Abstract
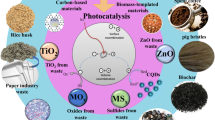
Urbanization and a massive population boom have immensely increased the solid wastes (SWs) generation and are expected to reach 3.40 billion tons by 2050. In many developed and emerging nations, SWs are prevalent in both major and small cities. As a result, in the current context, the reusability of SWs through various applications has taken on added importance. Carbon-based quantum dots (Cb-QDs) and their many variants are synthesized from SWs in a straightforward and practical method. Cb-QDs are a new type of semiconductor that has attracted the interest of researchers due to their wide range of applications, which include everything from energy storage, chemical sensing, to drug delivery. This review is primarily focused on the conversion of SWs into useful materials, which is an essential aspect of waste management for pollution reduction. In this context, the goal of the current review is to investigate the sustainable synthesis routes of carbon quantum dots (CQDs), graphene quantum dots (GQDs), and graphene oxide quantum dots (GOQDs) from various types SWs. The applications of CQDs, GQDs, and GOQDs in the different areas are also been discussed. Finally, the challenges in implementing the existing synthesis methods and future research directions are highlighted.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The generation of solid wastes (SWs) has increased dramatically as a result of urbanization and significant population growth and is anticipated to reach 3.40 billion tons by 2050 (“Solid Waste Management” n.d.). Thus, SWs treatment and disposal is a critical issue that must be addressed by both develo** and developed countries, particularly in metropolitan areas (Bui et al. 2022; Khan et al. 2021). Generally, these SWs are a collection of agricultural, paper, food, animal, yard trimming, plastics, metals, rubber, leather, textiles, wood, glass wastes, and so on (Fig. 1) (Abdel-Shafy and Mansour 2018). The continuous rise in ecologically harmful and hazardous SWs is currently a major concern, necessitating either proper trash disposal or reuse (Meyer et al. 2020). According to reports, over 70% of the global SWs are disposed of in landfills, while only 20% are recycled. As a result, it is imperative to enhance the proportion of SWs recycled into valuable goods.
Because of the high cost, lack of organization, and difficulties, sustainable management of these SWs is the most significant impediment to increasing urbanization and an improvement in the standard of living (Chien et al. 2021; Khan et al. 2022). Thus, the proposed management method must be cost-effective, simple, sustainable, and ecologically appealing, as well as legally and socially permissible. Because most agricultural, household, human, and animal wastes are rich in proteins, minerals, and carbohydrates, thus, these SWs can be used as raw materials in material science and other related areas.
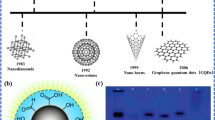
Owing to their wide range of applications in physical, chemical, biological, and materials research, nanomaterials are a revolutionary finding of the late twentieth century (Das et al. 2020; 2022; Kolahalam et al. 2019; Sasidharan et al. 2019; Talapin and Shevchenko 2016; Rajabi et al. 2020, 2018; Wei et al. 2017; Zang et al. 2017; Liu et al. 2018; Vatanpour et al. 2022; Fakhraie et al. 2023). Generally, they have different physicochemical properties compared to their bulk counterparts. Quantum dots (QDs) are a subset of nanomaterials that were envisioned in the 1980s, when charge transporters were confined to three-dimensional semiconductor materials (Das et al. 2018; Lv et al. 2020). Carbon onions (Dalal et al. 2021; Lettieri et al. 2017a, b), carbon nanotubes (Gao et al. 2014; Li and Shi 2014; Spreinat et al. 2021; Welsher et al. 2009), carbon nanoribbons (Lu et al. 2009; Wang et al. 2019b). Processed black liquid from the Kraft pul** of eucalyptus in the pulp paper industry was treated with concentrated sulfuric acid, to get the alkali lignin. The process of converting alkali lignin to GQDs was divided into two steps: the first was fractionation into lignin nanoparticles doped with nitrogen and sulphur, and the second was a hydrothermal approach to convert the produced lignin nanoparticles into GQDs. As indicated by a dynamic light scattering study, the average diameter range of the synthesized lignin-based GQDs (LGQDs) was 500–800 nm, and the QY was 21% under an excitation wavelength of 380 nm.
In another method, Ding et al. reported the synthesis of GQDs from alkali lignin (Ding et al. 2018). Here, alkali lignin was first treated with 67% nitric acid under ultrasonication to change it into the black carbon-based solution, and the resultant dispersed solution was hydrothermally treated for 12 h at 180 °C. The size range of GQDs was found to be 2–6 nm with a QY of 21%.
Table 1 displays different approaches for synthesizing GQDs from various SWs precursors. The top-down technique for GQD synthesis requires a certain type of carbon skeleton in the starting material, making it less prevalent than CQDs (Zhao et al. 2020). Since majority of SWs lack this sort of structure, therefore mostly GQDs have lower QY than CQDs.
Synthesis of graphene oxide quantum dots
Unlike CQDs and GQDs, GOQDs are produced under a strong oxidizing environment, hence a large number of COOH, -OH, and epoxy functional groups are usually available on their surfaces, making them highly water soluble (Kang et al. 2019). To the best of our knowledge, GOQDs have been reported to be synthesized from waste toner (Xu et al. 2019) and waste paper as SWs sources (Adolfsson et al. 2015) only.
Xu et al. (2019) hydrothermally synthesized GOQDs from waste toner at 180 °C for 4 h in an oxidizing atmosphere of 5% (w/w) hydrogen peroxide. They optimized the experimental conditions to achieve the best results. They further modified the synthesized GOQDs by heating them with polyethyleneimine (PEI) at 60 °C for 3 h to synthesize PEI@GOQDs. The diameter of the synthesized GOQDs, evaluated by HRTEM, was between 2 and 3.5 nm, and the QY was found to be 10.6% under illumination at 340 nm using quinine sulphate as a reference.
Adolfsson et al. developed a microwave-assisted method to synthesize GOQDs from cellulose-enriched waste-paper (Adolfsson et al. 2015). They synthesized the GOQDs in two stages: during the first stage, carbon nanospheres (CNs) were synthesized as an intermediate material directly from waste paper, followed by the conversion of CNs to GOQDs during the second stage. The second stage was sub-divided into two parts, the first being the disintegration of CNs and the second being the oxidation-degradation process. Finally, the heating was performed for various durations (30–60 min) to obtain different sizes of GOQDs. For 30 min and 60 min heating, the final HRTEM analyzed sizes for GOQDs were 3 nm and 1 nm, respectively.
Table 1 summarizes the available literature on GOQDs preparation from SWs. Due to the complexity of the synthesis process and the lower fluorescence intensity compared to the other two QDs, there have not been many reports of GOQDs from SWs. However, conjugated GOQDs are progressively gaining popularity among researchers due to the presence of various functional groups in their structure.
Figure 2 summarizes all the current methods of Cb-QDs synthesis from SWs, with hydrothermal being used in most cases. Hydrothermal synthesis is a simple and efficient approach for producing these Cb-QDs, and the QY obtained from this process is often the highest when compared to the other methods. The microwave is the second-best method for producing Cb-QDs. Both methods are environmentally sustainable but expensive. Reflux, sonochemical, pyrolysis, and other processes are still in their early stages and require further development. Some of the existing methods utilize toxic chemicals, while others demand high temperatures conditions. Thus, efforts should be directed toward the development of efficient, eco-friendly, cost-effective and low temperature procedures for producing Cb-QDs from SWs.
The temperature of the preparation, the presence of an oxidizing/reducing environment, the structure of starting material’s (in some cases), and the synthesis procedure are some vital factors that affect the formation of Cb-QDs. For example, Ye et al. (2013).
Characterization of carbon-based quantum dots
There are three types of characterization methodologies for identifying relevant Cb-QDs:
-
Chemical composition or structural characterizations,
-
Morphological characterization,
-
Optical characterization.
Chemical composition or structural characterizations
Various characterization approaches may be used to identify information about the chemical composition of the QDs and the groups present in various Cb-QDs or functionalized Cb-QDs. Among them, Fourier transform infrared (FT-IR) spectroscopy, X-ray photoelectron spectroscopy (XPS), Raman spectroscopy (RS), energy dispersive X-ray (EDX) analysis, and thermogravimetric analysis (TGA) are most common techniques. Nevertheless, we will limit our discussion only to the characterization of unmodified Cb-QDs as the surface modification will result in many complicated peaks of the spectra.
Fourier transform infrared (FT-IR) analysis
For CQDs
The FT-IR analysis data of CQDs prepared from lemon peel waste is shown in Fig. 3a (Tyagi et al. 2016). A broad peak at 3414 cm−1 was found in this FT-IR spectrum, which might be due to O–H stretching for water in CQDs. The vibrational frequency of C–H was found to be 2940 cm−1, C = O stretching was observed at 1715 cm−1, and the -COO− exhibited two peaks at 1605 and 1405 cm−1.
For GQDs
GQDs, on the other hand, produce more distinct peaks in FT-IR spectrum than GOQDs (Fig. 3b). Stretching vibration of C = C (for aromatic ring) was detected at 1590 cm−1. The vibrational frequencies of in-plane and out-plane C–H (aromatic) groups appeared at 1041, 870, and 762 cm−1. The presence of O–H, C = O, and C–O groups throughout the periphery of each GQDs sheet resulted in broad maxima at 3360, 1697, and 1190 cm−1. Ding et al. reported that the peak for C–O–C at 1261 cm−1 in the starting materials (alkali lignin) was missing in GQDs and it was replaced by a C–N bond (due to nitric acid oxidation), which appeared at 1149 cm−1(Ding et al. 2018).
For GOQDs
GOQDs are rich in oxygen containing functional groups on its surface, so due to that polar crown, GOQDs were highly dispersible in water. In FT-IR spectrum for uncoated GOQDs (Fig. 3c), O–H group on the GOQDs surface (or from the water) appeared at 3438 cm−1 and two weak C–H bending peaks were found at 2923 and 2849 cm−1. Moreover, one weak and one strong C = O stretching frequency were obtained at 1717 and 1635 cm−1, a weak C–H bending vibration was reported to be at 1385 cm−1 in addition to a broad and quite strong type of C–OH stretching frequency (Xu et al. 2019).
X-ray photoelectron spectrum (XPS) analysis
XPS determines the energy of the bonds present on the Cb-QDs. This provides information related to types of the bonding present (i.e., C–O, C = O, C = C, C–O–C) over the specific Cb-QD.
For CQDs
Prasannan and Imae (2013) described XPS of orange waste peel–derived CQDs to confirm the various functional groups. The C1s spectral analysis (Fig. 4a) exhibited total of five peaks at 284.9, 285.9, 287.3, and 288.8 eV, respectively for the C = C/C–C, C–OH/C–O–C, C = O, and O = C–O groups. On the other hand, three peaks were found from O1s spectrum (Fig. 4b), at 530.5, 531.9, and 533.2 eV for C–O, C = O, and C–OH/C–O–C species, respectively.
XPS-spectra of (a) C1s and (b) O1s type of CQDs, derived from orange waste peel (Prasannan and Imae 2013); (c) C1s, (d) N1s, and (e) O1s of GQDs-derived from spent tea (Abbas et al. 2020); (f) C1s, and (g) O1s are the and part of the XPS spectra in case of carboxymethyl cellulose–derived GOQDs (Adolfsson et al. 2015)
For GQDs
The XPS spectra for the spent tea–derived GQDs showed three peaks at 285.08, 400.08, and 532.08 eV for C1s (Fig. 4c), N1s (Fig. 4d), and O1s (Fig. 4e), respectively, with a small peak at 347.08 eV for Ca 2p (Abbas et al. 2020). The elemental analysis data of GQDs (produced under 500 W) showed C = 56.45%, O = 36.73%, N = 4.76%, and traces of Ca. C1s spectral analysis under high resolution XPS showed that they had three peaks at 284.9, 286.3, and 288.4 eV, for C–C/C = C, C–O–C, and O-C = O groups. N1s spectral analysis revealed three peaks at 399.89, 402.01, and 406.05 eV, for C–N/N–H (pyridinic or pyrrolic), N–C (3°-amine), and N–O (nitro) bonds, respectively. O1s spectrum on the other hand possessed two peaks at 531.58 and 533.08 eV, for O = C and O–C species, respectively. They also found that no structural change occurred in GQDs after 2 months, demonstrating excellent GQDs stability.
For GOQDs
The XPS C1s spectral analysis (Fig. 4f) of carbon nanospheres showed four peaks at 285 (C–C/C = C), 286.3 (C–O), 287.5 (C–O–C), 289.2 (O = C–O), and 290.1 eV (π-π* of aromatic ring) and three for GOQDs, viz. 285 (C–C/C = C), 286 (C–O), 287.3 (C–O–C), and 288.3 eV (O = C–O), respectively (Adolfsson et al. 2015), while two O1s spectral analysis peaks (Fig. 4g) were found at 532 and 533.4 eV for C = O and C–O groups. After the conversion of CNs to GOQDs, the increase in oxygen containing groups can be attributed to the decrease in C/O ratio from 4.2 to 2.0.
All three types of Cb-QDs might contain similar or different types of groups, depending on the synthetic procedures. If highly polar groups (e.g., COOH, C = O, OH, N–H) are present at the edge or exterior surface of the Cb-QDs, then those QDs would be highly soluble in water. With modification, we can tune the solubility as well as other properties of the QDs.
Miscellaneous skeletal characterizations
Other characterization techniques include RS, EDX analysis, TGA analysis, solid state cross polarization/magnetic angle spinning 13C nuclear magnetic resonance spectroscopy (CP-MAS-13C-NMR) analysis, two-dimensional NMR (2D-NMR) analysis, heteronuclear single quantum coherence (HSQC) spectroscopic analysis, gas chromatography–mass spectrometry (GC–MS), and high-performance liquid chromatography (HPLC).
Like FT-IR, RS detects the functional groups present over Cb-QDs. Although RS is more time consuming and expensive than FT-IR, it has the advantage of not being affected by the presence of water molecules in the spectra. The CP-MAS-13C-NMR spectra can distinguish between processed and unprocessed materials (David et al. 2009). When compared to 1D-NMR, 2D-NMR provides more information on the type of protonic environment, but HSQC provides information about proton coupling with heteronuclei, i.e., we may find out the interaction of a proton and a heteronuclei using this form of NMR spectroscopy. The elemental composition of the various QDs is determined using EDX analysis. TGA confirms the deposition of various molecules, drugs, or nanomaterials on the surface of Cb-QDs sheet. GC–MS identifies the components in QDs from the starting materials/final products mixtures and clearly reveals about whether the conversion is completed or not. Unlike GC–MS, HPLC can detect the components from the same mixtures but in the liquid phase and with varied time intervals.
Morphological analysis
For morphological analysis, the preferential characterization technique is transmission electron microscopy (TEM) and its high-resolution version (HRTEM), which simultaneously furnishes the details about size, shape, layers, and thickness. Morphological analysis is discussed below for the three unmodified Cb-QDs.
Transmission electron microscopy (TEM) analysis
The CQDs synthesized from peanut shell were analyzed by TEM (Xue et al. 2016). The CQDs were spherical in shape with 2–4 nm diameter (Fig. 5a). From single crystalline structural analysis by HRTEM, the lattice spacing was estimated to be 0.338 nm (Fig. 5b), corresponding to the (002) graphitic plane.
TEM image (resolution 10 nm) (a) and corresponding HRTEM of a single CQDs crystal of peanut shell–derived CQDs (b) (Xue et al. 2016); TEM (resolution 50 nm) (c) and HRTEM (2 nm) images of rice husk–derived GQDs (d) (Wang et al. 2016c); TEM (resolution 20 nm) (e), corresponding size analysis histogram (f) (inset) and HRTEM (2 nm) image (g) (inset) of waste toner–derived GOQDs (Xu et al. 2019)
In one study, HRTEM, employed for rice husk–derived GQDs analysis indicated its highly crystalline nature with spacing of 0.24 nm i.e., (1120) diffraction plane (Fig. 5c) (Wang et al. 2016b). TEM analysis of the GQDs revealed the size range 3–6 nm (Fig. 5d).
Xu et al. (2019) reported that the GOQDs produced from waste toner had a size range of 2 – 3.5 nm (histogram, Fig. 5f) using TEM analysis (Fig. 5e). The space in a single GOQDs crystal was estimated to be roughly 0.2 nm using HRTEM, resulting in a (102) plane of diffraction (graphitic sp2 carbon, JCPDS no.- 26–1076) (Fig. 5g).
Miscellaneous techniques related to morphological analysis
Dynamic light scattering (DLS) data partially offers an idea of the size of the QDs. Scanning electron microscopy (SEM) and atomic force microscopy (AFM) are also used to analyze the morphology of diverse nanomaterials, which includes the size, shape, thickness of the layers, and surface stability. To confirm the structures of crystalline nanomaterials, powder X-ray diffractometry (XRD) is used. Selected area electron diffraction (SAED) pattern is employed to validate the crystallinity data obtained by powder XRD. Brunauer–Emmett–Teller (BET) surface area analysis explores the specific surface area (in m2/g) of Cb-QDs via gas (inert type, viz. nitrogen) adsorption analysis, over a solid sample.
Optical characterization analysis
UV–visible and photoluminescence spectral analysis
UV–Visible spectroscopy is one of the most effective tools to obtain preliminary information for the efficient fluorescence characteristics of Cb-QDs. Furthermore, the presence of conjugation or electron excessive groups/atoms on the QDs can be concluded by UV–Vis spectral analysis (Table 2). Photoluminescence (PL) spectroscopy is used to examine the fluorescence and phosphorescence abilities of QDs, as well as the QY, which indicates the successful conversion of a carbon source into QDs, at a maximum excitation wavelength (Table 2).
According to Wang and Hu (2014), the color of fluorescence varies with the size of CQDs. The red shift for emissive radiation occurs when the size of Cb-QDs increases, due to a decrease in the HOMO–LUMO gap.
Miscellaneous optical characterizations
There are a variety of different optical techniques that can be used to fully comprehend the fluorescence properties of QDs. Time-correlated photon counting (TCSPC) is an optical characterization technique that measures the fluorescence decays and directly tells us about the lifetime of a QDs. Time-resolved photoluminescence (TRPL) is an extension of normal fluorescence spectroscopy that produces an excitation spectrum as a function of time. Furthermore, Fluorocube time-correlated single photon counting (TCSPC) fluorimetry is a well-developed method of measuring fluorescence lifetime with high data accuracy and high sensitivity.
Based on all the characterization techniques, the following conclusions can be drawn:
-
UV–Vis, PL, XPS, EDX, and FTIR spectroscopy can be used to identify all three Cb-QDs.
-
Carbon sheets are organized into layers to form a complete spherical CQD crystal, whereas GQDs and GOQDs exhibit a sheet-like structure.
-
CQDs and GQDs are generally less polar than GOQDs, regardless of modification, and so the latter have distinct water solubility.
-
CQDs and GQDs, on average, have a higher C/O ratio than GOQDs.
-
When exposed to UV light, all three types of Cb-QDs emit light at the same frequency for example, blue emission occurs for all three types of Cb-QDs at 365 nm (Fig. 6).
-
All the three types of Cb-QDs would have the same surface functionalities if they were synthesized from the same carbon source.
-
In CQDs and GQDs, the numbers of conjugated mobile (π-bonded) electrons are higher than in GOQDs. As a result, GOQDs are not probably exploited as popular fluorescent materials.
(a) Peanut shell–derived CQDs before excitation (left) and after excitation at 365 nm (right) (Xue et al. 2016); (b) GQDs from rice husk biomass under visible (left) and at 365 nm UV irradiation (right) (Wang et al. 2016c); (c) waste toner–derived GOQDs under day light (left) and 365 nm UV irradiation (right) (Xu et al. 2019)
Applications of carbon-based quantum dots
Cb-QDs, with and without surface functionalization, have gained immense attention among researchers in the past decades due to their wide range of applications. Because of their small size and perfect shape, they are used in various areas like bioimaging, in vitro sensing, drug delivery, chemical sensing, environmental applications, electrical devices, and catalysis. Herein, the various applications of Cb-QDs derived from SWs in the fields of biomedicine, electrical devices, environmental, and catalysis are discussed.
Biomedical applications
Cytotoxicity is a serious issue in the development of medications and therapeutic procedures since it can have a negative impact on both infected and normal tissues. Cb-QDs are now emerging as one of the important materials for biomedical applications because of their lower toxicity than other inorganic-based QDs. Cb-CDs at various concentrations, with or without surface modifications or do**, have been shown to be less cytotoxic, and thus more biocompatible on various cell lines. CQDs derived from alkali lignin showed more than 83% viability of HeLa cell lines even after 24 h incubation with a dosage of 50 mg/L (Zhang et al. 2019). Similarly, the cell viability of HeLa cells was above 90% when incubated with a high concentration of waste frying oil–derived sulphur-doped CQDs (600 μg/L) for 48 h. Likewise, a higher concentration of CQDs (1 mg/L) from the pseudo-stem of a banana plant on incubation with Hela and MCF-7 cell lines for 24 h revealed more than 85% cell viability. Similar types of high cell viability were observed for other cell lines (like A549, HepG2, Vero, HCT116, C6, T24) even after incubation for ≥ 24 h with a higher concentration of functionalized/doped/uncoated CQDs (Table 3). Like CQDs, functionalized/doped/uncoated GQDs also showed similar types of HeLa, L929, 3T3, HepG2, HEK293, etc., cell viability when incubated for ≥ 24 h. There is no information on the cytotoxicity of SWs-derived GOQDs on any types of cells. A live-cell imaging and sensing of cholesterol is illustrated in Fig. 7.
Drug delivery, sensors, magnetic hypothermia, photothermal therapy, in vivo imaging, and in vitro biosensing are currently well-developed techniques with Cb-QDs. Despite the fact that a nanomaterial’s ability to penetrate the blood–brain barrier (BBB) is challenging, several articles have reported that the relevant Cb-QDs can be effective in overcoming the problem. Kim et al. (2018) synthesized unfunctionalized GQDs and showed their ability to prevent synucleinopathy in Parkinson’s disease. Because of the strong fluorescence, water solubility, photo-stability, low toxicity, cell-membrane permeability, and excellent biocompatibility, CQDs produced from the pseudo-stem of the banana plant were used as fluorescent probes for imaging of HeLa and MCF-7 cells in three colors (blue, green, and red) (Vandarkuzhali et al. 2017). The highly luminous r-CQDs-GS from processed white rice has been effectively proven for in vitro and in vivo bioimaging of A549 cells (Anthony et al. 2020). Apart from that, fabricated N-doped CQDs derived from Platanus biomass have been widely used for cellular imaging of HeLa cells, L02 cells, and macrophage cells. There are other reports where CQDs derived from SWs were used for imaging different carcinoma cells like HeLa, C6, MC3T3 HUVEC, MDA-MB-231, Caco-2, DU145, and more (Zhang et al. 2019). Apart from biosensing of cells, fluorescent CQDs from diesel engine soot have been successfully utilized for the imaging of Escherichia coli (E. Coli) along with the sensing of cholesterol (Tripathi et al. 2014). Several other researchers have used SWs-derived CQDs for a variety of other applications, including bacterial cell imaging (Ang et al. 2020; Tripathi et al. 2014), drug sensing (Yu et al. 2019), drug delivery (D’souza et al. 2018), and antioxidant (Rajamanikandan et al. 2021). Unlike CQDs, applications of GQDs derived from SWs are yet to be explored in their wider range. Internalization of GQDs derived from waste molasses has also demonstrated in DF-1, HepG2, and HEK293 cells (Sangam et al. 2018). There are studies on the use of GQDs derived from SWs for bioimaging of HeLa (Wang et al. 2016b, 2016c), L929 and 3T3 (Wang et al. 2015a). When another strong chelating compound (ligand) is introduced to the metal ion − Cb-QDs complex solution, it triggers a chelation competition and, as a result, separates the “alien” metal ions by chelation. Thus, the fluorescent activity is regained (“OFF–ON” part). A description of “ON–OFF-ON” pathway is given in Fig. 9e. The total process is supported by Dexter energy transfer mechanism.
While sensing organic molecules including DNA and TNT, π* (antibonding orbital) of the acceptor molecule is involved (Demchenko and Dekaliuk 2013) in the reduction of PL intensity and causes the turn- “OFF” the fluorescent signal. Now if another molecule possing strong binding efficiency with that organic molecule added to that organic molecule − Cb-QDs complex solution, then the fluorescence signal (turn- “ON”) will be restored. Thus, as electron deficiency or electron accepting inclination increases, electron transfer from donor Cb-QDs to metal ions/organic molecules increases, resulting in a greater reduction of PL intensity. As a result, increase in sensitivity occurs with a significant increase in LOD efficiency.
The second mechanism is photoinduced electron transfer, or PET, which includes charge separation and excitation of the donor side via irradiation (Jose et al. 2017), resulting in either fluorescence quenching or amplification. Here, the electron transfer between the metal ion and the fluorophore, or inside the self-fluorophore-chelate unit, can also result in fluorescence property modulation (Fig. 9c). Direct electronic transitions between fluorophores (excited) and metal ions, containing low energy d-orbitals (empty or partially filled) are usually accompanied by quenching. PET can also enhance the fluorescence for fluorophore-electron rich metal chelate. Without metal ions, excitation leads to the separation of charges, and hence, the PET in between excited fluorophore and chelate goes in for emission, thereby PET gives rise to systematic relaxation pathway and resulting a decrease in QY for that fluorophore. When a metal ion binds to an electron-rich chelating site, a shifting of charge density occurs and thus effectively quenches the PET decay pathway, resulting in the increase in QY.
Another mechanism that involves radiation-free energy transfer through dipole–dipole coupling between a photoexcited donor and an acceptor is known as Forster resonance energy transfer, FRET (Masters 2014) (Fig. 9d). FRET efficiency of energy transfer is highly dependent on the distance (inversely and to the sixth power) between the donor and acceptor. The acceptor can be a chromophore (capable of absorbing energy) or a fluorophore, in which a photon is irradiated by a high energy molecule during relaxation to the low energy state due to sensitized emission. In general, FRET reduces donor emissions, resulting in a shorter lifetime. Metal binding results in a change in molecule structure and can affect distance and/or orientation that can assist or hinder FRET (Carter et al. 2014).
The mechanism for the fluorescence emission is still ambiguous due to the different theories among the researchers. The bioimaging within the cells are primarily due to the fluorescence emission property of CQDs. In general, two mechanisms are widely accepted for CQDs: excitation dependent and excitation independent. The excitation dependent fluorescence is primarily due to band gap emissions in π-domain where strong absorption in the UV range and a weak emission is observed. Surface defects in the structure of CQDs induce excitation independent fluorescence emission, which exhibits mild absorption and high emission in the visible region.
Defects in GQDs containing sp3 carbons are structurally comparable to those observed on the surface of tiny CQDs with an unusually high surface-to-volume ratio (Cao et al. 2013). According to Molaei group, the PL mechanism observed in GQDs is primarily due to surface defects and band gap transitions associated with conjugated π -domains (Molaei 2019). It is possible to use GQDs with near infrared (NIR) emission for bioimaging because tissues often exhibit autofluorescence and low light absorption in the NIR region (Younis et al. 2020).
Comparison of CQDs, GQDs, and GOQDs based on their synthesis, structures, physical properties, and chemical properties
The summary of SW-based Cb-QDs in terms of synthesis, structures as well as their physical and chemical properties are given in Table 5. It is evident that CQDs do not demonstrate any selectivity in their synthesis, but the structure of SWs plays a significant role during the synthesis of both GQDs and GOQDs. Though the surface functionalities of GOQDs are more compared to CQDs and GQDs, yet it is less explored because of its low fluorescent intensity than other two QDs.
Limitations and future research prospects of carbon-based quantum dots
Cb-QDs have been employed as a selective or non-selective medication, gene, and drug delivery agent for in vivo or in vitro studies on a certain kind of cell. Because of their stable PL, these materials are highly suitable for sensing in vivo and in vitro cells. Aside from medicinal uses, these materials are also employed for energy storage or batteries, as well as for the detection of toxic elements, ions or molecules, and explosives due to their high fluorescence and low and adjustable band gap.
Apart from these, there are many other applications of Cb-QDs. Lu et al. (2015) reported better photocatalytic activity of zinc-porphyrin modified GQDs compared to zinc-porphyrin towards the degradation of methylene blue (MB) under visible-light. On the other hand, Dang et al. (2022) used Fe-doped CQDs to generate methanol by CO2 reduction. A three-fold increase in photocatalytic activity of S and N co-doped CQDs with TiO2 NPs was observed towards acid red 88 degradation (Rahbar et al. 2019). Tammina et al. (2019) reported the application of N and P co-doped CQDs as dopamine sensor with a detection limit of 0.021 M. In another study, the use of N-doped GQDs, as a sensor, was reported for the detection of Fe3+ with high selectivity and sensitivity (Tam et al. 2014). Malček et al. (2022), through theoretical modelling, reported that Mn and Cr doped GQDs were highly effective at adsorbing H2 gas.
The fuel cell applications of doped CQDs and GQDs were also reported. N-doped CQDs decorated on the carbon paper surface were used to develop microbial fuel cell anode (Shaari et al. 2021). According to Yun et al. ( SW-derived Cb-QDs are inexpensive, reducing the cost of imaging, sensing, and detection applications. Carbon enriched SWs are the best raw materials for the production of Cb-QDs while agricultural and food wastes are top** up the list. Among the SWs-derived Cb-QDs, CQDs are the best QDs, as they can be produced with higher QY than the GQDs and GOQDs. Hence, SWs can be utilized in the form of CQDs with versatile applicability. Because of the low QY associated with them, to date, GQDs and GOQDs have not had broad utility. As of now, Cb-QDs are not very popular materials for practical applications because of their poor reproducibility during large scale synthesis. Therefore, a lot of opportunities are still there for future researchers to develop advanced pathways for their synthesis and applications. There is also a potential for converting tons of SWs generated daily in rural and urban areas into environmentally benign Cb-QDs for more practical utilization.
Conclusions
Abbreviations
- 1D-NMR:
-
One-dimensional nuclear magnetic resonance
- 2D-NMR:
-
Two-dimensional nuclear magnetic resonance
- AFM:
-
Atomic force microscope
- BET:
-
Brunauer-Emmett-Teller
- Cb-QDs:
-
Carbon-based quantum dots
- CFU:
-
Colony-forming unit
- CNTs:
-
Carbon nanotubes
- -COOH:
-
Carboxyl group
- CP-MAS-13C-NMR:
-
Solid state cross polarization/magnetic angle spinning 13C nuclear magnetic resonance spectroscopy
- CQDs:
-
Carbon quantum dots
- DLS:
-
Dynamic light scattering
- DNA:
-
Deoxyribonucleic acid
- EDX:
-
Energy dispersive X-ray
- EPA:
-
Environmental protection agency
- FTIR:
-
Fourier transform infrared
- GO:
-
Graphene oxide
- GOQDs:
-
Graphene oxide quantum dots
- GQDs:
-
Graphene quantum dots
- GSH:
-
Glutathione
- GSH@rCQDs:
-
Glutathione modified reduced carbon quantum dots
- HOMO:
-
Highest occupied molecular orbital
- HRTEM:
-
High-resolution transmission electron microscopy
- HSQC:
-
Heteronuclear single quantum coherence
- LGQDs:
-
Lignin-based graphene quantum dots
- LPEI:
-
Linear-polyethyleneimine
- LUMO:
-
Lowest unoccupied molecular orbital
- N@CQDs:
-
Nitrogen-doped carbon quantum dots
- N@mCQDs:
-
Nitrogen-doped microporous carbon quantum dots
- NaBH4 :
-
Sodium borohydride
- -NH2 :
-
Amino
- -OH:
-
Hydroxyl group
- PEI:
-
Branched polyethyleneimine
- PA:
-
Picric acid
- PL:
-
Photoluminescence
- ppb:
-
Parts per billion
- PXRD:
-
Powder X-ray diffractometry
- QD:
-
Quantum dot
- QDs:
-
Quantum dots
- QY:
-
Quantum yield
- rCQDs:
-
Reduced carbon quantum dots
- ROS:
-
Reactive oxygen species
- RS:
-
Raman spectroscopy
- S@GQDs:
-
Sulphur-doped graphene uantum dots
- SAED:
-
Selected area electron diffraction
- SEM:
-
Scanning electron microscopy
- -SO2-:
-
Sulfur-containing groups
- -SO3H:
-
Sulphonic acid group
- w/w:
-
Weight/weight ratio
- SW-materials:
-
Solid waste-materials
- SWs:
-
Solid wastes
- TCSPC:
-
Fluorocube time-correlated single photon counting
- TCSPC:
-
Time correlated photon counting
- TGA:
-
Thermogravimetric analysis
- TRPL:
-
Time resolved photoluminescence
- UV-Vis:
-
UV-visible absorption
- XPS:
-
X-ray photoelectron spectroscopy
References
Abbas A, Tabish TA, Bull SJ, Lim TM, Phan AN (2020) High yield synthesis of graphene quantum dots from biomass waste as a highly selective probe for Fe3+ sensing. Sci Rep 10:21262. https://doi.org/10.1038/s41598-020-78070-2
Abdel-Shafy HI, Mansour MSM (2018) Solid waste issue: sources, composition, disposal, recycling, and valorization. Egypt J Pet 27:1275–1290. https://doi.org/10.1016/j.ejpe.2018.07.003
Abdullah Issa MZ, Abidin Z, Sobri S, Rashid S, Adzir Mahdi M, Azowa Ibrahim N, Pudza YM (2019) Facile synthesis of nitrogen-doped carbon dots from lignocellulosic waste. Nanomaterials 9:1500. https://doi.org/10.3390/nano9101500
Adebayo (2011) Spectrophotometric determination of iron (III) in tap water using 8-hydoxyquinoline as a chromogenic reagent. AFRICAN J Biotechnol 10(16051):16057. https://doi.org/10.5897/AJB10.1840
Adolfsson KH, Hassanzadeh S, Hakkarainen M (2015) Valorization of cellulose and waste paper to graphene oxide quantum dots. RSC Adv 5:26550–26558. https://doi.org/10.1039/C5RA01805F
Ahirwar S, Mallick S, Bahadur D (2017) Electrochemical method to prepare graphene quantum dots and graphene oxide quantum dots. ACS Omega 2:8343–8353. https://doi.org/10.1021/acsomega.7b01539
Ahn J, Song Y, Kwon JE, Lee SH, Park KS, Kim S, Woo J, Kim H (2019) Food waste-driven N-doped carbon dots: applications for Fe3+ sensing and cell imaging. Mater Sci Eng C 102:106–112. https://doi.org/10.1016/j.msec.2019.04.019
Al Jahdaly BA, Elsadek MF, Ahmed BM, Farahat MF, Taher MM, Khalil AM (2021) Outstanding graphene quantum dots from carbon source for biomedical and corrosion inhibition applications: a review. Sustainability 13:2127. https://doi.org/10.3390/su13042127
Alaghmandfard A, Sedighi O, TabatabaeiRezaei N, Abedini AA, MalekKhachatourian A, Toprak MS, Seifalian A (2021) Recent advances in the modification of carbon-based quantum dots for biomedical applications. Mater Sci Eng C 120:111756. https://doi.org/10.1016/j.msec.2020.111756
Algar WR, Massey M, Rees K, Higgins R, Krause KD, Darwish GH, Peveler WJ, **ao Z, Tsai H-Y, Gupta R, Lix K, Tran MV, Kim H (2021) Photoluminescent nanoparticles for chemical and biological analysis and imaging. Chem Rev 121:9243–9358. https://doi.org/10.1021/acs.chemrev.0c01176
Alkian I, Sutanto H, Hadiyanto, (2022) Quantum yield optimization of carbon dots using response surface methodology and its application as control of Fe3+ ion levels in drinking water. Mater Res Express 9:015702. https://doi.org/10.1088/2053-1591/ac3f60
Alvand ZM, Rajabi HR, Mirzaei A, Sajadiasl F (2021) Combination of plant-mediated and sonochemical-assisted synthesis for preparation of low-toxic cadmium selenide semiconductor nanoparticles: study of the effect of extraction techniques, characterization, comparative study of biological activities. Surf Interfaces 25:101182. https://doi.org/10.1016/j.surfin.2021.101182
Ang WL, Boon Mee CAL, Sambudi NS, Mohammad AW, Leo CP, Mahmoudi E, Ba-Abbad M, Benamor A (2020) Microwave-assisted conversion of palm kernel shell biomass waste to photoluminescent carbon dots. Sci Rep 10:21199. https://doi.org/10.1038/s41598-020-78322-1
Anthony AM, Murugan R, Subramanian R, Selvarangan GK, Pandurangan P, Dhanasekaran A, Sohrab A (2020) Ultra-radiant photoluminescence of glutathione rigidified reduced carbon quantum dots (r-CQDs) derived from ice-biryani for in vitro and in vivo bioimaging applications. Colloids Surfaces A Physicochem Eng Asp 586:124266. https://doi.org/10.1016/j.colsurfa.2019.124266
Arias Velasco V, Caicedo Chacón WD, Carvajal Soto AM, Ayala Valencia G, Granada Echeverri JC, Agudelo Henao AC (2021) Carbon quantum dots based on carbohydrates as nano sensors for food quality and safety. Starch - Stärke 73:2100044. https://doi.org/10.1002/star.202100044
Atchudan R, Edison TNJI, Perumal S, Vinodh R, Sundramoorthy AK, Babu RS, Lee YR (2021) Leftover kiwi fruit peel-derived carbon dots as a highly selective fluorescent sensor for detection of ferric ion. Chemosensors 9:166. https://doi.org/10.3390/chemosensors9070166
Athika M, Prasath A, Duraisamy E, Sankar Devi V, Selva Sharma A, Elumalai P (2019) Carbon-quantum dots derived from denatured milk for efficient chromium-ion sensing and supercapacitor applications. Mater Lett 241:156–159. https://doi.org/10.1016/j.matlet.2019.01.064
Bagheri Z, Ehtesabi H, Hallaji Z, Latifi H, Behroodi E (2018) Investigation the cytotoxicity and photo-induced toxicity of carbon dot on yeast cell. Ecotoxicol Environ Saf 161:245–250. https://doi.org/10.1016/j.ecoenv.2018.05.071
Bankoti K, Rameshbabu AP, Datta S, Das B, Mitra A, Dhara S (2017) Onion derived carbon nanodots for live cell imaging and accelerated skin wound healing. J Mater Chem B 5:6579–6592. https://doi.org/10.1039/C7TB00869D
Baweja H, Jeet K (2019) Economical and green synthesis of graphene and carbon quantum dots from agricultural waste. Mater Res Express 6:0850g8. https://doi.org/10.1088/2053-1591/ab28e5
Bruno F, Sciortino A, Buscarino G, Soriano ML, Ríos Á, Cannas M, Gelardi F, Messina F, Agnello S (2021) A comparative study of top-down and bottom-up carbon nanodots and their interaction with mercury ions. Nanomaterials 11:1265. https://doi.org/10.3390/nano11051265
Bui T-D, Tseng J-W, Tseng M-L, Lim MK (2022) Opportunities and challenges for solid waste reuse and recycling in emerging economies: a hybrid analysis. Resour Conserv Recycl 177:105968. https://doi.org/10.1016/j.resconrec.2021.105968
Buzaglo M, Shtein M, Regev O (2016) Graphene quantum dots produced by microfluidization. Chem Mater 28:21–24. https://doi.org/10.1021/acs.chemmater.5b03301
Cao L, Meziani MJ, Sahu S, Sun Y-P (2013) Photoluminescence properties of graphene versus other carbon nanomaterials. Acc Chem Res 46:171–180. https://doi.org/10.1021/ar300128j
Carter KP, Young AM, Palmer AE (2014) Fluorescent sensors for measuring metal ions in living systems. Chem Rev 114:4564–4601. https://doi.org/10.1021/cr400546e
Chen F, Gao W, Qiu X, Zhang H, Liu L, Liao P, Fu W, Luo Y (2017) Graphene quantum dots in biomedical applications: recent advances and future challenges. Front Lab Med 1:192–199. https://doi.org/10.1016/j.flm.2017.12.006
Chen K, Qing W, Hu W, Lu M, Wang Y, Liu X (2019) On-off-on fluorescent carbon dots from waste tea: their properties, antioxidant and selective detection of CrO42−, Fe3+, ascorbic acid and L-cysteine in real samples. Spectrochim. Acta Part A Mol Biomol Spectrosc 213:228–234. https://doi.org/10.1016/j.saa.2019.01.066
Chen BB, Liu ML, Huang CZ (2020) Carbon dot-based composites for catalytic applications. Green Chem 22:4034–4054. https://doi.org/10.1039/D0GC01014F
Cheng C, Shi Y, Li M, **ng M, Wu Q (2017) Carbon quantum dots from carbonized walnut shells: structural evolution, fluorescence characteristics, and intracellular bioimaging. Mater Sci Eng C 79:473–480. https://doi.org/10.1016/j.msec.2017.05.094
Chien C-F, Aviso K, Tseng M-L, Fujii M, Lim MK (2021) Solid waste management in emerging economies: opportunities and challenges for reuse and recycling. Resour Conserv Recycl 172:105677. https://doi.org/10.1016/j.resconrec.2021.105677
Chiu S-H, Gedda G, Girma WM, Chen J-K, Ling Y-C, Ghule AV, Ou K-L, Chang J-Y (2016) Rapid fabrication of carbon quantum dots as multifunctional nanovehicles for dual-modal targeted imaging and chemotherapy. Acta Biomater 46:151–164. https://doi.org/10.1016/j.actbio.2016.09.027
Chromium in Drinking Water | US EPA [WWW Document] (n.d.) URL https://www.epa.gov/sdwa/chromium-drinking-water (accessed 1.9.22)
Chu X, Wang S, Cao Y (2020) A new fluorescence probe comprising nitrogen-doped graphene quantum dots for the selective and quantitative determination of cerium(iv). New J Chem 44:797–806. https://doi.org/10.1039/C9NJ04518J
Cunci L, González-Colón V, Lee Vargas-Pérez B, Ortiz-Santiago J, Pagán M, Carrion P, Cruz J, Molina-Ontoria A, Martinez N, Silva W, Echegoyen L, Cabrera CR (2021) Multicolor fluorescent graphene oxide quantum dots for sensing cancer cell biomarkers. ACS Appl Nano Mater 4:211–219. https://doi.org/10.1021/acsanm.0c02526
D’Angelis do E. S. Barbosa C, Corrêa JR, Medeiros GA, Barreto G, Magalhães KG, de Oliveira AL, Spencer J, Rodrigues MO, Neto BAD (2015) Carbon dots (C-dots) from cow manure with impressive subcellular selectivity tuned by simple chemical modification. Chem-A Eur J 21:5055–5060. https://doi.org/10.1002/chem.201406330
D’souza SL, Chettiar SS, Koduru JR, Kailasa SK (2018) Synthesis of fluorescent carbon dots using Daucus carota subsp. sativus roots for mitomycin drug delivery. Optik (stuttg) 158:893–900. https://doi.org/10.1016/j.ijleo.2017.12.200
Dalal C, Saini D, Garg AK, Sonkar SK (2021) Fluorescent carbon nano-onion as bioimaging probe. ACS Appl Bio Mater 4:252–266. https://doi.org/10.1021/acsabm.0c01192
Dang Y, Li B, Feng X, Jia J, Li K, Zhang Y (2022) Preparation of iron-doped carbon dots and their application in photocatalytic reduction of carbon dioxide. ChemPhotoChem. https://doi.org/10.1002/cptc.202200156
Das R, Bandyopadhyay R, Pramanik P (2018) Carbon quantum dots from natural resource: a review. Mater Today Chem 8:96–109. https://doi.org/10.1016/j.mtchem.2018.03.003
Das C, Paul SS, Saha A, Singh T, Saha A, Im J, Biswas G (2020) Silver-based nanomaterials as therapeutic agents against coronaviruses: a review. Int J Nanomedicine 15:9301–9315. https://doi.org/10.2147/IJN.S280976
Das C, Singh S, Bhakta S, Mishra P, Biswas G (2022) Bio-modified magnetic nanoparticles with Terminalia arjuna bark extract for the removal of methylene blue and lead (II) from simulated wastewater. Chemosphere 291:132673. https://doi.org/10.1016/j.chemosphere.2021.132673
David K, Pu Y, Foston M, Muzzy J, Ragauskas A (2009) Cross-polarization/magic angle spinning (CP/MAS) 13C nuclear magnetic resonance (NMR) analysis of chars from alkaline-treated pyrolyzed softwood. Energy Fuels 23:498–501. https://doi.org/10.1021/ef8004527
de Boëver R, Town JR, Li X, Claverie JP (2022) Carbon dots for carbon dummies: the quantum and the molecular questions among some others. Chem–A Eur J 28:e202200748. https://doi.org/10.1002/chem.202200748
Demchenko AP, Dekaliuk MO (2013) Novel fluorescent carbonic nanomaterials for sensing and imaging. Methods Appl Fluoresc 1:042001. https://doi.org/10.1088/2050-6120/1/4/042001
Desmond LJ, Phan AN, Gentile P (2021) Critical overview on the green synthesis of carbon quantum dots and their application for cancer therapy. Environ Sci Nano 8:848–862. https://doi.org/10.1039/D1EN00017A
Devi P, Kaur G, Thakur A, Kaur N, Grewal A, Kumar P (2017) Waste derivitized blue luminescent carbon quantum dots for selenite sensing in water. Talanta 170:49–55. https://doi.org/10.1016/j.talanta.2017.03.069
Devi S, Gupta RK, Paul AK, Tyagi S (2018) Waste carbon paper derivatized carbon quantum dots/(3-Aminopropyl)triethoxysilane based fluorescent probe for trinitrotoluene detection. Mater. Res. Express 6:025605. https://doi.org/10.1088/2053-1591/aaf03c
Ding Z, Li F, Wen J, Wang X, Sun R (2018) Gram-scale synthesis of single-crystalline graphene quantum dots derived from lignin biomass. Green Chem 20:1383–1390. https://doi.org/10.1039/C7GC03218H
Dong Y, Shao J, Chen C, Li H, Wang R, Chi Y, Lin X, Chen G (2012) Blue luminescent graphene quantum dots and graphene oxide prepared by tuning the carbonization degree of citric acid. Carbon n y 50:4738–4743. https://doi.org/10.1016/j.carbon.2012.06.002
Facure MHM, Schneider R, Mercante LA, Correa DS (2020) A review on graphene quantum dots and their nanocomposites: from laboratory synthesis towards agricultural and environmental applications. Environ Sci Nano 7:3710–3734. https://doi.org/10.1039/D0EN00787K
Fakhraie S, Rajabi HR, Rashidi A (2023) Fabrication and application of novel core–shell MIL-101(Cr)@UiO-66(Zr) nanocrystals for highly selective separation of H2S and CO2. Chem Eng J 452:139001. https://doi.org/10.1016/j.cej.2022.139001
Gao L, Li R, Sui X, Li R, Chen C, Chen Q (2014) Conversion of chicken feather waste to N-doped carbon nanotubes for the catalytic reduction of 4-nitrophenol. Environ Sci Technol 48:10191–10197. https://doi.org/10.1021/es5021839
Gedda G, Lee C-Y, Lin Y-C, Wu H (2016) Green synthesis of carbon dots from prawn shells for highly selective and sensitive detection of copper ions. Sensors Actuators B Chem 224:396–403. https://doi.org/10.1016/j.snb.2015.09.065
Gomes MF, Gomes YF, Lopes-Moriyama A, de Barros Neto EL, de Souza CP (2019) Design of carbon quantum dots via hydrothermal carbonization synthesis from renewable precursors. Biomass Convers Biorefinery 9:689–694. https://doi.org/10.1007/s13399-019-00387-4
Guo Y, Zhang L, Cao F, Leng Y (2016) Thermal treatment of hair for the synthesis of sustainable carbon quantum dots and the applications for sensing Hg2+. Sci Rep 6:35795. https://doi.org/10.1038/srep35795
Halder A, Godoy-Gallardo M, Ashley J, Feng X, Zhou T, Hosta-Rigau L, Sun Y (2018) One-pot green synthesis of biocompatible graphene quantum dots and their cell uptake studies. ACS Appl Bio Mater 1:452–461. https://doi.org/10.1021/acsabm.8b00170
Horst FH, da Silva Rodrigues CV, Carvalho PHPR, Leite AM, Azevedo RB, Neto BAD, Corrêa JR, Garcia MP, Alotaibi S, Henini M, Chaves SB, Rodrigues MO (2021) From cow manure to bioactive carbon dots: a light-up probe for bioimaging investigations, glucose detection and potential immunotherapy agent for melanoma skin cancer. RSC Adv 11:6346–6352. https://doi.org/10.1039/D0RA10859F
Hu L, Sun Y, Li S, Wang X, Hu K, Wang L, Liang X, Wu Y (2014a) Multifunctional carbon dots with high quantum yield for imaging and gene delivery. Carbon n y 67:508–513. https://doi.org/10.1016/j.carbon.2013.10.023
Hu Y, Yang J, Tian J, Jia L, Yu J-S (2014b) Waste frying oil as a precursor for one-step synthesis of sulfur-doped carbon dots with pH-sensitive photoluminescence. Carbon n y 77:775–782. https://doi.org/10.1016/j.carbon.2014.05.081
Hu C, Su T-R, Lin T-J, Chang C-W, Tung K-L (2018) Yellowish and blue luminescent graphene oxide quantum dots prepared via a microwave-assisted hydrothermal route using H 2 O 2 and KMnO 4 as oxidizing agents. New J Chem 42:3999–4007. https://doi.org/10.1039/C7NJ03337K
Hu X, Li Yanxiao, Xu Y, Gan Z, Zou X, Shi J, Huang X, Li Z, Li Yahui (2021) Green one-step synthesis of carbon quantum dots from orange peel for fluorescent detection of Escherichia coli in milk. Food Chem 339:127775. https://doi.org/10.1016/j.foodchem.2020.127775
Huang C, Li X, Tung C, Wu L (2018) Photocatalysis with quantum dots and visible light for effective organic synthesis. Chem–A Eur J 24:11530–11534. https://doi.org/10.1002/chem.201800391
Jeong Y, Moon K, Jeong S, Koh W-G, Lee K (2018) Converting waste papers to fluorescent carbon dots in the recycling process without loss of ionic liquids and bioimaging applications. ACS Sustain Chem Eng 6:4510–4515. https://doi.org/10.1021/acssuschemeng.8b00353
Jose AR, Vikraman AE, Girish Kumar K (2017) Photoinduced electron transfer between quantum dots and pralidoxime: an efficient sensing strategy. New J Chem 41:10828–10834. https://doi.org/10.1039/C7NJ00795G
Kang S, Kim KM, Jung K, Son Y, Mhin S, Ryu JH, Shim KB, Lee B, Han H, Song T (2019) Graphene oxide quantum dots derived from coal for bioimaging: facile and green approach. Sci Rep 9:4101. https://doi.org/10.1038/s41598-018-37479-6
Khan WU, Wang D, Zhang W, Tang Z, Ma X, Ding X, Du S, Wang Y (2017) High quantum yield green-emitting carbon dots for Fe(III) detection, biocompatible fluorescent ink and cellular imaging. Sci Rep 7:14866. https://doi.org/10.1038/s41598-017-15054-9
Khan AH, López-Maldonado EA, Khan NA, Villarreal-Gómez LJ, Munshi FM, Alsabhan AH, Perveen K (2021) Current solid waste management strategies and energy recovery in develo** countrihes - state of art review. Chemosphere 291:133088. https://doi.org/10.1016/j.chemosphere.2021.133088
Khan S, Anjum R, Raza ST, Ahmed Bazai N, Ihtisham M (2022) Technologies for municipal solid waste management: current status, challenges, and future perspectives. Chemosphere 288:132403. https://doi.org/10.1016/j.chemosphere.2021.132403
Kim D, Yoo JM, Hwang H, Lee J, Lee SH, Yun SP, Park MJ, Lee M, Choi S, Kwon SH, Lee Saebom, Kwon S-H, Kim S, Park YJ, Kinoshita M, Lee Y-H, Shin S, Paik SR, Lee SJ, Lee S, Hong BH, Ko HS (2018) Graphene quantum dots prevent α-synucleinopathy in Parkinson’s disease. Nat Nanotechnol 13:812–818. https://doi.org/10.1038/s41565-018-0179-y
Kolahalam LA, Kasi Viswanath IV, Diwakar BS, Govindh B, Reddy V, Murthy YLN (2019) Review on nanomaterials: synthesis and applications. Mater Today Proc 18:2182–2190. https://doi.org/10.1016/j.matpr.2019.07.371
Kumar R, Kumar VB, Gedanken A (2020a) Sonochemical synthesis of carbon dots, mechanism, effect of parameters, and catalytic, energy, biomedical and tissue engineering applications. Ultrason Sonochem 64:105009. https://doi.org/10.1016/j.ultsonch.2020.105009
Kumar YR, Deshmukh K, Sadasivuni KK, Pasha SKK (2020b) Graphene quantum dot based materials for sensing, bio-imaging and energy storage applications: a review. RSC Adv 10:23861–23898. https://doi.org/10.1039/D0RA03938A
Kusic H, Leszczynska D, Koprivanac N, Peternel I (2011) Role of quantum dots nanoparticles in the chemical treatment of colored wastewater: catalysts or additional pollutants. J Environ Sci 23:1479–1485. https://doi.org/10.1016/S1001-0742(10)60609-2
Lettieri S, Camisasca A, D’Amora M, Diaspro A, Uchida T, Nakajima Y, Yanagisawa K, Maekawa T, Giordani S (2017a) Far-red fluorescent carbon nano-onions as a biocompatible platform for cellular imaging. RSC Adv 7:45676–45681. https://doi.org/10.1039/C7RA09442F
Lettieri S, D’Amora M, Camisasca A, Diaspro A, Giordani S (2017b) Carbon nano-onions as fluorescent on/off modulated nanoprobes for diagnostics. Beilstein J Nanotechnol 8:1878–1888. https://doi.org/10.3762/bjnano.8.188
Li C, Shi G (2014) Carbon nanotube-based fluorescence sensors. J Photochem Photobiol C Photochem Rev 19:20–34. https://doi.org/10.1016/j.jphotochemrev.2013.10.005
Li M, Chen T, Gooding JJ, Liu J (2019) Review of carbon and graphene quantum dots for sensing. ACS Sensors 4:1732–1748. https://doi.org/10.1021/acssensors.9b00514
Lin Y, Shen Q, Kawabata Y, Segawa J, Cao X, Guan K, Istirokhatun T, Yoshioka T, Matsuyama H (2021) Graphene quantum dots (GQDs)-assembled membranes with intrinsic functionalized nanochannels for high-performance nanofiltration. Chem Eng J 420:127602. https://doi.org/10.1016/j.cej.2020.127602
Liu S-S, Wang C-F, Li C-X, Wang J, Mao L-H, Chen S (2014) Hair-derived carbon dots toward versatile multidimensional fluorescent materials. J Mater Chem C 2:6477–6483. https://doi.org/10.1039/C4TC00636D
Liu R, Zhang J, Gao M, Li Z, Chen J, Wu D, Liu P (2015) A facile microwave-hydrothermal approach towards highly photoluminescent carbon dots from goose feathers. RSC Adv 5:4428–4433. https://doi.org/10.1039/C4RA12077A
Liu M, Xu Y, Niu F, Gooding JJ, Liu J (2016) Carbon quantum dots directly generated from electrochemical oxidation of graphite electrodes in alkaline alcohols and the applications for specific ferric ion detection and cell imaging. Analyst 141:2657–2664. https://doi.org/10.1039/C5AN02231B
Liu X, Xu T, Li Y, Zang Z, Peng X, Wei H, Zha W, Wang F (2018) Enhanced X-ray photon response in solution-synthesized CsPbBr3 nanoparticles wrapped by reduced graphene oxide. Sol Energy Mater Sol Cells 187:249–254. https://doi.org/10.1016/j.solmat.2018.08.009
Liu J, Li R, Yang B (2020) Carbon dots: a new type of carbon-based nanomaterial with wide applications. ACS Cent Sci 6:2179–2195. https://doi.org/10.1021/acscentsci.0c01306
Liu S, Liu Z, Li Q, **a H, Yang W, Wang R, Li Y, Zhao H, Tian B (2021a) Facile synthesis of carbon dots from wheat straw for colorimetric and fluorescent detection of fluoride and cellular imaging. Spectrochim Acta Part A Mol Biomol Spectrosc 246:118964. https://doi.org/10.1016/j.saa.2020.118964
Liu Y-Y, Yu N-Y, Fang W-D, Tan Q-G, Ji R, Yang L-Y, Wei S, Zhang X-W, Miao A-J (2021b) Photodegradation of carbon dots cause cytotoxicity. Nat Commun 12:812. https://doi.org/10.1038/s41467-021-21080-z
Lu J, Yang J, Wang J, Lim A, Wang S, Loh KP (2009) One-pot synthesis of fluorescent carbon nanoribbons, nanoparticles, and graphene by the exfoliation of graphite in ionic liquids. ACS Nano 3:2367–2375. https://doi.org/10.1021/nn900546b
Lu Q, Zhang Y, Liu S (2015) Graphene quantum dots enhanced photocatalytic activity of zinc porphyrin toward the degradation of methylene blue under visible-light irradiation. J Mater Chem A 3:8552–8558. https://doi.org/10.1039/C5TA00525F
Lv Z, Wang Y, Chen J, Wang J, Zhou Y, Han S-T (2020) Semiconductor quantum dots for memories and neuromorphic computing systems. Chem Rev 120:3941–4006. https://doi.org/10.1021/acs.chemrev.9b00730
Mahat NA, Shamsudin SA (2020) Transformation of oil palm biomass to optical carbon quantum dots by carbonisation-activation and low temperature hydrothermal processes. Diam Relat Mater 102:107660. https://doi.org/10.1016/j.diamond.2019.107660
Mahat NA, Shamsudin SA, Jullok N, Ma’Radzi, A.H., (2020) Carbon quantum dots embedded polysulfone membranes for antibacterial performance in the process of forward osmosis. Desalination 493:114618. https://doi.org/10.1016/j.desal.2020.114618
Malček M, Müllerová S, Bučinský L (2022) Theoretical study of hydrogen adsorption on the graphene quantum dots doped with various first row transition metals: switch of spin state as a way to improve H2 adsorption. Phys. E Low-dimensional Syst. Nanostructures 139:115144. https://doi.org/10.1016/j.physe.2022.115144
Markovic ZM, Ristic BZ, Arsikin KM, Klisic DG, Harhaji-Trajkovic LM, Todorovic-Markovic BM, Kepic DP, Kravic-Stevovic TK, Jovanovic SP, Milenkovic MM, Milivojevic DD, Bumbasirevic VZ, Dramicanin MD, Trajkovic VS (2012) Graphene quantum dots as autophagy-inducing photodynamic agents. Biomaterials 33:7084–7092. https://doi.org/10.1016/j.biomaterials.2012.06.060
Masters BR (2014) Paths to Förster’s resonance energy transfer (FRET) theory. Eur Phys J H 39:87–139. https://doi.org/10.1140/epjh/e2013-40007-9
Meyer DE, Li M, Ingwersen WW (2020) Analyzing economy-scale solid waste generation using the United States environmentally-extended input-output model. Resour Conserv Recycl 157:104795. https://doi.org/10.1016/j.resconrec.2020.104795
Meziani MJ, Dong X, Zhu L, Jones LP, LeCroy GE, Yang F, Wang S, Wang P, Zhao Y, Yang L, Tripp RA, Sun Y-P (2016) Visible-light-activated bactericidal functions of carbon “quantum” dots. ACS Appl Mater Interfaces 8:10761–10766. https://doi.org/10.1021/acsami.6b01765
Molaei MJ (2019) Carbon quantum dots and their biomedical and therapeutic applications: a review. RSC Adv 9:6460–6481. https://doi.org/10.1039/C8RA08088G
Moradi Alvand Z, Rajabi HR, Mirzaei A, Masoumiasl A (2019a) Ultrasonic and microwave assisted extraction as rapid and efficient techniques for plant mediated synthesis of quantum dots: green synthesis, characterization of zinc telluride and comparison study of some biological activities. New J Chem 43:15126–15138. https://doi.org/10.1039/C9NJ03144H
Moradi Alvand Z, Rajabi HR, Mirzaei A, Masoumiasl A, Sadatfaraji H (2019b) Rapid and green synthesis of cadmium telluride quantum dots with low toxicity based on a plant-mediated approach after microwave and ultrasonic assisted extraction: synthesis, characterization, biological potentials and comparison study. Mater Sci Eng C 98:535–544. https://doi.org/10.1016/j.msec.2019.01.010
Muñoz R, Santos EM, Galan-Vidal CA, Miranda JM, Lopez-Santamarina A, Rodriguez JA (2021) Ternary quantum dots in chemical analysis. synthesis and detection mechanisms. Molecules 26:2764. https://doi.org/10.3390/molecules26092764
Nair A, Haponiuk JT, Thomas S, Gopi S (2020) Natural carbon-based quantum dots and their applications in drug delivery: a review. Biomed Pharmacother 132:110834. https://doi.org/10.1016/j.biopha.2020.110834
Nasrollahzadeh M, Sajjadi M, Iravani S, Varma RS (2021) Carbon-based sustainable nanomaterials for water treatment: state-of-art and future perspectives. Chemosphere 263:128005. https://doi.org/10.1016/j.chemosphere.2020.128005
National Primary Drinking Water Regulations | US EPA [WWW Document] (n.d.) URL https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations#Radionuclides (accessed 1.9.22)
Ng HKM, Lim GK, Leo CP (2021) Comparison between hydrothermal and microwave-assisted synthesis of carbon dots from biowaste and chemical for heavy metal detection: a review. Microchem J 165:106116. https://doi.org/10.1016/j.microc.2021.106116
Nguyen N-A, Le T-H, Trinh V-H, Ngo Q-T, Nguyen V-T, Lee G, Choi H-S, Chen G (2021) Au/Cdot-nanohybrid electrocatalyst synthesized by rice-straw-derived carbon dots as a reducing agent for improved hydrogen evolution reactions. J Electrochem Soc 168:044509. https://doi.org/10.1149/1945-7111/abf267
Nilewski L, Mendoza K, Jalilov AS, Berka V, Wu G, Sikkema WKA, Metzger A, Ye R, Zhang R, Luong DX, Wang T, McHugh E, Derry PJ, Samuel EL, Kent TA, Tsai A-L, Tour JM (2019) Highly oxidized graphene quantum dots from coal as efficient antioxidants. ACS Appl Mater Interfaces 11:16815–16821. https://doi.org/10.1021/acsami.9b01082
Pandiyan S, Arumugam L, Srirengan SP, Pitchan R, Sevugan P, Kannan K, Pitchan G, Hegde TA, Gandhirajan V (2020) Biocompatible carbon quantum dots derived from sugarcane industrial wastes for effective nonlinear optical behavior and antimicrobial activity applications. ACS Omega 5:30363–30372. https://doi.org/10.1021/acsomega.0c03290
Park SJ, Park JY, Chung JW, Yang HK, Moon BK, Yi SS (2020) Color tunable carbon quantum dots from wasted paper by different solvents for anti-counterfeiting and fluorescent flexible film. Chem Eng J 383:123200. https://doi.org/10.1016/j.cej.2019.123200
Pooja D, Singh L, Thakur A, Kumar P (2019) Green synthesis of glowing carbon dots from Carica papaya waste pulp and their application as a label-freechemo probe for chromium detection in water. Sensors Actuators B Chem 283:363–372. https://doi.org/10.1016/j.snb.2018.12.027
Pramanik S, Chatterjee S, Suresh Kumar G, Sujatha Devi P (2018) Egg-shell derived carbon dots for base pair selective DNA binding and recognition. Phys Chem Chem Phys 20:20476–20488. https://doi.org/10.1039/C8CP02872A
Prasannan A, Imae T (2013) One-pot synthesis of fluorescent carbon dots from orange waste peels. Ind Eng Chem Res 52:15673–15678. https://doi.org/10.1021/ie402421s
Qureshi WA, Vivekanandan B, Jayaprasath JA, Ali D, Alarifi S, Deshmukh K (2021) Antimicrobial activity and characterization of pomegranate peel-based carbon dots. J Nanomater 2021:1–6. https://doi.org/10.1155/2021/9096838
Raber L (1998) Chloroform is safe at levels found in tap water, says EPA. Chem Eng News Arch 76:28. https://doi.org/10.1021/cen-v076n023.p028
Rahbar M, Mehrzad M, Behpour M, Mohammadi-Aghdam S, Ashrafi M (2019) S, N co-doped carbon quantum dots/TiO2 nanocomposite as highly efficient visible light photocatalyst. Nanotechnology 30:505702. https://doi.org/10.1088/1361-6528/ab40dc
Rajabi HR, Karimi F, Kazemdehdashti H, Kavoshi L (2018) Fast sonochemically-assisted synthesis of pure and doped zinc sulfide quantum dots and their applicability in organic dye removal from aqueous media. J Photochem Photobiol B Biol 181:98–105. https://doi.org/10.1016/j.jphotobiol.2018.02.016
Rajabi HR, Sajadiasl F, Karimi H, Alvand ZM (2020) Green synthesis of zinc sulfide nanophotocatalysts using aqueous extract of Ficus Johannis plant for efficient photodegradation of some pollutants. J Mater Res Technol 9:15638–15647. https://doi.org/10.1016/j.jmrt.2020.11.017
Rajamanikandan S, Biruntha M, Ramalingam G (2021) Blue emissive carbon quantum dots (CQDs) from bio-waste peels and its antioxidant activity. J Clust Sci. https://doi.org/10.1007/s10876-021-02029-0
Rani UA, Ng LY, Ng CY, Mahmoudi E (2020) A review of carbon quantum dots and their applications in wastewater treatment. Adv Colloid Interface Sci 278:102124. https://doi.org/10.1016/j.cis.2020.102124
Rasal AS, Yadav S, Yadav A, Kashale AA, Manjunatha ST, Altaee A, Chang J-Y (2021) Carbon quantum dots for energy applications: a review. ACS Appl Nano Mater 4:6515–6541. https://doi.org/10.1021/acsanm.1c01372
Ren X, Zhang F, Guo B, Gao N, Zhang X (2019) Synthesis of N-doped micropore carbon quantum dots with high quantum yield and dual-wavelength photoluminescence emission from biomass for cellular imaging. Nanomaterials 9:495. https://doi.org/10.3390/nano9040495
Reshak AH (2017) Quantum dots in photocatalytic applications: efficiently enhancing visible light photocatalytic activity by integrating CdO quantum dots as sensitizers. Phys Chem Chem Phys 19:24915–24927. https://doi.org/10.1039/C7CP05312F
Rodríguez-Padrón D, Algarra M, Tarelho LAC, Frade J, Franco A, de Miguel G, Jiménez J, Rodríguez-Castellón E, Luque R (2018) Catalyzed microwave-assisted preparation of carbon quantum dots from lignocellulosic residues. ACS Sustain Chem Eng 6:7200–7205. https://doi.org/10.1021/acssuschemeng.7b03848
Rosso C, Filippini G, Prato M (2020) Carbon dots as nano-organocatalysts for synthetic applications. ACS Catal 10:8090–8105. https://doi.org/10.1021/acscatal.0c01989
Sangam S, Gupta A, Shakeel A, Bhattacharya R, Sharma AK, Suhag D, Chakrabarti S, Garg SK, Chattopadhyay S, Basu B, Kumar V, Rajput SK, Dutta MK, Mukherjee M (2018) Sustainable synthesis of single crystalline sulphur-doped graphene quantum dots for bioimaging and beyond. Green Chem 20:4245–4259. https://doi.org/10.1039/C8GC01638K
Sasidharan S, Raj S, Sonawane, Shirish, Sonawane, Shriram, Pinjari D, Pandit AB, Saudagar P (2019) Nanomaterial synthesis: chemical and biological route and applications, in: Nanomaterials Synthesis. Elsevier, pp. 27–51. https://doi.org/10.1016/B978-0-12-815751-0.00002-X
Selenium in Drinking-water Background document for development of WHO Guidelines for Drinking-water Quality, 2011
Shaari N, Kamarudin SK, Bahru R (2021) Carbon and graphene quantum dots in fuel cell application: an overview. Int J Energy Res 45:1396–1424. https://doi.org/10.1002/er.5889
Singh A, Eftekhari E, Scott J, Kaur J, Yambem S, Leusch F, Wellings R, Gould T, Ostrikov K, Sonar P, Li Q (2020) Carbon dots derived from human hair for ppb level chloroform sensing in water. Sustain Mater Technol 25:e00159. https://doi.org/10.1016/j.susmat.2020.e00159
Skourtis SS, Liu C, Antoniou P, Virshup AM, Beratan DN (2016) Dexter energy transfer pathways. Proc Natl Acad Sci 113:8115–8120. https://doi.org/10.1073/pnas.1517189113
Solid Waste Management [WWW Document], n.d. URL https://www.worldbank.org/en/topic/urbandevelopment/brief/solid-waste-management. Accessed 1.21.22
Spreinat A, Dohmen MM, Lüttgens J, Herrmann N, Klepzig LF, Nißler R, Weber S, Mann FA, Lauth J, Kruss S (2021) Quantum defects in fluorescent carbon nanotubes for sensing and mechanistic studies. J Phys Chem C 125:18341–18351. https://doi.org/10.1021/acs.jpcc.1c05432
Stanković NK, Bodik M, Šiffalovič P, Kotlar M, Mičušik M, Špitalsky Z, Danko M, Milivojević DD, Kleinova A, Kubat P, Capakova Z, Humpoliček P, Lehocky M, Todorović Marković BM, Marković ZM (2018) Antibacterial and antibiofouling properties of light triggered fluorescent hydrophobic carbon quantum dots langmuir–blodgett thin films. ACS Sustain Chem Eng 6:4154–4163. https://doi.org/10.1021/acssuschemeng.7b04566
Su R, Wang D, Liu M, Yan J, Wang J-X, Zhan Q, Pu Y, Foster NR, Chen J-F (2018) Subgram-scale synthesis of biomass waste-derived fluorescent carbon dots in subcritical water for bioimaging, sensing, and solid-state patterning. ACS Omega 3:13211–13218. https://doi.org/10.1021/acsomega.8b01919
Surendran P, Lakshmanan A, Vinitha G, Ramalingam G, Rameshkumar P (2020) Facile preparation of high fluorescent carbon quantum dots from orange waste peels for nonlinear optical applications. Luminescence 35:196–202. https://doi.org/10.1002/bio.3713
Suryawanshi A, Biswal M, Mhamane D, Gokhale R, Patil S, Guin D, Ogale S (2014) Large scale synthesis of graphene quantum dots (GQDs) from waste biomass and their use as an efficient and selective photoluminescence on–off–on probe for Ag + ions. Nanoscale 6:11664–11670. https://doi.org/10.1039/C4NR02494J
Tabish TA, Zhang S (2016) Graphene quantum dots: syntheses, properties, and biological applications, in: comprehensive nanoscience and nanotechnology. Elsevier, pp. 171–192. https://doi.org/10.1016/B978-0-12-803581-8.04133-3
Tajik S, Dourandish Z, Zhang K, Beitollahi H, Le QV, Jang HW, Shokouhimehr M (2020) Carbon and graphene quantum dots: a review on syntheses, characterization, biological and sensing applications for neurotransmitter determination. RSC Adv 10:15406–15429. https://doi.org/10.1039/D0RA00799D
Talapin DV, Shevchenko EV (2016) Introduction: nanoparticle chemistry. Chem Rev 116:10343–10345. https://doi.org/10.1021/acs.chemrev.6b00566
Tam TV, Trung NB, Kim HR, Chung JS, Choi WM (2014) One-pot synthesis of N-doped graphene quantum dots as a fluorescent sensing platform for Fe3+ ions detection. Sensors Actuators B Chem 202:568–573. https://doi.org/10.1016/j.snb.2014.05.045
Tammina SK, Yang D, Koppala S, Cheng C, Yang Y (2019) Highly photoluminescent N, P doped carbon quantum dots as a fluorescent sensor for the detection of dopamine and temperature. J Photochem Photobiol B Biol 194:61–70. https://doi.org/10.1016/j.jphotobiol.2019.01.004
Tang D, Liu J, Yan X, Kang L (2016) Graphene oxide derived graphene quantum dots with different photoluminescence properties and peroxidase-like catalytic activity. RSC Adv 6:50609–50617. https://doi.org/10.1039/C5RA26279H
Tao S, Feng T, Zheng C, Zhu S, Yang B (2019) Carbonized polymer dots: a brand new perspective to recognize luminescent carbon-based nanomaterials. J Phys Chem Lett 10:5182–5188. https://doi.org/10.1021/acs.jpclett.9b01384
Tatrari G, Karakoti M, Tewari C, Pandey S, Bohra BS, Dandapat A, Sahoo NG (2021) Solid waste-derived carbon nanomaterials for supercapacitor applications: a recent overview. Mater Adv 2:1454–1484. https://doi.org/10.1039/D0MA00871K
Thulasi S, Kathiravan A, Asha Jhonsi M (2020) Fluorescent carbon dots derived from vehicle exhaust soot and sensing of tartrazine in soft drinks. ACS Omega 5:7025–7031. https://doi.org/10.1021/acsomega.0c00707
Tian P, Tang L, Teng KS, Lau SP (2018) Graphene quantum dots from chemistry to applications. Mater Today Chem 10:221–258. https://doi.org/10.1016/j.mtchem.2018.09.007
Tian L, Li Z, Wang P, Zhai X, Wang X, Li T (2021) Carbon quantum dots for advanced electrocatalysis. J Energy Chem 55:279–294. https://doi.org/10.1016/j.jechem.2020.06.057
Tripathi KM, Sonker AK, Sonkar SK, Sarkar S (2014) Pollutant soot of diesel engine exhaust transformed to carbon dots for multicoloured imaging of E. coli and sensing cholesterol. RSC Adv 4:30100. https://doi.org/10.1039/C4RA03720K
Tyagi A, Tripathi KM, Singh N, Choudhary S, Gupta RK (2016) Green synthesis of carbon quantum dots from lemon peel waste: applications in sensing and photocatalysis. RSC Adv 6:72423–72432. https://doi.org/10.1039/C6RA10488F
Valizadeh A, Mikaeili H, Samiei M, Farkhani SM, Zarghami N, Kouhi M, Akbarzadeh A, Davaran S (2012) Quantum dots: synthesis, bioapplications, and toxicity. Nanoscale Res Lett 7:480. https://doi.org/10.1186/1556-276X-7-480
Vandarkuzhali SAA, Jeyalakshmi V, Sivaraman G, Singaravadivel S, Krishnamurthy KR, Viswanathan B (2017) Highly fluorescent carbon dots from Pseudo-stem of banana plant: applications as nanosensor and bio-imaging agents. Sensors Actuators B Chem 252:894–900. https://doi.org/10.1016/j.snb.2017.06.088
Vandarkuzhali SAA, Natarajan S, Jeyabalan S, Sivaraman G, Singaravadivel S, Muthusubramanian S, Viswanathan B (2018) Pineapple peel-derived carbon dots: applications as sensor, molecular keypad lock, and memory device. ACS Omega 3:12584–12592. https://doi.org/10.1021/acsomega.8b01146
Vatanpour V, Karatas O, Amiri S, Rajabi HR, Koyuncu I, Khataee A (2022) Different metal-doped ZnS quantum dots photocatalysts for enhancing the permeability and antifouling performances of polysulfone membranes with and without UV irradiation. Chemosphere 294:133705. https://doi.org/10.1016/j.chemosphere.2022.133705
Venkatesan S, Mariadoss AJ, Arunkumar K, Muthupandian A (2019) Fuel waste to fluorescent carbon dots and its multifarious applications. Sensors Actuators B Chem 282:972–983. https://doi.org/10.1016/j.snb.2018.11.144
Wang Q, Liu X, Zhang L, Lv Y (2012) Microwave-assisted synthesis of carbon nanodots through an eggshell membrane and their fluorescent application. Analyst 137:5392. https://doi.org/10.1039/c2an36059d
Wang D, Zhu L, Chen J-F, Dai L (2015a) Can graphene quantum dots cause DNA damage in cells? Nanoscale 7:9894–9901. https://doi.org/10.1039/C5NR01734C
Wang Y, Wu W, Wu M, Sun H, **e H, Hu C, Wu X, Qiu J (2015b) Yellow-visual fluorescent carbon quantum dots from petroleum coke for the efficient detection of Cu2+ ions. New Carbon Mater 30:550–559. https://doi.org/10.1016/S1872-5805(15)60204-9
Wang L, Li W, Wu B, Li Z, Wang S, Liu Y, Pan D, Wu M (2016a) Facile synthesis of fluorescent graphene quantum dots from coffee grounds for bioimaging and sensing. Chem Eng J 300:75–82. https://doi.org/10.1016/j.cej.2016.04.123
Wang Y, Hu A (2014) Carbon quantum dots: synthesis, properties and applications. J Mater Chem C 2:6921. https://doi.org/10.1039/C4TC00988F
Wang S, Cole IS, Li Q (2016b) The toxicity of graphene quantum dots. RSC Adv 6:89867–89878. https://doi.org/10.1039/C6RA16516H
Wang Z, Fu B, Zou S, Duan B, Chang C, Yang B, Zhou X, Zhang L (2016c) Facile construction of carbon dots via acid catalytic hydrothermal method and their application for target imaging of cancer cells. Nano Res 9:214–223. https://doi.org/10.1007/s12274-016-0992-2
Wang Z, Yu J, Zhang X, Li N, Liu B, Li Y, Wang Y, Wang W, Li Y, Zhang L, Dissanayake S, Suib SL, Sun L (2016d) Large-scale and controllable synthesis of graphene quantum dots from rice husk biomass: a comprehensive utilization strategy. ACS Appl Mater Interfaces 8:1434–1439. https://doi.org/10.1021/acsami.5b10660
Wang R, **a G, Zhong W, Chen L, Chen L, Wang Y, Min Y, Li K (2019a) Direct transformation of lignin into fluorescence-switchable graphene quantum dots and their application in ultrasensitive profiling of a physiological oxidant. Green Chem 21:3343–3352. https://doi.org/10.1039/C9GC01012B
Wang Z-X, Gao Y-F, ** X, Yu X-H, Tao X, Kong F-Y, Fan D-H, Wang W (2019b) Excitation-independent emission carbon nanoribbon polymer as a ratiometric photoluminescent probe for highly selective and sensitive detection of quercetin. Analyst 144:2256–2263. https://doi.org/10.1039/C9AN00094A
Wang C, Shi H, Yang M, Yan Y, Liu E, Ji Z, Fan J (2020) Facile synthesis of novel carbon quantum dots from biomass waste for highly sensitive detection of iron ions. Mater Res Bull 124:110730. https://doi.org/10.1016/j.materresbull.2019.110730
Wang X, Ma Y, Wu Q, Wang Z, Tao Y, Zhao Y, Wang B, Cao J, Wang H, Gu X, Huang H, Li S, Wang X, Hu F, Shao M, Liao L, Sham T, Liu Y, Kang Z (2021) Ultra-bright and stable pure blue light-emitting diode from O, N Co-doped carbon dots. Laser Photon Rev 15:2000412. https://doi.org/10.1002/lpor.202000412
Wareing TC, Gentile P, Phan AN (2021) Biomass-based carbon dots: current development and future perspectives. ACS Nano 15:15471–15501. https://doi.org/10.1021/acsnano.1c03886
Wei J, Zang Z, Zhang Y, Wang M, Du J, Tang X (2017) Enhanced performance of light-controlled conductive switching in hybrid cuprous oxide/reduced graphene oxide (Cu_2O/rGO) nanocomposites. Opt Lett 42:911. https://doi.org/10.1364/OL.42.000911
Welsher K, Liu Z, Sherlock SP, Robinson JT, Chen Z, Daranciang D, Dai H (2009) A route to brightly fluorescent carbon nanotubes for near-infrared imaging in mice. Nat Nanotechnol 4:773–780. https://doi.org/10.1038/nnano.2009.294
Wu P, Li W, Wu Q, Liu Y, Liu S (2017) Hydrothermal synthesis of nitrogen-doped carbon quantum dots from microcrystalline cellulose for the detection of Fe3+ ions in an acidic environment. RSC Adv 7:44144–44153. https://doi.org/10.1039/C7RA08400E
Xu X, Ray R, Gu Y, Ploehn HJ, Gearheart L, Raker K, Scrivens WA (2004) Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J Am Chem Soc 126:12736–12737. https://doi.org/10.1021/ja040082h
Xu Q, Gong Y, Zhang Z, Miao Y, Li D, Yan G (2019) Preparation of graphene oxide quantum dots from waste toner, and their application to a fluorometric DNA hybridization assay. Microchim Acta 186:483. https://doi.org/10.1007/s00604-019-3539-x
Xu D, Lin Q, Chang H (2020) Recent advances and sensing applications of carbon dots. Small Methods 4:1900387. https://doi.org/10.1002/smtd.201900387
Xu-Cheng F, Xuan-Hua L, ** J-Z, Zhang J, Wei G (2018) Facile synthesis of bagasse waste derived carbon dots for trace mercury detection. Mater Res Express 5:065044. https://doi.org/10.1088/2053-1591/aaccb7
Xue M, Zhan Z, Zou M, Zhang L, Zhao S (2016) Green synthesis of stable and biocompatible fluorescent carbon dots from peanut shells for multicolor living cell imaging. New J Chem 40:1698–1703. https://doi.org/10.1039/C5NJ02181B
Yan C, Hu X, Guan P, Hou T, Chen P, Wan D, Zhang X, Wang J, Wang C (2020) Highly biocompatible graphene quantum dots: green synthesis, toxicity comparison and fluorescence imaging. J Mater Sci 55:1198–1215. https://doi.org/10.1007/s10853-019-04079-2
Yang HB, Miao J, Hung S-F, Huo F, Chen HM, Liu B (2014) Stable quantum dot photoelectrolysis cell for unassisted visible light solar water splitting. ACS Nano 8:10403–10413. https://doi.org/10.1021/nn503751s
Yang H, Zhou B, Zhang Y, Liu H, Liu Y, He Y, **a S (2021) Valorization of expired passion fruit shell by hydrothermal conversion into carbon quantum dot: physical and optical properties. Waste and Biomass Valorization 12:2109–2117. https://doi.org/10.1007/s12649-020-01132-z
Yao Y-Y, Gedda G, Girma WM, Yen C-L, Ling Y-C, Chang J-Y (2017) Magnetofluorescent carbon dots derived from crab shell for targeted dual-modality bioimaging and drug delivery. ACS Appl Mater Interfaces 9:13887–13899. https://doi.org/10.1021/acsami.7b01599
Ye R, **ang C, Lin J, Peng Z, Huang K, Yan Z, Cook NP, Samuel ELG, Hwang C-C, Ruan G, Ceriotti G, Raji A-RO, Martí AA, Tour JM (2013) Coal as an abundant source of graphene quantum dots. Nat Commun 4:2943. https://doi.org/10.1038/ncomms3943
Ye Q, Yan F, Luo Y, Wang Y, Zhou X, Chen L (2017) Formation of N, S-codoped fluorescent carbon dots from biomass and their application for the selective detection of mercury and iron ion. Spectrochim. Acta Part A Mol Biomol Spectrosc 173:854–862. https://doi.org/10.1016/j.saa.2016.10.039
Ye Z, Zhang Y, Li G, Li B (2020) Fluorescent determination of mercury(II) by green carbon quantum dots synthesized from eggshell membrane. Anal Lett 53:2841–2853. https://doi.org/10.1080/00032719.2020.1759618
Yoo D, Park Y, Cheon B, Park M-H (2019) Carbon dots as an effective fluorescent sensing platform for metal ion detection. Nanoscale Res Lett 14:272. https://doi.org/10.1186/s11671-019-3088-6
Youh M-J, Jiang M-Y, Chung M-C, Tai H-C, Li Y-Y (2020) Formation of graphene quantum dots by ball-milling technique using carbon nanocapsules and sodium carbonate. Inorg Chem Commun 119:108061. https://doi.org/10.1016/j.inoche.2020.108061
Younis MR, He G, Lin J, Huang P (2020) Recent advances on graphene quantum dots for bioimaging applications. Front Chem 8:424. https://doi.org/10.3389/fchem.2020.00424
Yu C, Jiang X, Qin D, Mo G, Zheng X, Deng B (2019) Facile syntheses of s, n-codoped carbon quantum dots and their applications to a novel off–on nanoprobe for detection of 6-thioguanine and its bioimaging. ACS Sustain Chem Eng 7:16112–16120. https://doi.org/10.1021/acssuschemeng.9b02886
Yuan M, Zhong R, Gao H, Li W, Yun X, Liu J, Zhao X, Zhao G, Zhang F (2015) One-step, green, and economic synthesis of water-soluble photoluminescent carbon dots by hydrothermal treatment of wheat straw, and their bio-applications in labeling, imaging, and sensing. Appl Surf Sci 355:1136–1144. https://doi.org/10.1016/j.apsusc.2015.07.095
Yun X, Li J, Chen X, Chen H, **ao L, **ang K, Chen W, Liao H, Zhu Y (2019) Porous Fe 2 O 3 modified by nitrogen-doped carbon quantum dots/reduced graphene oxide composite aerogel as a high-capacity and high-rate anode material for alkaline aqueous batteries. ACS Appl Mater Interfaces 11:36970–36984. https://doi.org/10.1021/acsami.9b12827
Zammataro A, Sfrazzetto GT (2019) Carbon dots as catalysts: a new class of nanozymes. Curr Organocatalysis 7:3–6. https://doi.org/10.2174/2213337206666190702165008
Zang Z, Zeng X, Wang M, Hu W, Liu C, Tang X (2017) Tunable photoluminescence of water-soluble AgInZnS–graphene oxide (GO) nanocomposites and their application in-vivo bioimaging. Sensors Actuators B Chem 252:1179–1186. https://doi.org/10.1016/j.snb.2017.07.144
Zdrazil L, Zahradnicek R, Mohan R, Sedlacek P, Nejdl L, Schmiedova V, Pospisil J, Horak M, Weiter M, Zmeskal O, Hubalek J (2018) Preparation of graphene quantum dots through liquid phase exfoliation method. J Lumin 204:203–208. https://doi.org/10.1016/j.jlumin.2018.08.017
Zhang B, Liu Y, Ren M, Li W, Zhang X, Vajtai R, Ajayan PM, Tour JM, Wang L (2019) Sustainable synthesis of bright green fluorescent nitrogen-doped carbon quantum dots from alkali lignin. Chemsuschem 12:4202–4210. https://doi.org/10.1002/cssc.201901693
Zhao S, Rondin L, Delport G, Voisin C, Beser U, Hu Y, Feng X, Müllen K, Narita A, Campidelli S, Lauret JS (2017) Fluorescence from graphene nanoribbons of well-defined structure. Carbon n y 119:235–240. https://doi.org/10.1016/j.carbon.2017.04.043
Zhao C, Song X, Liu Y, Fu Y, Ye L, Wang N, Wang F, Li L, Mohammadniaei M, Zhang M, Zhang Q, Liu J (2020) Synthesis of graphene quantum dots and their applications in drug delivery. J Nanobiotechnology 18:142. https://doi.org/10.1186/s12951-020-00698-z
Zhu J, Zhu F, Yue X, Chen P, Sun Y, Zhang L, Mu D, Ke F (2019) Waste utilization of synthetic carbon quantum dots based on tea and peanut shell. J Nanomater 2019:1–7. https://doi.org/10.1155/2019/7965756
Funding
Chanchal Das and Goutam Biswas received grants from the Department of Chemistry, Cooch Behar Panchanan Barma University.
Author information
Authors and Affiliations
Contributions
Chanchal Das: conceptualization, methodology, data curation, and writing—original draft. Mika Sillanpää: writing—reviewing and editing. Shabi Abbas Zaidi: writing—reviewing and editing. Moonis Ali Khan: conceptualization, methodology, and writing—reviewing and editing. Goutam Biswas: conceptualization, methodology, validation, and writing—reviewing and editing.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: George Z. Kyzas
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Das, C., Sillanpää, M., Zaidi, S.A. et al. Current trends in carbon-based quantum dots development from solid wastes and their applications. Environ Sci Pollut Res 30, 45528–45554 (2023). https://doi.org/10.1007/s11356-023-25822-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-25822-y