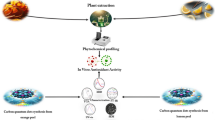
Abstract
In this work, Carbon Quantum Dots (CQDs) are synthesized from agro-waste Ananas comosus by facile hydrothermal treatment. The synthesized CQDs are characterized using powder X-ray diffraction analysis, Fourier-transform infrared, UV–Visible spectral analysis and quantum yield (QY) measurements. The formation of CQDs with a mean particle size of 2.4 nm is measured using HR-TEM analysis. The fluorescence properties of CQDs show strong blue emission radiation with a QY of 10.65%. Antioxidant assay of the CQDs were assessed against DPPH (2, 2-diphenyl-1-picrylhydrazyl), Super oxide anion radical scavenging, hydroxyl radical scavenging and hydrogen peroxide radical scavenging activities. The results suggest that agro-waste is also one of the promising materials for optical switching devices and also it have good medicinal value by extracting drugs.









Similar content being viewed by others
References
A. Roda, D. M. De Faveri, S. Giacosa, R. Dordoni, and M. Lambri (2016). Effect of pre-treatments on the saccharification of pineapple waste as a potential source for vinegar production. J. Clean. Prod. 112, 4477–4484. https://doi.org/10.1016/j.jclepro.2015.07.019.
A. M. Goula and H. N. Lazarides (2015). Integrated processes can turn industrial food waste into valuable food by-products and/or ingredients: the cases of olive mill and pomegranate wastes. J. Food Eng. 167, 45–50. https://doi.org/10.1016/j.jfoodeng.2015.01.003.
A. Schieber, F. C. Stintzing, and R. Carle (2001). By-products of plant food processing as a source of functional compounds - recent developments. Trends Food Sci. Technol. 12, 401–413. https://doi.org/10.1016/S0924-2244(02)00012-2.
V. Oreopoulou and W. Russ, Utilization of By-Products and Treatment of Waste in the Food. (Springer, New York, 2007), p. 316.
A. Abdullah and H. Mat (2008). Characterisation of solid and liquid pineapple waste. Reaktor 12, 48–52. https://doi.org/10.14710/reaktor.12.1.48-52.
S. B. Imandi, V. V. R. Bandaru, S. R. Somalanka, S. R. Bandaru, and H. R. Garapati (2008). Application of statistical experimental designs for the optimization of medium constituents for the production of citric acid from pineapple waste. Bioresour. Technol. 99, 4445–4450. https://doi.org/10.1016/j.biortech.2007.08.071.
K. Tanaka, Z. D. Hilary, and A. Ishizaki (1999). Investigation of the utility of pineapple juice and pineapple waste material as low-cost substrate for ethanol fermentation by Zymomonas mobilis. J. Biosci. Bioeng. 87, 642–646. https://doi.org/10.1016/S1389-1723(99)80128-5.
D. Kumar, V. K. Jain, G. Shanker, and A. Srivastava (2003). Utilisation of fruits waste for citric acid production by solid state fermentation. Process Biochem. 38, 1725–1729. https://doi.org/10.1016/S0032-9592(02)00253-4.
J. N. Nigam (2000). Continuous ethanol production from pineapple cannery waste using immobilized yeast cells. J. Biotechnol. 80, 189–193. https://doi.org/10.1016/S0168-1656(00)00246-7.
S. Ketnawa, P. Chaiwut, and S. Rawdkuen (2012). Pineapple wastes: a potential source for bromelain extraction. Food Bioprod. Process. 90, 385–391. https://doi.org/10.1016/j.fbp.2011.12.006.
P. G. Lozano-De-Gonzalez, D. M. Barrett, R. E. Wrolstad, and R. W. Durst (1993). Enzymatic browning inhibited in fresh and dried apple rings by pineapple juice. J. Food Sci. 58, 399–404. https://doi.org/10.1111/j.1365-2621.1993.tb04284.x.
M. A. Hossain and S. M. M. Rahman (2011). Total phenolics, flavonoids and antioxidant activity of tropical fruit pineapple. Food Res. Int. 44, 672–676. https://doi.org/10.1016/j.foodres.2010.11.036.
S. Zhu, Y. Song, X. Zhao, J. Shao, J. Zhang, and B. Yang (2015). The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): current state and future perspective. Nano Res. 8, 355–381. https://doi.org/10.1007/s12274-014-0644-3.
D. Qu, M. Zheng, L. Zhang, H. Zhao, Z. **e, X. **g, R. E. Haddad, H. Fan, and Z. Sun (2015). Formation mechanism and optimization of highly luminescent N-doped graphene quantum dots. Sci. Rep. 4, 5294. https://doi.org/10.1038/srep05294.
Yi. Zhang, Y. Wang, X. Feng, F. Zhang, Y. Yang, and X. Liu (2016). Effect of reaction temperature on structure and fluorescence properties of nitrogen-doped carbon dots. Appl. Surf. Sci. 387, 1236–1246. https://doi.org/10.1016/j.apsusc.2016.07.048.
V. Lesnyak, N. Gaponik, and A. Eychmüller (2013). Colloidal semiconductor nanocrystals: the aqueous approach. Chem. Soc. Rev. 42, 2905–2929. https://doi.org/10.1039/C2CS35285K.
Z. Moslemi, E. Soheyli, M. H. Majles Ara, and R. Sahraei (2018). Facile preparation of yellow and red emitting ZnCdSeS quantum dots and their third-order nonlinear optical properties. J. Phys. Chem. Solids 120, 64–70. https://doi.org/10.1016/j.jpcs.2018.04.017.
Z. Tan, F. Zhang, T. Zhu, J. Xu, A. Y. Wang, J. D. Dixon, L. Li, Qi. Zhang, S. E. Mohney, and J. Ruzyllo (2007). Bright and color-saturated emission from blue light-emitting diodes based on solution-processed colloidal nanocrystal quantum dots. Nano Lett. 7, 3803–3807. https://doi.org/10.1021/nl072370s.
H. Li, X. He, Y. Liu, H. Huang, S. Lian, S. T. Lee, and Z. Kang (2011). One-step ultrasonic synthesis of water-soluble carbon nanoparticles with excellent photoluminescent properties. Carbon N. Y. 49, 605–609. https://doi.org/10.1016/j.carbon.2010.10.004.
J. Shen, Y. Zhu, X. Yang, and C. Li (2012). Graphene quantum dots: emergent nanolights for bioimaging, sensors, catalysis and photovoltaic devices. Chem. Commun. 48, 3686–3699. https://doi.org/10.1039/c2cc00110a.
A. Prasannan and T. Imae (2013). One-pot synthesis of fluorescent carbon dots from orange waste peels. Ind. Eng. Chem. Res. 52, 15673–15678. https://doi.org/10.1021/ie402421s.
B. Zhu, S. Sun, Y. Wang, S. Deng, G. Qian, M. Wang, and A. Hu (2013). Preparation of carbon nanodots from single chain polymeric nanoparticles and theoretical investigation of the photoluminescence mechanism. J. Mater. Chem. C 1, 580–586. https://doi.org/10.1039/C2TC00140C.
T. Chatzimitakos, A. Kasouni, L. Sygellou, A. Avgeropoulos, A. Troganis, and C. Stalikas (2017). Two of a kind but different: luminescent carbon quantum dots from Citrus peels for iron and tartrazine sensing and cell imaging. Talanta 175, 305–312. https://doi.org/10.1016/j.talanta.2017.07.053.
P. Surendran, A. Lakshmanan, G. Vinitha, G. Ramalingam, and P. Rameshkumar (2019). Facile preparation of high fluorescent carbon quantum dots from orange waste peels for nonlinear optical applications. Luminescence 35, 196–202. https://doi.org/10.1002/bio.3713.
Y. X. Sun, Z. W. He, X. B. Sun, and Z. D. Zhao (2013). Synthesis of water-soluble fluorescent carbon dots from a one-step hydrothermal method with potato. Adv. Mater. Res. 873, 770–776.
W. Li, Z. Yue, C. Wang, W. Zhang, and G. Liu (2013). An absolutely green approach to fabricate carbon nanodots from soya bean grounds. RSC Adv. 3, 20662–20665. https://doi.org/10.1039/c3ra43330g.
J. Zhou, Z. Sheng, H. Han, M. Zou, and C. Li (2012). Facile synthesis of fluorescent carbon dots using watermelon peel as a carbon source. Mater. Lett. 66, 222–224. https://doi.org/10.1016/j.matlet.2011.08.081.
X. Wen, L. Shi, G. Wen, Y. Li, C. Dong, J. Yang, and S. Shuang (2015). Green synthesis of carbon nanodots from cotton for multicolor imaging, patterning, and sensing. Sens. Actuators B Chem. 221, 769–776. https://doi.org/10.1016/j.snb.2015.07.019.
G. García-Rosales, L. C. Longoria-Gándara, S. Martínez-Gallegos, and J. González-Juárez (2013). Synthesis and characterization of carbon conditioned with iron nanoparticles using pineapple-peel. Adv. Nanopart. 2, 384–390. https://doi.org/10.4236/anp.2013.24053.
S. Sharma, A. Umar, S. K. Mehta, and S. K. Kansal (2017). Fluorescent spongy carbon nanoglobules derived from pineapple juice: a potential sensing probe for specific and selective detection of chromium (VI) ions. Ceram. Int. 43, 7011–7019. https://doi.org/10.1016/j.ceramint.2017.02.127.
A. L. Himaja, P. S. Karthik, B. Sreedhar, and S. P. Singh (2014). Synthesis of carbon dots from kitchen waste: conversion of waste to value added product. J. Fluoresc. 24, 1767–1773. https://doi.org/10.1007/s10895-014-1465-1.
I. Jirapornvaree, T. Suppadit, and A. Popan (2017). Use of pineapple waste for production of decomposable pots. Int. J. Recycl. Org. Waste Agric. 6, 345–350. https://doi.org/10.1007/s40093-017-0183-5.
S. T. Chang, J. H. Wu, S. Y. Wang, P. L. Kang, N. S. Yang, and F. Y. Shyur (2001). Antioxidant activity of extracts from acacia confusa bark and heartwood. J. Agric. Food Chem. 49, 3420–3424.
F. Wang, M. Kreiter, B. He, S. Pang, and C. Liu (2010). Synthesis of direct white-light emitting carbogenic quantum dots. Chem. Commun. 46, 3309–3311. https://doi.org/10.1039/c002206c.
J. Y. Li, Y. Liu, Q. W. Shu, J. M. Liang, F. Zhang, X. P. Chen, X. Y. Deng, M. T. Swihart, and K. J. Tan (2017). One-pot hydrothermal synthesis of carbon dots with efficient up- and down-converted photoluminescence for the sensitive detection of morin in a dual-readout assay. Langmuir 33, 1043–1050. https://doi.org/10.1021/acs.langmuir.6b04225.
H. Lin, L. Ding, B. Zhang, and J. Huang (2018). Detection of nitrite based on fluorescent carbon dots by the hydrothermal method with folic acid. R. Soc. Open Sci. 5, 172149. https://doi.org/10.1098/rsos.172149.
A. Kumar, A. R. Chowdhuri, D. Laha, T. K. Mahto, P. Karmakar, and S. K. Sahu (2017). Green synthesis of carbon dots from Ocimum sanctum for effective fluorescent sensing of Pb2+ions and live cell imaging. Sens. Actuators B Chem. 242, 679–686. https://doi.org/10.1016/j.snb.2016.11.109.
T. A. Tabish, F. A. Memon, D. E. Gomez, D. W. Horsell, and S. Zhang (2018). A facile synthesis of porous graphene for efficient water and wastewater treatment. Sci. Rep. 8, 1817. https://doi.org/10.1038/s41598-018-19978-8.
P. **, J. Song, X. C. Wang, and X. ** (2018). Two-dimensional correlation spectroscopic analysis on the interaction between humic acids and aluminum coagulant. J. Environ. Sci. 64, 181–189. https://doi.org/10.1016/j.jes.2017.06.018.
S. Suresh, S. Karthikeyan, and K. Jayamoorthy (2016). FTIR and multivariate analysis to study the effect of bulk and nano copper oxide on peanut plant leaves. J. Sci. Adv. Mater. Devices 1, 343–350. https://doi.org/10.1016/j.jsamd.2016.08.004.
S. Chandra, D. Laha, A. Pramanik, A. R. Chowdhuri, P. Karmakar, and S. K. Sahu (2016). Synthesis of highly fluorescent nitrogen and phosphorus doped carbon dots for the detection of Fe3+ions in cancer cells. Luminescence 31, 81–87. https://doi.org/10.1002/bio.2927.
C. Zheng, L. Huang, Q. Guo, W. Chen, W. Li, and H. Wang (2018). Facile one-step fabrication of upconversion fluorescence carbon quantum dots anchored on graphene with enhanced nonlinear optical responses. RSC Adv. 8, 10267–10276. https://doi.org/10.1039/C8RA00390D.
S. Chandra, A. R. Chowdhuri, T. K. Mahto, A. Samui, and S. K. Sahu (2016). One-step synthesis of amikacin modified fluorescent carbon dots for the detection of Gram-negative bacteria like Escherichia coli. RSC Adv. 6, 72471–72478. https://doi.org/10.1039/C6RA15778E.
S. Y. Lim, W. Shen, and Z. Gao (2015). Carbon quantum dots and their applications. Chem. Soc. Rev. 4, 362–381. https://doi.org/10.1039/C4CS00269E.
T. T. Meiling, P. J. Cywiński, and I. Bald (2016). White carbon: fluorescent carbon nanoparticles with tunable quantum yield in a reproducible green synthesis. Sci. Rep. 6, 28557. https://doi.org/10.1038/srep28557.
K. Suzuki, L. Malfatti, M. Takahashi, D. Carboni, F. Messina, Y. Tokudome, M. Takemoto, and P. Innocenzi (2017). Design of carbon dots photoluminescence through organo-functional silane grafting for solid-state emitting devices. Sci. Rep. 7, 5469. https://doi.org/10.1038/s41598-017-05540-5.
B. De and N. Karak (2013). A green and facile approach for the synthesis of water soluble fluorescent carbon dots from banana juice. RSC Adv. 3, 8286–8290. https://doi.org/10.1039/c3ra00088e.
M. Nishikimi, N. A. Rao, and K. Yagi (1972). The occurrence of superoxide anion in the reaction of reduced. Biochem. Biophys. Res. Commun. 46, 849–854. https://doi.org/10.1016/S0006-291X(72)80218-3.
B. Halliwell and J. M. C. Gutteridge (1981). Formation of a thiobarbituric-acid-reactive substance from deoxyribose in the presence of iron salt. FEBS Lett. 128, 347–352. https://doi.org/10.1016/0014-5793(81)80114-7.
L. Zhao, Y. Wang, and Y. Li (2019). Antioxidant activity of graphene quantum dots prepared in different electrolyte environments. Nanomaterials 9, 1708. https://doi.org/10.3390/nano9121708.
L. A. Chunduri, A. Kurdekar, S. Patnaik, B. V. Dev, T. M. Rattan, and V. Kamisetti (2016). Carbon quantum dots from coconut hust: evaluation for antioxidant and cytotoxic activity. Mater. Focus 5, 55–61. https://doi.org/10.1016/0014-5793(81)80114-7.
Z. T. Rosenkrans, T. Sun, D. Jiang, W. Chen, T. E. Barnhart, Z. Zhang, C. A. Ferreira, X. Wang, J. W. Engle, P. Huang, and W. Cai (2020). Selenium-doped carbon quantum dots act as braod spectrum antioxidants for acute kidney injury management. Adv. Sci. 7, 2000420. https://doi.org/10.1002/advs.202000420.
Acknowledgements
The corresponding author (Dr. G. Ramalingam & Dr. M. Biruntha) acknowledges the economic support from RUSA 2.0 Grant No.F.24-51/2014-U, Policy (TNMulti-Gen) Govt. of India.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
We declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rajamanikandan, S., Biruntha, M. & Ramalingam, G. Blue Emissive Carbon Quantum Dots (CQDs) from Bio-waste Peels and Its Antioxidant Activity. J Clust Sci 33, 1045–1053 (2022). https://doi.org/10.1007/s10876-021-02029-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-021-02029-0