Abstract
Emerging pollutants, also referred to as emerging contaminants, are substances that have recently been recognized or are gaining attention due to their potential adverse impacts on the environment, human health, or ecosystems. These pollutants present a significant threat to both environmental and human well-being and are challenging to eliminate using conventional remediation methods. Extremophiles, organisms adapted to extreme environmental conditions like high or low temperatures, high pressure, and elevated salt concentrations, play a crucial role in this context. They produce a diverse array of enzymes capable of breaking down complex organic compounds, some of which remain stable and functional even in harsh environmental conditions, making extremophiles well-suited for use in bioremediation applications. Numerous studies have demonstrated the capability of extremophiles to degrade various pollutants, including toxic solvents, heavy metals, and industrial chemicals. Halophilic archaea, a type of extremophile, have particularly shown promise in degrading emerging contaminants in salt marsh sediments. Despite their potential, there are challenges associated with using extremophiles in bioremediation, such as the limited availability of extremophilic microorganisms capable of degrading specific pollutants and a reduction in enzyme stability when operating outside their optimum range. Nevertheless, ongoing research in this field is anticipated to result in the development of new and innovative bioremediation strategies for effectively removing emerging pollutants from the environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Emerging pollutants (EPs), also known as contaminants of emerging concerns (CECs), are commonly defined as pollutants that have recently appeared or have existed for some time, with concerns arising not long ago [134]. However, ambiguity often arises when categorizing a compound as EPs. For instance, a pollutant with concrete evidence of a known adverse effect and requiring a regular monitoring system by the authorities may still be considered as EPs. Depending on each region or country, their EPs could be different depending on their economics activities, pollutant treatment technologies, policies and regulations, education levels and lifestyle of their citizens [1]. For example, uranium-238 and thorium-232 has been considered as EPs in Malaysia due to the operation of Lynas Advanced Materials Plant (rare earth refinery) in Kuantan start from year 2012, and it is suspected to cause pollution in Balok River [2]. In the case of Indonesia, the discharge limits of thorium-232 was not set by the Indonesia government and no concrete evidence was found on its related pollution, and thus not considered as EPs in Indonesia [3]. Besides that, the EP status for Dichlorodiphenyltrichloroethane (DDT), which is a widely used organochlorine pesticide in the mid-1940s, could be different between countries as well. The US Environmental Protection Agency (EPA) totally banned usage of DDT in year 1972, and after decades of time, it can no longer be considered as EPs in the US although some of the DTTs and its metabolites still presence in the US environment [4]. However, in the European Union (EU) listed priority ‘emerging contaminants’, DDT is still considered as EPs, and it is still widely utilised and produced in India [5]. Thus, with the incorporation of the above statements, EPs could be newly found or existed substances, that appear in the natural environment naturally or anthropogenically, that originally did not contain the said substances, which concerns arise recently with or without concrete evidences to prove their impacts, and lack of proper regulations or enforcement in monitoring or controlling their presence in the environment of the specific region or country [6, 7].
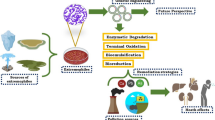
Industry and home activities are frequently the most significant contributors to water pollution, and similarly to other pollutants, EPs make their way to the environment through point source and non-point source discharge. Some examples of ECs point source pollution could be personnel care products (PCPs) and pharmaceutical compounds leak or discharge from production facilities; biocides and estrogenic chemicals such as steroid hormones from livestock farms; mixed matrices of ECs from leachate of landfills; and heavy metals or radioactive substances runoff from active mines. For non-point source, the examples include pesticide, herbicides and fertilizers runoff from agriculture farmland; heavy metals and radioactive substances flow from abandoned mines; as well as ECs suspended in air flow into natural water bodies due to precipitations [8, 9]. Figure 1 demonstrated the EPs pathway to the environment. Polycyclic aromatic hydrocarbons, various pesticides and fungicide, flame retardants, per- and polyfluoroalkyl substances (PFAS), endocrine disrupting chemicals (EDCs), and pharmaceutical and PCPs are some examples of commonly spotted and observed emerging pollutants in water environment [10]. The continuous production and utilization of novel items, including industrial chemicals, agrochemicals, electronics, fragrances, pharmaceutical products, and others, have led to the ongoing discharge of numerous newly introduced chemical pollutants into aquatic ecosystems.
The necessity to access the risk and monitor their discharge into the environment lead to the establishment of the NORMAN project, which target to create a permanent network between references bodies, researches centres, industries, non-government organisations and other parties, in order to promote more rapid, broad and transparent information and data exchange, standardisation of measuring and monitoring methods for EPs, and the establishment of independent and competent forum for scientific debate on EPs related matters [11]. The original NORMAN list for EPs only consists only around 900 compounds, while in year 2018, the NORMAN Suspect List Exchange (SUSDAT) database have included more than 40,000 compounds. Currently, the SUSDAT list consist of almost 106,000 compounds with different validation level, and the increment of EPs proved the increasing in types of compounds synthesised or the reemphasize on compounds that previously ignored or being paid less attention on [12]. Dembrexine is used as seccretolytic drug to treat respiratory disease for horses [13]; Salinazid which used as antituberculous, antiasthmatic and anti-inflammatory drugs [14]; Atropine methyl often used to restrict production of saliva and bronchial fluids, and to treat organophosphorus agent poisoning [15]. Among the compounds above, atropine methyl and dembrexine can be considered as existing compounds that added into the suspect list recently as they have been registered in the PubChem library for a long time. Some of the EPs, including but not limited to pharmaceutically active compounds, personal care products, plasticizers, and brominated flame retard are restricted as “legacy persistent organic pollutants (POPs) “ by The Stockholm Convention of 2001 [16,17,18,19,20]. Due to their inherent physical and chemical characteristics, POPs exhibit persistence in the environment, widespread distribution, bioaccumulation in the food chain (including humans), and pose risks to both wildlife and human health [6, 10]. From this point onwards, the utilisation of extremophiles for the degradation of these compounds could come in handy. Table 1 below summarised some of the EPs and their adverse effect towards human and the environment.
Extremophiles are categorized into various groups, including thermophiles, acidophiles, psychrophiles, halophiles, alkaliphiles, xerophiles, barophiles, and endoliths. Recently, researchers have shown considerable interest in extremophiles due to their ability to produce a multitude of enzymes called extremozymes, which can function under extreme conditions, affecting their substrates [21, 22]. Extremozymes showed some of the properties such as highly charged exterior surfaces, rigid folds sustained by several ion-pair networks and tightly packed hydrophobic core. Compare to normal enzymes, they are more dense and compact protein structures which partly caused by the high amount of acidic and basic amino acids [23]. In the biodegradation of organic pollutants, it is necessary to produce enzymes or microorganisms that can withstand extreme salinity, acidity, alkalinity, temperature, high pressure, and the toxicity of chemical pollutants. Extremophiles and extremozymes play a crucial role in lignin bioprocessing. These specialized microorganisms and enzymes have the unique ability to thrive and function under extreme conditions, making them ideal candidates for lignin degradation and utilization. Their involvement in lignin bioprocessing holds great promise for the development of sustainable and efficient methods to break down lignin and extract valuable products. By harnessing the power of extremophiles and extremozymes, researchers aim to enhance lignin valorization, biofuel production, and other applications in lignin-based industries [24, 25]. The production of active extremozymes enables extremophiles to carry out essential biochemical processes necessary for their survival in their extreme habitats. For example, thermophilic extremophiles living in high-temperature environments produce heat-stable enzymes that can continue to function even at temperatures that would render most enzymes inactive. Similarly, acidophilic extremophiles in highly acidic conditions produce acid-stable enzymes, while halophilic extremophiles in highly saline environments produce salt-tolerant enzymes [26].
Cultivating extremophiles necessitates specific nutrient, salt concentration, and temperature parameters. Microorganisms with moderate thermophilic, extremely thermophilic, and hyperthermophilic traits can thrive and propagate at temperatures above 45 ℃, 70–80 ℃, and beyond 80 ℃, respectively [27]. Some hyperthermophilic archaea able to thrive and flourish at temperatures exceeding 100 ℃. Extremophiles have distinct adaptations that enable their survival in environments with extreme heat, such as hydrothermal vents and hot springs. One of the most significant contributions of hyperthermophilic archaea lies in their production of thermostable enzymes. These enzymes can maintain their functionality and structural integrity even at extremely high temperatures, making them invaluable for various industrial applications [28]. Halophiles are organisms that thrive at high salinity conditions. The halotolerant and halophilic bacteria growing salinity condition can be 0.5–2.5 M and 2.5–5.2 M NaCl, respectively [29]. Alkaliphiles require a pH range of 8.5 to 11, with an optimum pH of 10. Haloalkaliphiles can survive and growth under alkali and high saline condition. A growing medium with a pH of 5 or less is suitable for isolating acidophilic and extreme acidophilic microorganisms [30, 31]. However, depending on the environment condition of the sample source, the setting for growth medium must be changed accordingly for the culture of extremophiles. For optimal growth, polyextremophilic bacteria require specific combinations of these extreme conditions, which vary depending on the species [32]. Scientists were intrigued by the possibility in commercial applications of extremophiles to accelerate processes under extreme environments. Extremozymes that exhibit excellent activity throughout a broad pH, temperature, and gasification range are utilized extensively in various industries, including dairy and beverages, bioenergy, and sustainable manufacturing of valuable chemicals [33, 34].
2 Extremophile and Industry
One of the most significant industries that use extremophiles is the pharmaceutical industry. Many of the compounds produced by extremophiles have potential medical benefits. For example, Thermus aquaticus, a thermophilic bacterium, produces Taq polymerase, which is used in the polymerase chain reaction (PCR), a widely used laboratory technique in molecular biology. The discovery of Taq polymerase has revolutionized the field of genetics and helped to diagnose and treat genetic disorders [35]. Similarly, the discovery of archaeosomes, which are new generation of liposomes made by natural ether lipids produced from archaea, has potential medical applications due to their stability in various condition and unique properties, such as their ability to transport drugs through the blood-brain barrier [36]. Extremophiles also have applications in the biotechnology industry. Extremophilic bacteria are known to produce enzymes known as extremozymes that exhibit remarkable stability in extreme environments and possess unique properties. Notably, some of these enzymes are capable of breaking down cellulose, which happens to be the most abundant organic polymer found on Earth. Extremozymes have found wide applications in various industries, including the production of detergents, textiles, paper, and biofuel [37]. The use of extremophile-derived enzymes in these industries not only enhances the efficiency of various processes but also contributes to more sustainable and eco-friendly practices, as they often require lower energy consumption and generate fewer harmful byproducts. The environmental industry also benefits from extremophiles. Some extremophiles have unique abilities to clean up polluted environments. For example, certain bacteria can degrade oil spills and heavy metals [38]. The discovery of these bacteria has led to the development of bioremediation techniques, which use microorganisms to break down pollutants in soil and water. Table 2 summarized the extremophiles applications in various industries. Despite the benefits of extremophiles, there are also some potential risks associated with their use in industry. One risk is the spread of these organisms to non-native environments. If an extremophile is introduced into a new environment, it could outcompete native organisms, leading to ecological imbalances. Additionally, some extremophiles are pathogenic and could pose a risk to human health if not handled properly [37, 39].
One potential application of thermophile amylase in environmental remediation is in the degradation of starch-based pollutants. Starch is a common pollutant found in many industrial wastewaters, and its degradation can be difficult due to its complex structure. However, thermophile amylase has been shown to be effective in breaking down starch into simpler, more easily degraded molecules [40]. This can lead to more efficient and cost-effective removal of starch-based pollutants from wastewater. Another potential application of thermophile amylase in environmental remediation is the bioremediation of contaminated soil. Bioremediation is a bio-based remediation process that harnesses the power of microorganisms to degrade and eliminate contaminants from soil. However, the effectiveness of bioremediation can be limited by the temperature of the soil, as many microorganisms are unable to function at high temperatures [41]. Thermophile amylase, on the other hand, can function at high temperatures and can be used to improve the effectiveness of bioremediation in high-temperature environments. By breaking down starch-based pollutants in contaminated soil, thermophile amylase can help to facilitate the removal of other contaminants as well [35, 39]. Psychrophiles are organisms that thrive in cold environments, including polar regions, high-altitude locations, and deep-sea waters. One example of a psychrophile protease that has been used for environmental remediation is a protease isolated from a psychrophilic bacterium, Pseudoalteromonas sp. strain A28. This protease has been shown to effectively degrade several emerging pollutants, including naproxen, ibuprofen, and diclofenac, at low temperatures. Previous study proved that the Pseudoalteromonas sp. strain A28 protease was able to degrade over 80% of the naproxen in an aquatic environment within 24 hours at 10°C [42, 43].
3 Adaptation Mechanisms of Extremophiles and Their Enzymatic Processes Towards Pollutants
3.1 Halophiles
Halophilic microbes can survive in a high-saline environment, which impedes the survival of organisms due to osmolar imbalance and metabolic issues [31, 42]. Prior research on halophilic microbes identified two main adaption methods for survival in situations of extremely high salt concentration. The first is to employ a “salt-in” strategy, which refers to the accumulation of inorganic osmoprotectants such as KCl within the cell to preserve osmotic equilibrium both within and outside the cell [44]. By utilizing the adenosine triphosphate (ATP)-dependent potassium ion (K+) transport system (the KdpFABC complex), cationic amino acid transporter-3 (Cat3) and Na+ efflux antiporters (NhaC) for balancing the osmotic gradient between cell and environment under high-salt conditions, Halobacterium salinarum has been shown to store up to 3.97 M and 4.57 M of K+ and Cl ions respectively [45, 46]. Furthermore, halophilic microorganisms have developed various negatively charged aspartate and glutamate residues on the surfaces of their proteins. This feature allows them to retain water molecules around the cells, preventing protein precipitation and dehydration. By doing so, halophilic microbes effectively avoid drying out in their high-salt environments [46, 47]. The ‘compatible solutes adaption’ technique was adopted by certain halophilic and halotolerant bacteria. They make use of compatible organic solutes, such as polyols, glucosylglycerol, sucrose, trehalose, ectoine, and betaine, to maintain osmotic equilibrium in their high-salt environments. This allows osmotic equilibrium to be preserved by the bacteria in extreme conditions, and it is facilitated by the utilization of compatible organic solutes. [48, 49]. When exposed to a high salt concentration, the halophilic bacteria Spiribacter salinus M19-40 secretes increased quantity of suitable solutes which play a vital function in lowering the thermodynamic activity of water in order to compensate for the external osmotic pressure [44, 48]. Table 3 listed the halophiles and their enzymes in the degradation of various pollutants.
3.2 Acidophiles
Acidophilic microbes can survive in environments with an extremely low pH (less than pH 3) via managing proton permeation. Microorganisms from the genera of Thermoplasma, Ferroplasma, and Sulfolobus, for instance, have highly impermeable cell membrane primarily made up of tetraether lipids with a diverse formation of polar head groups and a bulky isoprenoid core, allow them to control proton permeation under extremely low pH environment [46]. Survival at low pH requires the control of proton influx through the proton pump system, putative proton pump proteins such as plasma membrane H + -ATPase, symporters, and antiporters such as NahP-/NhaA-type Na + /H + exchangers from Ferroplasma type II and Leptospirillum group II are implicated in pH homeostasis. Furthermore, it is known that F0F1-type ATP synthase is crucial for the regulation of proton permeation, while they can be found in acidophiles such as Bacillus acidocaldarus, Thermoplasma acidophilum, and Leptospirillum ferriphilum. Additionally, a few auxiliary mechanisms, such as chaperone which participates in protein refolding; and cytoplasmic buffering capacity, preserve intracellular molecules such as DNA, RNA, and proteins under extremely acidic situations [46, 50, 51]. With those surviving abilities, acidophiles has been utilized and studied for the treatment of various pollutants (Table 4).
3.3 Alkaliphiles
Alkaliphilic microbes are tolerant to high pH, as opposed to acidophilic microorganisms. Up to present, three important biological pathways have been found in these microbes as survival strategies. Under extreme alkaline conditions, certain alkaliphilic Bacillus species can boost proton motive force generation by synthesizing an acidic cell membrane, composed mostly of peptidoglycan, teichuronic acid (TUA), and teichuronopeptide (TUP), which benefits both energy production and pH balance. In Bacillus lentus C-125, the contents of TUA and TUP in cell wall greatly increase as the surrounding pH increase [52]. Secondly, sodium motive force can increase pH equilibrium in settings of excessively high pH. The intracellular pH is altered through the Na + /H + antiporter, Na + channels or stator force generator that triggers Na + -dependent motility, reduce the dependency on H + -potential base transport systems. It is useful when the alkaliphile is exposed to neutral conditions when the concentration of H + is very low [53]. Lastly, the generation of organic acids such as succinate, lactate and acetate by alkaliphiles for pH calibration is a crucial biological activity for pH maintenance [25]. Table 5 summarized the alkaliphiles utilized in the degradation of several pollutants.
3.4 Thermophiles
Similar survival mechanisms are activated by thermophilies with a favoured temperature above 60 °C. In order to tolerate high temperatures, B. acidocalidus, stabilized the membrane lipid fluidity by increase the production of hopanoids (a subclass of triterpenoids). Thermophiles might also alter the iso or anteiso fatty acid composition in cell depending on the surrounding temperature. It is reported that the iso fatty acids in Bacillus spp. increase according to the increment of growth temperature while anteisofatty acid content increase with the decrement of temperature [54]. By the regulation of membrane lipid content, the thermophilic archaeon Metahnocaldococcus jannaschii can withstand extreme temperature. Diether lipids (archaeol-based) reduced from 80 to 20% when these microorganisms were subjected to high temperatures, but caldarchaeol- and cyclic archaeol-based lipids will increase by 30% to a total of 40% content in the cell [55]. Moreover, thermophilic microbes have evolved several biomolecules to induce heat stability, such as by increasing the guanine or cytosine concentration of DNA, or generating a positive supercoiled DNA shape. In addition to possessing an abundance of ribosomal proteins, these organisms have a proficient heat-shock response that permits the generation of normal protein even at elevated temperatures [46]. Thus, thermophiles have been utilized in several processes that run on elevated temperatures (Table 6).
3.5 Psychrophiles
A superabundance of genetic redundancy is typically observed in psychrophilic microorganisms. This means that they encode multiple copies of tRNA species responsible for the biosynthesis of amino acids and have a high quantity and variety of chaperones. This genetic redundancy suggests that a high capacity for translation and post-translational processing is crucial for these organisms to thrive in low-temperature environments. Additionally, psychrophilic enzymes tend to exhibit higher structural flexibility, lower thermostability, and greater specific activity at low temperatures compared to their mesophilic counterparts [56]. Cold-adapted enzymes have higher catalytic activity and more productive than mesophilic and thermophilic enzymes under low temperature, thus provides an energy saving and economical pathways for low temperature processes. Some potential applications including biodegradation, biodiesel production, and food and beverage production through psychrophilic xylanase, lipase, protease and peroxidase [57]. Psychrophilic enzymes including proteases, lipases and chitinases have been applied in food processing activity including descaling of fish, skin and grease removal, and oil extraction, while some proteases are involved in meat tenderization and flavor enhancement [58]. Study found that psychrophilic protease synthesized from Pseudoalteromonas sp. SM9913 have a higher catalytic efficiency and released more taste amino acids and essential amino acids on the exterior of sea fishes, shrimps, and pork than their mesophilic counterparts during refrigerated storage [59].
3.6 Metalophiles
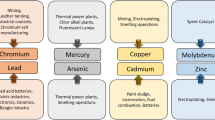
As minute amounts can be hazardous to public health and the environment, concerns over the toxicity of heavy metals have increased dramatically. In addition, chemical remediation of dangerous heavy metals under harsh environments are sometimes hindered by their limited accessibility. Thus, throughout the past several decades, the advancement of sustainable bioremediation technologies that employ extremophilic bacteria for the treatment of heavy metals has been researched. Acidophilic bacteria that can flourish under low pH circumstances have been employed in biomining processes including bioleaching and bio-oxidation as well as detoxification of heavy metals in the event of severely acidic environments [60, 61]. Metalophilic or some acidophilic microorganisms had evolved sharpened metal detoxification pathways and mechanisms to activate and/or inhibit anti-oxidativestress, metal-binding and transport, and membrane-permeability responses [38]. The most prevalent acidophilic and chemolithotrophic microorganisms are Acidothiobacillus strains, which have been used in the development of bioremediation techniques. For instance, Acidothiobacillus ferrooxidans has been utilized for bioleaching on an industrial scale for extracting metals out of sulfide ores. There are also growing interest to utilise the species for develo** of in situ resource utilization (ISRU) techniques such as mining from other celestial bodies under microgravity condition [60, 62, 63]. Previous studies reported the ex-situ bioremediation of Uranium (IV) from polluted mine water utilizing Acidithiobacillus ferrooxidans through bioadsoprtion, as well as applying both Acidithiobacillus ferrooxidans and Acidothiobacillus ferrivorans strains for sulfidogenesis process for the selective precipitation of copper from a zinc-copper solution under pH ranged from 2.6 to 2.6. Besides that, Acidocella aromatica PFBC were found able to efficiently reduce vanadium ions [vanadate; V(V)] to V(IV) under pH 2 or pH 4.5, aerobically or anaerobic condition [64, 65]. Table 7 concludes a few species that have been used in detoxification and removal of heavy metals.
4 Potential of Utilizing Extremophiles for Biodegradation of EPs in Water
From previous part, it is mentioned that utilization of extremophiles has been widely commercialized in various industries including food and beverages, pulp and paper and biodiesel. However, very little is studied and utilized in water and wastewater treatment industries, especially on the degradation of EPs. Current conventional water treatment plants majorly relied on physical and chemical treatment methods in the removal of various pollutants, while wastewater treatment plants utilized both the methods together with biological processes [66]. However, conventional treatment methods are not specifically design for the removal of EPs, especially with most of them appears on trace level [67]. Their efficiency varies depending on the treatment methods and types of EPs, could be as low as around 20% to more than 98% removal [68]. Besides, conventional treatment methods bring their own limitations such as energy-intensive and the production of harmful by-product. Among the processes, biological therapy for pollutants removal appears to be more favourable and widely accepted as they are regard as environmentally friendly and sustainable [69]. Although processes such as settling, aerating volatilization, chemical precipitation and sludge absorption contribute to the removal of EPs, but biodegradation process plays the major role [67]. Harmful organic contaminants, such as petroleum hydrocarbons, aromatic petrochemicals, and various halogenated compounds, can be converted into non-toxic or less toxic molecules by a variety of microbes. This extensive transformation requires not only strong resistance to exposure to toxic organic pollutants but also the ability to utilize these harmful contaminants for cellular metabolism [46].
Industrial wastewater discharge is one of the main sources for EPs contamination. Industrial production plants including pharmaceutical, food and beverages, personal care products and pesticides production factories generally produce wastewater contained with EPs due to production waste, leakage, and by-products formation, contaminates the surrounding environment due to incomplete removal [70]. The wastewater produced from these industries often has broad range of compositions and characteristics, with some comes with extreme conditions such as low pH, high temperature and high in toxicity. Pharmaceutical wastewater normally characterized with high in organic matter, high salt concentration, microbial toxicity, high fluctuations in quantity and complicated compositions due to verities of raw material used during batch production process. The temperature of wastewater could range from 25 to 80℃, and pH from 1 to 8 [71]. Pesticides production industry wastewater contains high amount of toxic components, with high chemical and biochemical oxygen demand, high total dissolved solids, and generally low in pH [23]. The pH could range from 1.5 to 14, and chemical oxygen demand from 17,000 to 35,800 mg/L [72]. For personal care products (PCPs) industries, a case study on Brazilian cosmetic industries proved that the pH could range from 1 to 12, with maximum salinity of 2.4 parts per thousands (ppt) [10]. Moreover, the condition of incoming raw water or wastewater for treatment always fluctuates depending on different causes such as weather or season, incoming water flowrate, saline intrusion, time of a day, adjustment in production processes and much more [16, 44]. Conventional treatment methods often incorporate multiple processes to adjust the influent conditions such as pH and temperature as commonly used chemicals (coagulant, flocculant) and microbes in water treatment industry usually require a “sweet spot”, which commonly close to normal condition for optimal performance. These indicates extra capital and operation cost are needed to change the condition of influent for maximum treatment efficiency.
Hence, extremophilic microorganisms that have adapted over a long period of time to harsh habitats such as extreme temperatures, pH and salinity have the potential to be exploited extensively for the treatment of hazardous EPs under the corresponding conditions, without additional processes to change the characteristics of influent [42]. Only very limited studies have been done on the EPs degradation by extremophiles, while many focus on petroleum products, hydrocarbon compounds, dyes, and heavy metals [53, 72]. In terms of hydrocarbon compounds, X. Lin et. al. found that close to 90% removal in 28 days was achieved for degradation of mixed crude oil and diesel by Pseudoalteromonas sp. P29 at temperature of 5 ℃ [73]. Another study isolated thermophilic Bacillus licheniformis WY2 which able to degrade hydrocarbon by 52% at temperature range from 52 to 80 ℃ [74]. Srivastava et. al. discovered that Bacillus albus DD1 able to degrade azo dye Reactive Black 5 up to 98% in 38 hours under condition of 40 ℃ and pH 7, the bacteria species also showed degradation efficiency of 73% at pH 8 and pH 9 [75]. These could prove that extremophiles could undergoes metabolism activities and their enzymes able to work efficiently even under extreme conditions. More researches could be done to discover their potentials in the bioremediation of EPs including but not limited to pesticide and fungicide, PCPs and pharmaceutical drugs due to their increment in usage as well as the pollution causes by them.
5 Extremozymes Activities Against EPs
From Sect. 3, it is found that enzymes synthesis by the extremophiles plays an important role in degrading various EPs. In bioremediation, enzymes such as hydrolases, lipases, oxygenases, laccases and oxidoreductase able to degrade or detoxify most of the hazardous pollutants [76]. Oxygenases catalyse the oxidation of reduced substrates through transmitting oxygen atom from oxygen molecule (O2), by using coenzyme such as Flavin Adenine Dinucleotide (FAD), Nicotinamide adenine dinucleotide hydrogen (NADH), or nicotinamide adenine dinucleotide phosphate (NADPH) [77]. Oxygenases actively participate in the metabolism of organic compound, through increasing their reactivity or water solubility or breaking of the aromatic ring, and thus able to degrade wide range of toxic pollutants such as aromatic hydrocarbons, halogenated compounds and chlorinated aliphatics [78]. Oxygenases can be categorised into 2 main groups, which are monooxygenase, and dioxygenase. Monooxygenases catalyse the infusion of one oxygen atom into substrate, while another form into water; while dioxygenase catalyse both O2 atoms into the substrate [79]. Multiple tables in Sect. 3 stated different types of extremophiles synthesised various oxygenases enzyme in the degradation of pollutants, including phenanthrene, phenol, aniline, trichloroethylene, 4-HBA and others. Monooxygenases often participates in processes including dehalogenation, dealkylation, denitrification and hydroxylation processes [79, 80]. Dehalogenation process reduce the toxicity and degradation-resistance of a compound by removal of halogenated groups; Dealkylation process involved the removal of alkyl groups in a substrate, breaking it into 2 smaller substances; Denitrification process involve the changing of nitro group attached to aromatic ring into hydroxyl group; while hydroxylation by oxygenase often involve bond attachment of hydroxyl group onto aromatic rings, usually for the purpose of aromatic ring cleavage [81]. Dioxygenase catalyse several reactions including breaking of aromatic rings, dihydroxylation, and S-oxygenation of thiols to sulfunic acid [79, 80]. (choose a EP and propose a metabolic path way)
6 Factors Affecting Stability of Extremozymes
Enzymes are highly efficient catalyst that account for catalysing various biological reactions in every living cell [82]. Enzymes able to carry out molecular recognition and selective catalysis which are the key chemical processes in life, while also capable of speeding up bioreactions up to 17 orders of magnitude [60, 82]. With those characteristics, enzymes able to synthesis various complex biomolecules with distinct structural feature [60]. However, enzymes efficiency is highly dependable on the surrounding conditions such as temperature, pH and toxicity. Extremophiles have to ability to produce extremozymes that could withstand and works efficiently even under extreme and high toxicity conditions, which play a major role in metabolism activities and survival of extremophiles. For instance, thermophiles such as T. thermophilus, Geobacillus, and Thermosediminibacter species able to produce extreme pH and temperature stable laccases and azoreductases, which are competent for the removal of various toxic dyes, while some capable to produce detoxifying thermozymes for the degradation of toxic organic pollutants [83].
Although having a better adaptation towards extreme conditions when comparing to normal enzymes, the stability of extremozyme could still be affected by the surrounding conditions. Thermodynamic stability of enzyme is the capability of protein to unfold or refold after being subjected to stresses including increase in temperature, extreme pH or high toxicity condition. Enzyme denaturation happens when the tertiary structure unfolds to a disordered polypeptide where the residues are not functional or carry out structural stabilizing interactions, and it can be reversible and irreversible [84]. One of the primary factors affecting the stability of extremozymes is temperature. Extremophilic enzymes are typically adapted to function in environments that are far outside the temperature range that is conducive to the activity of mesophilic enzymes. Thermophilic enzymes usually work best in the temperature range between 55 and 121℃, while psychrophilic enzymes work efficiently from −2 to 20 ℃ [63]. When comparing to their mesophilic counter parts, thermophilic enzymes showed similar three-dimensional structures, but have increased charged residues on the surface and have different amino acid contents. They also have shorter loops to prevent nonspecific interactions due to their high flexibility at an elevated temperature. The numbers of bisulphide bonds formed between two cysteine residues are also higher to increase their structural rigidity for preventing unfolding at high temperature [63]. Besides, the available of salt bridges is also a distinct feature of thermozyme comparing to their mesophilic variant. Salt bridges help in stabilise the enzyme by increasing rigidity, with the penalty of increment in activation energy, and thus explained that thermophilic enzyme is usually less active at lower temperature [85]. Figure 2 showed the presence of salt bridge in thermophilic acylphosphatase, connecting the active side and C-terminus of the enzyme [86]. Thermophilic enzyme tends to have better adaptation towards enzyme unfolding cause by extreme heat, as their highly charged surfaces and increment in bonds could hold the structure closer together, giving it greater possibility to regain stability when condition returns to normal [87] (Fig. 3).
Comparison of thermophilic AcP to its mesophilic counterparts [86]
Illustration on thermal decomposition on thermophilic and mesophilic enzyme [87]
Psychrophilic enzymes usually have a flexible structure and low stabilities, and it is due to higher proportion of α-helix than β-sheets in their protein. They maintain high catalytic rate at a low temperature through the enhancement of the solvent connection and structural flexibility [63]. By reducing the salt bridges, disulphide bond, aromatic face-to-edge interaction, and bidentate short hydrogen bond, as well as increment in hydrophilicity in core clusters, the psychrophilic enzymes tend to increase their structural flexibility, require lower activation energy and thus able to maintain active at lower temperature [88]. Figure 4 stated the difference between psychrophilic α-amylase synthesised from Pseudoalteromonas haloplanktis to their mesophilic counterparts, with cyan colour represent psychrophilic enzyme and red as mesophilic enzyme. N150D showed the presence of salt-bridge in mesophilic enzyme; V196F present the increment of aromatic ring in mesophilic protein which strengthen the aromatic face-to-edge interaction; Q58C/A99C showed the availability of disulphide bond in mesophilic protein; T232V and Q1641 demonstrated the substitution of hydrophobic to a hydrophilic protein in the core cluster of mesophilic enzyme; and K300R illustrated the availability of bidentate binding though short hydrogen bond in mesophilic enzyme [89].
Comparisons between psychrophilic and mesophilic α-amylase [89].. *Lys = Lysine; Asn = Asparagine; Asp = Aspartic Acid; Val = Valine; Phe = Phenylalanine; Tyr = Tyrosine; Cys = Cysteine; Ala = Alanine; Gln = Glutamine; Trp = Tryptophan; Leu = Leucine; Thr = Threonine; Pro = Proline; Ile = Isoleucine; Arg = Arginine
Although extremophiles can withstand greater range of temperature, they still subject to denature or become unstable when the temperature range exceeded their adaptation capability, either too hot or cold. The thermal unfolding of enzymes also often leads to aggregation [90]. In order to overcome this issue, extremozymes may be stabilized by adding certain chemicals such as trehalose, glycerol and dextran that can protect the enzyme from denaturation, or by modifying the enzyme’s amino acid composition to improve its stability at higher temperatures [69. 87]. Trehalose is usually used to prevent thermal denature of enzyme, with some theories explained its bioprotection action including vitrification, preferential exclusion, and water replacement theory. Vitrification theory stated trehalose forms a glassy matrix surround the enzyme or cell, physically protects it from abiotic stress; preferential exclusion theory explains that trehalose isolates enzyme away from water molecules, increase the enzyme stability by reducing its wetted radius and increasing its compactness; water replacement theory assumes the structure of enzyme is maintained through the replacement of water molecules by trehalose-forming hydrogen bonds [51]. Figure 5 demonstrated the stated theories.
Trehalose in stabilizing enzyme [11]
Another factor that can affect extremozyme stability is pH. At extreme pH condition, enzyme unfolding happens as the formation of same charges occur due to ionisation of functional groups, causing an increment in electrostatic repulsion. Most enzyme will form molten globule state, which consist of considerable amounts of secondary structure and totally disrupted tertiary structure as the protein denatures gradually [11]. Totally unfold of protein may happened under prolonged extreme condition. Figure 6 illustrate the pH induced denature of enzyme, in native state, the surface charges is at a balance state with zero or minimum net charge. When the condition turns acidic, more hydrogen ions (H+) will presence in the environment and interact with the negative ions of the enzyme, turning the net charges into positive state, along with the drop in ionic strength. When condition prolonged or worsen, more negatively charges will be dissipated and lead to unfolding of protein.
Generally, enzyme function optimally between range of 6 to 8, while extremophiles live in environments that often have extreme pH conditions, and their enzymes have evolved to function optimally under these conditions. For example, acidophilic enzymes function optimally at pH values below 5, while alkaliphilic enzymes function optimally at pH values above 9 [91]. Acidophilic enzyme usually demonstrated large area of negative charge at the active site, and overall have lesser positive charge compare to neutrophilic counter parts. As protonation of the negatively charged group happen more frequently at lower pH, this will balance out the charges of the enzyme and make it stable at lower pH [11, 90]. They also showed an increase in the number of hydrogen bond. Similar to acidophilic enzyme, alkaliphilic enzyme also showed more negatively charges on the protein surface. They also have an elevated number of hydrophobic residues and drop in polar residues to maintain better stability in alkali environment [89]. However, extreme pH conditions can also cause denaturation of the enzyme. To address this issue, extremozymes may be stabilized by altering their amino acid composition to improve their stability at extreme pH values, or by using buffering agents to maintain a stable pH in the reaction environment. Enzyme immobilization techniques such as nano-zeolite immobilization can be applied for increasing the pH stability of enzyme [92].
Salt concentration is another factor that can affect extremozyme stability. Enzyme are usually more soluble and active in a dilute salt concentration environment, as ionic-formed salt able to associate with opposite charges within the protein moiety, increase hydration on the protein surface. However, extreme salt concentration could remove the essential layer of water molecules from the enzyme, disrupting the salt bridge as well as certain level of ionic bond, which could lead to denature on quaternary and tertiary enzyme structure [31]. Halophilic enzymes are adapted to function in environments with high salt concentrations, and their stability is often dependent on the presence of salt ions. Halophilic enzymes thrive in high saline environment as the protein have higher ion-pair networks, reduction in hydrophobic surface patches, and abnormally high in number of ordered side chains [56]. The high negative charges could provide hydrated carboxylate groups that maintain the solubility of protein under high salinity condition, as well as offsets the stabilization from the salt’s enhancement of hydrophobic effect [43]. Figure 7 showed a comparison between halotolerant aminotransferase from Halomonas elongata and its counterparts from E. coli. Red parts indicate negatively charged aspartic and glutamic acid, while white parts indicate hydrophobic leucine, isoleucine, and phenylalanine protein. Halophilic aminotransferase showed a higher density of negatively charge residues, while its homologue showed a more evenly distribution of acidic and basic proteins, with net charge near to zero [93].
Comparison on the surface charge residues between halotolerant aminotransferase to its counterparts [93]
However, at extremely high salt concentrations or very low salts concentration, denaturation can occur due to the disruption of the enzyme’s structure. To improve the stability of halophilic enzymes, the salt concentration of the reaction environment may need to be optimized, or the enzyme compositions may need to be modified to enhance its stability under extremely saline or low in salt concentration [31]. The presence of other chemicals or contaminants in the reaction environment can also impact extremozyme stability. Enzymes can be sensitive to certain chemicals, such as detergents, solvents, or heavy metals, which can inhibit their activity or cause denaturation. Some but not all extremozyme showed resistance or able to degrade the toxic compounds lie within the chemicals. To improve the stability of extremozymes in the presence of these chemicals, they may need to be modified to reduce their sensitivity, or stabilizing agents may need to be added to the reaction environment [94].
7 Key Challenges in Utilizing in Industries, Conclusions, Future Prospective
Overall, extremophiles exhibited promising characteristics such as able to thrive in harsh environment of extreme temperature, pH, salinity, and some of the properties help them to survive and utilized and degrade toxic compounds as their energy sources. These make them an excellent candidate in the treatment of EPs which commonly showed high toxicity towards other organisms. Utilizing extremophiles in the treatment of EPs will also bring other advantages such as reduce energy consumption, capital cost and operation cost especially in industrial wastewater treatment plant as the ability of extremophiles to survive in harsh condition able to reduce some steps to alter water conditions in treatment process. Studies can be started by finding the source and isolate the potential strains of extremophiles that could survive and degrade EPs under extreme conditions. Further research including but not limited to determine the extremozymes that helps in degradation and the optimization of efficiency can be done for commercial application of extremophiles in the water and wastewater treatment industries.
Data Availability
Data available upon request.
Change history
27 May 2024
A Correction to this paper has been published: https://doi.org/10.1007/s11244-024-01948-2
References
References Karpińska J, Kotowska U (2021) New aspects of occurrence and removal of emerging pollutants. Water 13:2418. https://doi.org/10.3390/w13172418
Wahab Ab, Kalthum SU, Shaibullah S, Samah A, Armi M, Aris M, Shukri M (2016) An assessment of surface water quality and heavy metals involving the radioactive elements in Sungai Tunggak and Sungai Balok, Gebeng, Kuantan Pahang. Int J Appl Chem 12:146–151
Soeparna I, Tanega J (2022) A critical assessment on nuclear security measure in Indonesia. Yuridika, 37(2):317–344. https://doi.org/10.20473/ydk.v37i2.36279
Patterson GM (2016) Looking backward, looking forward: the long torturous struggle with mosquitoes. Insects 7:56. https://doi.org/10.3390/insects7040056
Feng W, Deng Y, Yang F, Miao Q, Ngien SK (2023) Systematic review of contaminants of emerging concern (CECs): distribution, risks, and implications for water quality and health. Water 15:3922. https://doi.org/10.3390/w15223922
Lapworth DJ, Baran N, Stuart ME, Ward RS (2012) Emerging organic contaminants in groundwater: a review of sources, fate and occurrence. Environ Pollut 163:287–303. https://doi.org/10.1016/j.envpol.2011.12.034
Richardson SD, Kimura SY (2019) Water analysis: emerging contaminants and current issues. Anal Chem 92(1):473–505. https://doi.org/10.1021/acs.analchem.9b05269
Vasilachi IC, Asiminicesei DM, Fertu DI, Gavrilescu M (2021) Occurrence and fate of emerging pollutants in water environment and options for their removal. Water 13:181. https://doi.org/10.3390/w13020181
Belle G, Schoeman Y, Oberholster P (2023) Potential toxic-element pollution in surface water and its implications for aquatic and human health: source–pathway–receptor model. Water 15:3100. https://doi.org/10.3390/w15173100
Puri M, Gandhi K, Kumar MS (2023) Emerging environmental contaminants: A global perspective on policies and regulations. J Environ Manage 332:Article 117344. https://doi.org/10.1016/j.jenvman.2023.117344
Vinciguerra D, Gelb M, Maynard H (2022) Synthesis and application of trehalose materials. JACS Au 2. https://doi.org/10.1021/jacsau.2c00309.
Mohammed Taha H, Aalizadeh R, Alygizakis N, Antignac JP, Arp HPH, Bade R, Baker N, Belova L, Bijlsma L, Bolton EE, Brack W, Celma A, Chen WL, Cheng T, Chirsir P et al (2022) The NORMAN Suspect List Exchange (NORMAN-SLE): facilitating European and worldwide collaboration on suspect screening in high resolution mass spectrometry. Environ Sci Eur 34(1):104. https://doi.org/10.1186/s12302-022-00680-6
Klier J, Fuchs S, Winter G, Gehlen H (2022) Inhalative nanoparticulate cpg immunotherapy in severe equine asthma: an innovative therapeutic concept and potential animal model for human asthma treatment. Animals 12:2087. https://doi.org/10.3390/ani12162087
Abdel-Aziz HA, Eldehna WM, Fares M, Al-Rashood STA, Al-Rashood KA, Abdel-Aziz MM, Soliman DH (2015) Synthesis, biological evaluation and 2D-QSAR study of halophenyl bis-hydrazones as antimicrobial and antitubercular agents. Int J Mol Sci 16:8719–8743. https://doi.org/10.3390/ijms16048719
Cristaldi M, Olivieri M, Pezzino S, Spampinato G, Lupo G, Anfuso CD, Rusciano D (2020) Atropine differentially modulates ecm production by ocular fibroblasts, and its ocular surface toxicity is blunted by colostrum. Biomedicines 8:78. https://doi.org/10.3390/biomedicines8040078
Huerta-Fontela M, Galceran MT, Ventura F (2011) Occurrence and removal of pharmaceuticals and hormones through drinking water treatment. Water Res 45(3):1432–1442. https://doi.org/10.1016/j.watres.2010.10.036
Asif MB, Hai FI, Kang J, van de Merwe JP, Leusch FDL, Price WE, Nghiem LD (2018) Biocatalytic degradation of pharmaceuticals, personal care products, industrial chemicals, steroid hormones and pesticides in a membrane distillation-enzymatic bioreactor. Biores Technol 247:528–536. https://doi.org/10.1016/j.biortech.2017.09.129
Chakraborty P, Shappell NW, Mukhopadhyay M, Onanong S, Rex KR, Snow D (2021) Surveillance of plasticizers, bisphenol A, steroids and caffeine in surface water of River Ganga and Sundarban wetland along the Bay of Bengal: occurrence, sources, estrogenicity screening and ecotoxicological risk assessment. Water Research 190:Article 116668. https://doi.org/10.1016/j.watres.2020.116668
Lv Y, ** J, Li R, Ma R, Huang W, Wang Y (2022) Photodegradation kinetics and solvent effect of new brominated flame retardants (NBFRS) in liquid medium. Int J Environ Res Public Health 19(18):11690–11690. https://doi.org/10.3390/ijerph191811690
Pavúková D, Fašková L, Melníková E, Mališová E, Híveš J, Štibrányi L, Hudec P, Naumowicz M, Gál M (2022) Removal of Environmentally harmful and hardly degradable pharmaceuticals sulfamethoxazole, diclofenac, and cetirizine by adsorption on activated charcoal. Water 14:3988. https://doi.org/10.3390/w14243988
Rampelotto PH (2013) Extremophiles and Extreme environments. Life 3(3):482–485. https://doi.org/10.3390/life3030482
Ye J-W, Lin Y-N, Yi X-Q, Yu Z-X, Liu X, Chen G-Q (2022) Synthetic biology of extremophiles: a new wave of biomanufacturing. Trends Biotechnol 41(3):342–357. https://doi.org/10.1016/j.tibtech.2022.11.010
Elleuche S, Schröder C, Sahm K, Antranikian G (2014) Extremozymes—biocatalysts with unique properties from extremophilic microorganisms. Curr Opin Biotechnol 29:116–123. https://doi.org/10.1016/j.copbio.2014.04.003
Merino N, Aronson HS, Bojanova DP, Feyhl-Buska J, Wong ML, Zhang S, Giovannelli D (2019) Living at the extremes: extremophiles and the limits of life in a planetary context. Front Microbiol 10:Article 780. https://doi.org/10.3389/fmicb.2019.00780
Wernick DG, Pontrelli S, Pollock AW, Liao JC (2016) Sustainable biorefining in wastewater by engineered extreme alkaliphile Bacillus marmarensis. Sci Rep 6(1):Article 20224. https://doi.org/10.1038/srep20224
Dhakar K, Pandey A (2016) Wide pH range tolerance in extremophiles: towards understanding an important phenomenon for future biotechnology. Appl Microbiol Biotechnol 100(6):2499–2510. https://doi.org/10.1007/s00253-016-7285-2
Trotsenko YA, Khmelenina VN (2002) Biology of extremophilic and extremotolerant methanotrophs. Arch Microbiol 177(2):123–131. https://doi.org/10.1007/s00203-001-0368-0
Vieille C, Zeikus GJ (2001) Hyperthermophilic enzymes: sources, uses, and molecular mechanisms for thermostability. Microbiol Mol Biol Rev 65(1):1–43. https://doi.org/10.1128/mmbr.65.1.1-43.2001
Zhang X, Lin Y, Chen GQ (2018) Halophiles as chassis for bioproduction. Adv Biosyst 2(11):Article 1800088. https://doi.org/10.1002/adbi.201800088
Atakav Y, Pinar O, Kazan D (2021) Investigation of the physiology of the obligate alkaliphilic Bacillus marmarensis GMBE 72T considering its alkaline adaptation mechanism for poly(3-hydroxybutyrate) synthesis. Microorganisms 9(2):462–462. https://doi.org/10.3390/microorganisms9020462
Śliżewska W, Struszczyk-Świta K, Marchut-Mikołajczyk O (2022) Metabolic potential of halophilic filamentous fungi—current perspective. Int J Mol Sci 23(8):Article 4189. https://doi.org/10.3390/ijms23084189
Bowers KJ, Mesbah NM, Wiegel J (2009) Biodiversity of poly-extremophilic bacteria: does combining the extremes of high salt, alkaline pH and elevated temperature approach a physico-chemical boundary for life? Saline Syst 5:Article 9. https://doi.org/10.1186/1746-1448-5-9
Zambare V, Bhalla A, Muthukumarappan K, Sani RK, Christopher LP (2011) Bioprocessing of agricultural residues to ethanol utilizing a cellulolytic extremophile. Extremophiles 15(5):611–618. https://doi.org/10.1007/s00792-011-0391-2
Zhu D, Adebisi WA, Ahmad F, Sethupathy S, Danso B, Sun J (2020) Recent development of extremophilic bacteria and their application in biorefinery. Front Bioeng Biotechnol 8:Article 483. https://doi.org/10.3389/fbioe.2020.00483
Demirjian DC, Morı́s-Varas F, Cassidy CS (2011) Enzymes from extremophiles. Curr Opin Chem Biol 5(2):144–151. https://doi.org/10.1016/s1367-5931(00)00183-6
Chong PL-G, Chang A, Yu A, Mammedova A (2022) Vesicular and planar membranes of archaea lipids: unusual physical properties and biomedical applications. Int J Mol Sci 23:7616. https://doi.org/10.3390/ijms23147616
Dumorne K, Cordova DC, Astorga-Elo M, Renganathan P (2017) Extremozymes: a potential source for industrial applications. J Microbiol Biotechnol 27(4):649–659. https://doi.org/10.4014/jmb.1611.11006
Marques CR (2018) Extremophilic microfactories: applications in metal and radionuclide bioremediation. Front Microbiol 9:Article 1191. https://doi.org/10.3389/fmicb.2018.01191
Ichiye T (2018) Enzymes from piezophiles. Semin Cell Dev Biol 84:138–146. https://doi.org/10.1016/j.semcdb.2018.01.004
Simair AA, Khushk I, Qureshi AS, Bhutto MA, Chaudhry HA, Ansari KA, Lu C (2017) Amylase production from thermophilic Bacillus sp. BCC 021–50 isolated from a marine environment. Fermentation 3:25. https://doi.org/10.3390/fermentation3020025
Gonzalez JM, Aranda B (2023) Microbial growth under limiting conditions-future perspectives. Microorganisms 11:1641. https://doi.org/10.3390/microorganisms11071641
** M, Gai Y, Guo X, Hou Y, Zeng R (2019) Properties and applications of extremozymes from deep-sea extremophilic microorganisms: a mini review. Marine Drugs 17(12):Article 656. https://doi.org/10.3390/md17120656
Moayad W, Zha G, Yan Y (2018) Metalophilic lipase from Ralstonia solanacearum: gene cloning, expression, and biochemical characterization. Biocatal Agric Biotechnol 13:31–37. https://doi.org/10.1016/j.bcab.2017.11.005
Czech L, Hermann L, Stöveken N, Richter A, Höppner A, Smits SHJ, Heider J, Bremer E (2018) Role of the extremolytes ectoine and hydroxyectoine as stress protectants and nutrients: genetics, phylogenomics, biochemistry, and structural analysis. Genes 9(4):Article 177. https://doi.org/10.3390/genes9040177
Engel MB, Catchpole HR (2005) A microprobe analysis of inorganic elements in. Cell Biol Int 29(8):616–622. https://doi.org/10.1016/j.cellbi.2005.03.024
Jeong S-W, Choi YJ (2020) Extremophilic microorganisms for the treatment of toxic pollutants in the environment. Molecules 25(21):Article 4916. https://doi.org/10.3390/molecules25214916
DasSarma S, DasSarma P (2015) Halophiles and their enzymes: negativity put to good use. Curr Opin Microbiol 25:120–126. https://doi.org/10.1016/j.mib.2015.05.009
Barrau C, Di Lorenzo F, Menes RJ, Lanzetta R, Molinaro A, Silipo A (2018) The structure of the lipid A from the halophilic bacterium spiribacter salinus M19–40T. Marine Drugs 16(4):Article 124. https://doi.org/10.3390/md16040124
Roberts MF (2005) Organic compatible solutes of halotolerant and halophilic microorganisms. Saline Syst 1(5):5. https://doi.org/10.1186/1746-1448-1-5
Baker-Austin C, Dopson M (2007) Life in acid: pH homeostasis in acidophiles. Trends Microbiol 15(4):165–171. https://doi.org/10.1016/j.tim.2007.02.005
Vergara E, Neira G, González C, Cortez D, Dopson M, Holmes DS (2020) Evolution of predicted acid resistance mechanisms in the extremely acidophilic Leptospirillum genus. Genes 11(4):Article 389. https://doi.org/10.3390/genes11040389
Aono R, Ito M, Machida T (1999) Contribution of the cell wall component teichuronopeptide to pH homeostasis and alkaliphily in the alkaliphile Bacillus lentus C-125. J Bacteriol 181(21):6600–6606. https://doi.org/10.1128/jb.181.21.6600-6606.1999
Matsuno T, Goto T, Ogami S, Morimoto H, Yamazaki K, Inoue N, Matsuyama H, Yoshimune K, Yumoto I (2018) Formation of proton motive force under low-aeration alkaline conditions in alkaliphilic bacteria. Front Microbiol 9:Article 2331. https://doi.org/10.3389/fmicb.2018.02331
Koga Y (2012) Thermal adaptation of the archaeal and bacterial lipid membranes. Archaea 2012:Article 789652. https://doi.org/10.1155/2012/789652
Ranawat P, Rawat S (2017) Stress response physiology of thermophiles. Arch Microbiol 199:391–414. https://doi.org/10.1007/s00203-016-1331-4
De Maayer P, Anderson D, Cary C, Cowan DA (2014) Some like it cold: understanding the survival strategies of psychrophiles. EMBO Rep 15(5):508–517. https://doi.org/10.1002/embr.201338170
Cavicchioli R, Charlton T, Ertan H, Omar SM, Siddiqui KS, Williams TJ (2011) Biotechnological uses of enzymes from psychrophiles. Microb Biotechnol 4(4):449–460. https://doi.org/10.1111/j.1751-7915.2011.00258.x
Mesbah NM (2022) Industrial biotechnology based on enzymes from extreme environments. Front Bioeng Biotechnol 10:Article 870083. https://doi.org/10.3389/fbioe.2022.870083
He H, Chen X, Li J, Zhang Y, Gao P (2004) Taste improvement of refrigerated meat treated with cold-adapted protease. Food Chem 84(2):307–311. https://doi.org/10.1016/s0308-8146(03)00242-5
Gumulya Y, Boxall NJ, Khaleque HN, Santala V, Carlson RP, Kaksonen AH (2018) In a quest for engineering acidophiles for biomining applications: challenges and opportunities. Genes 9(2):Article 116. https://doi.org/10.3390/genes9020116
Hong J, Silva RA, Park J, Lee E, Park J, Kim H (2016) Adaptation of a mixed culture of acidophiles for a tank biooxidation of refractory gold concentrates containing a high concentration of arsenic. J Biosci Bioeng 121(5):536–542. https://doi.org/10.1016/j.jbiosc.2015.09.009
Hassan S, Daryoush A, Sadegh OM (2011) Galena bio-oxidation by moderate sulphur bacterianktonic and biofilm-producing Staphylococcus aureus. African J Microbiol Res 5(24):4170–4174. https://doi.org/10.5897/ajmr11.626
Kaksonen AH, Deng X, Morris C, Khaleque HN, Zea L, Gumulya Y (2021) Potential of acidithiobacillus ferrooxidans to grow on and bioleach metals from mars and lunar regolith simulants under simulated microgravity conditions. Microorganisms 9(12):Article 2416. https://doi.org/10.3390/microorganisms9122416
Jameson E, Rowe OF, Hallberg KB, Johnson DB (2010) Sulfidogenesis and selective precipitation of metals at low pH mediated by Acidithiobacillus spp. and acidophilic sulfate-reducing bacteria. Hydrometallurgy 104(3–4):488–493. https://doi.org/10.1016/j.hydromet.2010.03.029
Okibe N, Maki M, Nakayama D, Sasaki K (2016) Microbial recovery of vanadium by the acidophilic bacterium Acidocella aromatica. Biotechnol Lett 38:1475–1481. https://doi.org/10.1007/s10529-016-2131-2
Kristanti RA, Bunrith S, Kumar R, Mohamed AO (2023) Municipal wastewater treatment technologies in malaysia: a short review. Ind Domest Waste Manage 3:38–46. https://doi.org/10.53623/idwm.v3i1.243
Rajasulochana P, Preethy V (2016) Comparison on efficiency of various techniques in treatment of waste and sewage water – a comprehensive review. Resource-Efficient Technol 2(4):175–184. https://doi.org/10.1016/j.reffit.2016.09.004
Almeida-Naranjo CE, Guerrero VH, Villamar Ayala CA (2023) Emerging contaminants and their removal from aqueous media using conventional/non-conventional adsorbents: a glance at the relationship between materials, processes, and technologies. Water 15:Article 1626. https://doi.org/10.3390/w15081626
Sharma P, Bano A, Singh SP, Dubey NK, Chandra R, Iqbal HMN (2022) Recent advancements in microbial-assisted remediation strategies for toxic contaminants. Clean Chem Eng 2:Article 100020. https://doi.org/10.1016/j.clce.2022.100020
Abdulrazaq Y, Abdulsalam A, Larayetan Rotimi A, Aliyu Abdulbasit A, Clifford O, Abdulazeez Abdulsalam O et al (2020) Classification, potential routes and risk of emerging pollutants/contaminant. IntechOpen. https://doi.org/10.5772/intechopen.94447
Hadibarata T, Kristanti RA, Bilal M, Yilmaz M, Sathishkumar P (2023) Biodegradation mechanism of chlorpyrifos by halophilic bacterium Hortaea sp. B15. Chemosphere 312:Article 137260. https://doi.org/10.1016/j.chemosphere.2022.137260
Pham VHT, Kim J, Chang S, Bang D (2023) Investigating Bio-inspired degradation of toxic dyes using potential multi-enzyme producing extremophiles. Microorganisms 11(5):Article 1273. https://doi.org/10.3390/microorganisms11051273
Liu N, Liu Z, Song D (2019) Degradation characteristics of catechol and sodium benzoate by a petroleum-degrading bacterium. Biotechnol Bull 35(9):156–164. https://doi.org/10.13560/j.cnki.biotech.bull.1985.2019-0578
Li H, Meng F, Duan W, Lin Y, Zheng Y (2019) Biodegradation of phenol in saline or hypersaline environments by bacteria: a review. Ecotoxicol Environ Safety 184:Article 109658. https://doi.org/10.1016/j.ecoenv.2019.109658
Sysoev M, Grötzinger SW, Renn D, Ep**er J, Rue** M, Karan R (2021) Bioprospecting of novel extremozymes from prokaryotes—the advent of culture-independent methods. Front Microbiol 12:Article 630013. https://doi.org/10.3389/fmicb.2021.630013
El-Gendi H, Saleh AK, Badierah R, Redwan EM, El-Maradny YA, El-Fakharany EM (2022) A comprehensive insight into fungal enzymes: structure, classification, and their role in mankind’s challenges. J Fungi 8:23. https://doi.org/10.3390/jof8010023
Napolitano G, Fasciolo G, Venditti P (2022) The ambiguous aspects of oxygen. Oxygen 2:382–409. https://doi.org/10.3390/oxygen2030027
Xu M, He L, Sun P, Wu M, Cui X, Liu D, Adomako-Bonsu AG, Geng M, **ong G, Guo L et al (1969) Critical role of monooxygenase in biodegradation of 2,4,6-trinitrotoluene by Buttiauxella sp. S19–1. Molecules 2023:28. https://doi.org/10.3390/molecules28041969
Matsui T, Nambu S, Goulding CW, Takahashi S, Fujii H, Ikeda-Saito M (2016) Unique coupling of mono- and dioxygenase chemistries in a single active site promotes heme degradation. Proc Natl Acad Sci U S A 113(14):3779–3784. https://doi.org/10.1073/pnas.1523333113
Torres Pazmiño DE, Winkler M, Glieder A, Fraaije MW (2010) Monooxygenases as biocatalysts: classification, mechanistic aspects and biotechnological applications. J Biotechnol 146(1–2):9–24. https://doi.org/10.1016/j.jbiotec.2010.01.021
Ang T-F, Maiangwa J, Salleh AB, Normi YM, Leow TC (2018) Dehalogenases: from improved performance to potential microbial dehalogenation applications. Molecules 23:1100. https://doi.org/10.3390/molecules23051100
Ahmed AT, Othman MA, Sarwade VD, Kachru GR (2012) Degradation of anthracene by alkaliphilic bacteria Bacillus badius. Environ Pollut 1(2):97–104. https://doi.org/10.5539/ep.v1n2p97
García-Hernández MA, Villarreal-Chiu JF, Garza-González MT (2017) Metallophilic fungi research: an alternative for its use in the bioremediation of hexavalent chromium. Int J Environ Sci Technol 14:2023–2038. https://doi.org/10.1007/s13762-017-1348-5
Bezalel L, Shoham Y, Rosenberg E (1993) Characterization and delignification activity of a thermostable α-L–arabinofuranosidase from Bacillus stearothermophilus. Appl Microbiol Biotechnol 40:57–62. https://doi.org/10.1007/bf00170429
Dachuri V, Jang S-H, Lee C (2022) Different effects of salt bridges near the active site of cold-adapted Proteus mirabilis lipase on thermal and organic solvent stabilities. Catalysts 12:761. https://doi.org/10.3390/catal12070761
Lam SY, Yeung RCY, Yu TH, Sze KH, Wong KB (2011) A rigidifying salt-bridge favors the activity of thermophilic enzyme at high temperatures at the expense of low-temperature activity. PLoS Biol 9(3):e1001027. https://doi.org/10.1371/journal.pbio.1001027
Finch AJ, Kim JR (2018) Thermophilic proteins as versatile scaffolds for protein engineering. Microorganisms 6(4):97. https://doi.org/10.3390/microorganisms6040097
Rapuano R, Graziano G (2022) Some clues about enzymes from psychrophilic microorganisms. Microorganisms 10:1161. https://doi.org/10.3390/microorganisms10061161
Cipolla A, D’Amico S, Barumandzadeh R, Matagne A, Feller G (2011) Stepwise adaptations to low temperature as revealed by multiple mutants of psychrophilic α-amylase from antarctic bacterium. J Biol Chem 286:38348–38355. https://doi.org/10.1074/jbc.M111.274423
Gallo G, Puopolo R, Carbonaro M, Maresca E, Fiorentino G (2021) Extremophiles, a nifty tool to face environmental pollution: from exploitation of metabolism to genome engineering. Int J Environ Res Public Health 18(10):5228. https://doi.org/10.3390/ijerph18105228
Lin X, Yang B, Shen J, Du N (2009) Biodegradation of crude oil by an arctic psychrotrophic bacterium pseudoalteromomas sp. P29. Curr Microbiol 59:341–345. https://doi.org/10.1007/s00284-009-9440-9
Tambat VS, Patel AK, Chen C-W, Raj T, Chang J-S, Singhania RR, Dong C-D (2023) A sustainable vanadium bioremediation strategy from aqueous media by two potential green microalgae. Environ Pollut 323:Article 21247. https://doi.org/10.1016/j.envpol.2023.121247
Benítez-Mateos A, Paradisi F (2023) Halomonas elongata: a microbial source of highly stable enzymes for applied biotechnology. Appl Microbiol Biotechnol 107:1–8. https://doi.org/10.1007/s00253-023-12510-7
Agarwal PK (2006) Enzymes: an integrated view of structure, dynamics and function. Microbial Cell Fact 5:Article 2. https://doi.org/10.1186/1475-2859-5-2
Mestre AS, Carvalho AP (2019) Photocatalytic degradation of pharmaceuticals carbamazepine, diclofenac, and sulfamethoxazole by semiconductor and carbon materials: a review. Molecules 24:3702. https://doi.org/10.3390/molecules24203702
Tudi M, Li H, Li H, Wang L, Lyu J, Yang L, Tong S, Yu QJ, Ruan HD, Atabila A et al (2022) Exposure routes and health risks associated with pesticide application. Toxics 10:335. https://doi.org/10.3390/toxics10060335
Roman DL, Voiculescu DI, Filip M, Ostafe V, Isvoran A (2021) Effects of triazole fungicides on soil microbiota and on the activities of enzymes found in soil: a review. Agriculture 11:893. https://doi.org/10.3390/agriculture11090893
Santander Ballestín S, Luesma Bartolomé MJ (2023) Toxicity of different chemical components in sun cream filters and their impact on human health: a review. Appl Sci 13:712. https://doi.org/10.3390/app13020712
Juliano C, Magrini GA (2017) Cosmetic ingredients as emerging pollutants of environmental and health concern. A Mini-Review Cosmet 4:11. https://doi.org/10.3390/cosmetics4020011
Potărniche I-A, Saroși C, Terebeș RM, Szolga L, Gălătuș R (2023) Classification of food additives using UV spectroscopy and one-dimensional convolutional neural network. Sensors 23:7517. https://doi.org/10.3390/s23177517
Radulescu M-C, Bucur B, Bucur M-P, Radu GL (2014) Bienzymatic biosensor for rapid detection of aspartame by flow injection analysis. Sensors 14:1028–1038. https://doi.org/10.3390/s140101028
Basu B (2022) The radiophiles of Deinococcaceae family: resourceful microbes for innovative biotechnological applications. Curr Res Microb Sci 3:Article 100153. https://doi.org/10.1016/j.crmicr.2022.100153
Niyonzima FN (2018) Detergent-compatible bacterial cellulases. J Basic Microbiol 59(2):134–147. https://doi.org/10.1002/jobm.201800436
Nadaroglu H, Polat M S (2021) 6. Microbial extremozymes: novel sources and industrial applications. In: Kuddus M (ed) Microbial extremozymes: novel sources and industrial applications. Academic Press, pp 67–82
Lade H, Kadam A, Paul D, Govindwar S (2015) Biodegradation and detoxification of textile azo dyes by bacterial consortium under sequential microaerophilic/aerobic processes. EXCLI J 14:158–174. https://doi.org/10.17179/excli2014-642
Wang C, Huang Y, Zhang Z, Wang H (2018) Salinity effect on the metabolic pathway and microbial function in phenanthrene degradation by a halophilic consortium. AMB Exp 8(1):Article 67. https://doi.org/10.1186/s13568-018-0594-3
Al Farraj DA, Hadibarata T, Yuniarto A, Syafiuddin A, Surtikanti HK, Elshikh MS, Al Khulaifi MM, Al-Kufaidy R (2019) Characterization of pyrene and chrysene degradation by halophilic Hortaea sp. B15. Bioprocess Biosyst Eng 42:963–969. https://doi.org/10.1007/s00449-019-02096-8
Kristanti RA, Hadibarata T, Al Farraj DA, Elshikh MS, Alkufeidy RM (2018) Biodegradation mechanism of phenanthrene by halophilic hortaea sp. B15. Water Air Soil Pollut 229:Article 324. https://doi.org/10.1007/s11270-018-3969-9
Arakaki R, Monteiro DA, Boscolo M, da Silva R, Gomes E (2013) Halotolerance, ligninase production and herbicide degradation ability of basidiomycetes strains. Braz J Microbiol 44(4):1207–1214. https://doi.org/10.1590/s1517-83822014005000014
Dalvi S, Nicholson CA, Najar FZ, Roe BA, Canaan P, Hartson SD, Fathepure BZ (2014) Arhodomonas sp. strain seminole and its genetic potential to degrade aromatic compounds under high-salinity conditions. Appl Environ Microbiol 80(21):6664–6676. https://doi.org/10.1128/aem.01509-14
Choi M, Yun T, Song MJ, Kim J, Lee B-H, Löffler FE, Yoon S (2021) Cometabolic vinyl chloride degradation at acidic pH catalyzed by acidophilic methanotrophs isolated from alpine peat bogs. Environ Sci Technol 55(9):5959–5969. https://doi.org/10.1021/acs.est.0c08766
Christen P, Vega A, Casalot L, Simon G, Auria R (2012) Kinetics of aerobic phenol biodegradation by the acidophilic and hyperthermophilic archaeon Sulfolobus solfataricus 98/2. Biochem Eng J 62:56–61. https://doi.org/10.1016/j.bej.2011.12.012
Shao Y (2019) Cometabolic biodegradation of groundwater contaminants by acidophilic methanotrophs. Doctoral dissertation, Texas A&M University, College Station, December 2019. https://uniskills.library.curtin.edu.au/assets/docs/APA_7th_referencing_guide_220726.pdf
Schelert J, Rudrappa D, Johnson T, Blum P (2013) Role of MerH in mercury resistance in the archaeon Sulfolobus solfataricus. Microbiology 159(Pt_6):1198–1208. https://doi.org/10.1099/mic.0.065854-0
Asoodeh A, Chamani J, Lagzian M (2010) A novel thermostable, acidophilic α-amylase from a new thermophilic “Bacillus sp. Ferdowsicous” isolated from Ferdows hot mineral spring in Iran: purification and biochemical characterization. Int J Biol Macromol 46(3):289–297. https://doi.org/10.1016/j.ijbiomac.2010.01.013
Shukla SK, Hariharan S, Rao TS (2020) Uranium bioremediation by acid phosphatase activity of Staphylococcus aureus biofilms: can a foe turn a friend? J Hazard Mater 384:Article 121316. https://doi.org/10.1016/j.jhazmat.2019.121316
Kanekar PP, Sarnaik SS, Kelkar AS (1998) Bioremediation of phenol by alkaliphilic bacteria isolated from alkaline lake of Lonar, India. J Appl Microbiol 85(S1):128S133S. https://doi.org/10.1111/j.1365-2672.1998.tb05291.x
Sarwade V, Gawai K (2014) Biodegradation of aniline by alkaliphilic strain Bacillus badius D1. IOSR J Environ Sci Toxicol Food Technol 8(5):71–78. https://doi.org/10.9790/2402-08527178
Maltseva O, McGowan C, Fulthorpe R, Oriel P (1996) Degradation of 2,4-dichlorophenoxyacetic acid by haloalkaliphilic bacteria. Microbiology 142(5):1115–1122. https://doi.org/10.1099/13500872-142-5-1115
Sridharan R, Krishnaswamy VG, Archana KM, Rajagopal R, Kumar DT, Doss CGP (2021) Integrated approach on azo dyes degradation using laccase enzyme and Cul nanoparticle. SN Appl Sci 3:Article 370. https://doi.org/10.1007/s42452-021-04164-9
Wicher K, Abou-Hachem M, Halldórsdóttir S, Thorbjarnadóttir S, Eggertsson G, Hreggvidsson G, Nordberg Karlsson E, Holst O (2001) Deletion of a cytotoxic, N-terminal putative signal peptide results in a significant increase in production yields in Escherichia coli and improved specific activity of Cel12A from Rhodothermus marinus. Appl Microbiol Biotechnol 55:578–584. https://doi.org/10.1007/s002530000559
Kaur G, Kumar S, Satyanarayana T (2004) Production, characterization and application of a thermostable polygalacturonase of a thermophilic mould Sporotrichum thermophile Apinis. Biores Technol 94(3):239–243. https://doi.org/10.1016/j.biortech.2003.05.003
Boyadzhieva I, Atanasova N, Paunova-Krasteva T, Kambourova M (2022) Ɛ-Polycaprolactone degradation by a thermostable lipase isolated from Brevibacillus thermoruber strain 7. Research Square. Preprint. https://doi.org/10.21203/rs.3.rs-2304161/v1
Qiao W, Zhang Y, **a H, Luo Y, Liu S, Wang S, Wang W (2019) Bioimmobilization of lead by Bacillus subtilis X3 biomass isolated from lead mine soil under promotion of multiple adsorption mechanisms. Roy Soc Open Sci 6(2):Article 181701. https://doi.org/10.1098/rsos.181701
Llorens I, Untereiner G, Jaillard D, Gouget B, Chapon V, Carriere M (2012) Uranium Interaction with two multi-resistant environmental bacteria: Cupriavidus metallidurans CH34 and Rhodopseudomonas palustris. PLoS ONE 7(12):Article e51783. https://doi.org/10.1371/journal.pone.0051783
Palanivel TM, Pracejus B, Novo LAB (2023) Bioremediation of copper using indigenous fungi Aspergillus species isolated from an abandoned copper mine soil. Chemosphere 314:Article 137688. https://doi.org/10.1016/j.chemosphere.2022.137688
Al-Dhabi NA, Esmail GA, Mohammed Ghilan A-K, Valan Arasu M (2019) Optimizing the management of cadmium bioremediation capacity of metal-resistant pseudomonas sp. strain Al-Dhabi-126 isolated from the industrial city of Saudi Arabian environment. Int J Environ Res Public Health 16(23):Article 4788. https://doi.org/10.3390/ijerph16234788
Wang X, Li D, Gao P, Gu W, He X, Yang W, Tang W (2020) Analysis of biosorption and biotransformation mechanism of Pseudomonas chengduensis strain MBR under Cd(II) stress from genomic perspective. Ecotoxicol Environ Safety 198:Article 110655. https://doi.org/10.1016/j.ecoenv.2020.110655
Acknowledgements
This research was supported by the Ministry of Higher Education, Malaysia, under the Fundamental Research Grant Scheme (FRGS/1/2022/TK06/CURTIN/02/4). The authors are grateful to the Sarawak Meteorological Department for providing the data necessary to complete this research. This research was also supported by the Higher Research Degree Scholarship (HRD) of Curtin University, Miri, Sarawak, Malaysia.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This research was funded by the Ministry of Higher Education, Malaysia, under the Fundamental Research Grant Scheme (FRGS/1/2022/TK06/CURTIN/02/4).
Author information
Authors and Affiliations
Contributions
XKC: original draft preparation, methodology, data curation. TH: original draft preparation, writing—review and editing, funding acquisition, validation, supervision. MNHJ: visualization, funding acquisition, supervision, LIS: review and editing, IST: visualization, supervision. HCYF: visualization, supervision.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Informed Consent Statement
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: “In this article, the Acknowledgements section was incorrectly given as “The authors are grateful to the Sarawak Meteorological Department for providing the data necessary to complete this research. This research was supported by the Higher Research Degree Scholarship (HRD) of Curtin University, Miri, Sarawak, Malaysia” But should have been “This research was supported by the Ministry of Higher Education, Malaysia, under the Fundamental Research Grant Scheme (FRGS/1/2022/TK06/ CURTIN/02/4). The authors are grateful to the Sarawak Meteorological Department for providing the data necessary to complete this research. This research was also supported by the Higher Research Degree Scholarship (HRD) of Curtin University, Miri, Sarawak, Malaysia”. And the statement in the Funding information section was incorrectly given as “This research was funded by Fundamental Research Grant Scheme, Ministry of High Education Malaysia No. 1134 and Curtin Malaysia Postgraduates Research Scheme 2022, Curtin University Malaysia.” and should have read “This research was funded by the Ministry of Higher Education, Malaysia, under the Fundamental Research Grant Scheme (FRGS/1/2022/TK06/ CURTIN/02/4).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chia, X.K., Hadibarata, T., Jusoh, M.N.H. et al. Role of Extremophiles in Biodegradation of Emerging Pollutants. Top Catal (2024). https://doi.org/10.1007/s11244-024-01919-7
Accepted:
Published:
DOI: https://doi.org/10.1007/s11244-024-01919-7