plant-derived active substances incorporated as antioxidant, antibacterial or antifungal components in coatings/films for food packaging applications. Food Biosci. 53, 102717 (2023). https://doi.org/10.1016/j.fbio.2023.102717
Z. Hu et al., Physiological characterization, antioxidant potential, and bacterial survival of soybean sprouts subjected to pre- and post-harvest low intensity ultrasound and exogenous ascorbic acid application. Postharvest Biol. Technol. 198, 112258 (2023). https://doi.org/10.1016/j.postharvbio.2023.112258
Q.A. Al-Maqtari et al., Evaluation of bioactive compounds and antibacterial activity of Pulicaria jaubertii extract obtained by supercritical and conventional methods. J. Food Measure. 15(1), 449–456 (2021). https://doi.org/10.1007/s11694-020-00652-5
F.J. Álvarez-Martínez et al., Antibacterial plant compounds, extracts and essential oils: an updated review on their effects and putative mechanisms of action. Phytomedicine. 90, 153626 (2021). https://doi.org/10.1016/j.phymed.2021.153626
L.-Y. Lin et al., Optimization of Bioactive compounds in Buckwheat sprouts and their effect on blood cholesterol in hamsters. J. Agric. Food Chem. 2008 56(4): p. 1216–1223. https://doi.org/10.1021/jf072886x
G. Zhao et al., Different thermal drying methods affect the phenolic profiles, their bioaccessibility and antioxidant activity in Rhodomyrtus tomentosa (Ait.) Hassk berries. LWT - Food Sci. Technol. 79, 260–266 (2017). https://doi.org/10.1016/j.lwt.2017.01.039
M.S. Lingua et al., Bioaccessibility of polyphenols and antioxidant properties of the white grape by simulated digestion and Caco-2 cell assays: comparative study with its winemaking product. Food Res. Int. 122, 496–505 (2019). https://doi.org/10.1016/j.foodres.2019.05.022
B.J. Prasad, P.S. Sharavanan, R. Sivaraj, Efficiency of Oryza punctata extract on glucose regulation: inhibition of α-amylase and α-glucosidase activities. Grain Oil Sci. Technol. 2(2), 44–48 (2019). https://doi.org/10.1016/j.gaost.2019.04.007
J. Verma et al., α-Amylase Inhibitor’s Performance in the Control of Diabetes Mellitus: An Application of Computational Biology, in Current trends in Bioinformatics: An Insight, G. Wadhwa, Editors. 2018, Springer Singapore: Singapore. pp. 307–332. https://doi.org/10.1007/978-981-10-7483-7_18
D. Stojkovic et al., An insight into antidiabetic properties of six medicinal and edible mushrooms: inhibition of α-amylase and α-glucosidase linked to type-2 diabetes. South. Afr. J. Bot. 120, 100–103 (2019). https://doi.org/10.1016/j.sajb.2018.01.007
H. Kızıltaş, L.C.-H.R.M.S. Profiling et al., and Antidiabetic, Anticholinergic, and Antioxidant Activities of Aerial Parts of Kınkor (Ferulago stellata). Molecules. 2021. 26(9): p. 2469. https://www.mdpi.com/1420-3049/26/9/2469
J.U. Obaroakpo et al., Bioactive assessment of the antioxidative and antidiabetic activities of oleanane triterpenoid isolates of sprouted quinoa yoghurt beverages and their anti-angiogenic effects on HUVECS line. J. Funct. Foods. 66, 103779 (2020). https://doi.org/10.1016/j.jff.2020.103779
V.B. Oliveira et al., Chemical composition and inhibitory activities on dipeptidyl peptidase IV and pancreatic lipase of two underutilized species from the Brazilian Savannah: Oxalis cordata A.St.-Hil. and Xylopia aromatica (Lam.) Mart. Food Res. Int. 2018. 105: pp. 989–995. https://doi.org/10.1016/j.foodres.2017.11.079
W.N. Hozzein et al., CO2 treatment improves the hypocholesterolemic and antioxidant properties of fenugreek seeds. Food Chem. 308, 125661 (2020). https://doi.org/10.1016/j.foodchem.2019.125661
T. Herrera et al., Inhibitory effect of quinoa and fenugreek extracts on pancreatic lipase and α-amylase under in vitro traditional conditions or intestinal simulated conditions. Food Chem. 270, 509–517 (2019b). https://doi.org/10.1016/j.foodchem.2018.07.145
H. Pandey, P. Awasthi, Effect of processing techniques on nutritional composition and antioxidant activity of fenugreek (Trigonella foenum-graecum) seed flour. J. Food Sci. Technol. 52(2), 1054–1060 (2015). https://doi.org/10.1007/s13197-013-1057-0
B. Zheng et al., Green extraction of phenolic compounds from foxtail millet bran by ultrasonic-assisted deep eutectic solvent extraction: optimization, comparison and bioactivities. LWT-Food Sci. Technol. 154, 112740 (2022). https://doi.org/10.1016/j.lwt.2021.112740
R.S. Mohamed et al., Hypoglycemic, hypolipidemic and antioxidant effects of green sprouts juice and functional dairy micronutrients against streptozotocin-induced oxidative stress and diabetes in rats. Heliyon. 5(2), e01197 (2019). https://doi.org/10.1016/j.heliyon.2019.e01197
S. Khole et al., Bioactive constituents of germinated fenugreek seeds with strong antioxidant potential. J. Funct. Foods. 6, 270–279 (2014). https://doi.org/10.1016/j.jff.2013.10.016
R. Najafi, A. Rezaei, D. Talei, Potential of ultrasound and nicotinic acid to improve physiological responses and trigonelline biosynthesis in fenugreek (Trigonella foenum-graecum L). Ind. Crops Prod. 182, 114815 (2022). https://doi.org/10.1016/j.indcrop.2022.114815
Acknowledgements
Thisproject was supported by the grants from Thirteen Five National Key Research and Development Program of China (2018YFD0400902) and Changji science and technology project (2019G02).
Author information
Authors and Affiliations
Contributions
Waqas Ashraf, conceptualization, experimentation, data analysis, writing and editing of the first draft. Zhang Lianfu, conceptualization, funding acquisition, supervision, reviewing and editing. Abdur Rehman, data interpretation, proofreading the paper and editing. Arif Hussain, methodology and formal data interpretations. Aiman Karim, methodology and formal analysis. Khubaib Ali, methodology, proofreading the original draft and editing, Hafiz Rizwan Sharif, data curation, proofreading and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ashraf, W., Rehman, A., Sharif, H.R. et al. Ultrasonication-assisted deep eutectic solvent extraction of flavonoids from pretreated fenugreek and their antidiabetic & hypo-lipidemic potential. Food Measure (2024). https://doi.org/10.1007/s11694-024-02575-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11694-024-02575-x