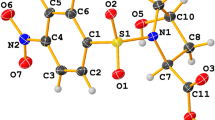
Abstract
Based on the X-ray crystallography and 1H NMR spectroscopy data and quantum chemical studies, it was found that 1(11)H-2, 3, 4, 5-tetrahydro[1, 3]diazepino[1, 2-a]benzimidazole (1) exists almost exclusively in the 1H-prototropic form. To prepare the fixed 11H-diazepinobenzimidazole forms of 1, 1-R-2-(4-chlorobutylamino)benzimidazoles (R = Me, N=CHAr) were synthesized, which underwent thermal cyclization with the formation of a mixture of 11-Rsubstituted diazepine 1 and 1-R-2-(pyrrolidin-1-yl)benzimidazole. Alkylation of diazepine 1 in a neutral medium regioselectively gave 11-R-diazepinobenzimidazoles in high yield. Their 1-substituted isomers were obtained by carrying out this reaction in the system NaH—THF. The N(11)-derivatives of diazepinobenzimidazole 1 were found to inhibit dipeptidyl peptidase 4 (DPP-4), but less actively than a comparator drug sitagliptin. The compounds under study did not exhibit antiglycation action in vitro and virtually did not affect activity of α-glucosidase and glycogen phosphorylase. However, they are characterized by a strong antiaggregant effect, making these derivatives promising for further studies.
Similar content being viewed by others
References
V. A. Anisimova, V. V. Kuźmenko, T. A. Kuźmenko, A. S. Morkovnik, Russ. Chem. Bull. (Int. Ed.), 2007, 56, 2315 [Izv. Akad. Nauk, Ser. Khim., 2007, 2237].
Pat. Fr. 2691462; http://v3.espacenet.com.
Pat. RF, 2061481; Byul. isobret. [Invention Bull.], 1996, No. 20 (in Russian).
V. A. Anisimova, I. E. Tolpygin, A. A. Spasov, V. A. Kosolapov, A. V. Stepanov, A. F. Kucheryavenko, Pharm. Chem. J. (Engl. Transl.), 2006, 40, 261 [Khim. Farm. Zh., 2006, 40, No. 5, 27].
V. A. Anisimova, A. A. Spasov, V. A. Kosolapov, I. E. Tolpygin, E. V. Tibiŕkova, O. A. Salaznikova, V. A. Kuznetsova, N. A. Gurova, K. V. Lenskaya, D. S. Yakovlev, D. V. Mal´tsev, N. A. Kolobrodova, T. M. Mitina, O. Yu. Grechko, Pharm. Chem. J. (Engl. Transl.), 2013, 46, 647 [Khim. Farm. Zh., 2012, 46, No. 11, 15].
Pat. RF No. 2386634; Byul. isobret. [Invention Bull.], 2010, No. 11 (in Russian).
V. I. Minkin, L. P. Olekhnovich, Yu. A. Zhdanov, Molekulyarnyi dizain tautomernykh sistem [Molecular Design of Tautomeric Systems], RGU Publ., Rostov-on-Don, 1977, p. 18 (in Russian).
S. Takeuchi, T. Tahara, Proc. Natl. Acad. Sci. USA, 2007, 104, 5285.
J. C. Hargis, E. Vöhringer-Martinez, H. L. Woodcock, A. Toro-Labbé, H. F. Schaefer, J. Phys. Chem. A, 2011, 115, 2650.
H. Lim, S.-Y. Park, D.-J. Jang, J. Phys. Chem. A, 2010, 114, 11432.
A. F. Pozharskii, Teoreticheskie osnovy khimii geterotsiklov [Basic Theoretical Chemistry of Heterocycles], Khimiya, Moscow, 1985, 279 pp. (in Russian).
N. E. Gel´man, E. A. Terentéva, T. M. Shanina, L. M. Kiparenko, Metody kolichestvennogo organicheskogo elementnogo analiza [Methods of Quantitative Organic Elemental Analysis], Khimiya, Moscow, 1987 (in Russian).
A. V. El´tsov, K. M. Krivozheiko, M. V. Kolesova, Zh. Org. Khim., 1967, 3, 1518 (in Russian).
T. A. Kuźmenko, V. V. Kuźmenko, A. F. Pozharskii, A. M. Simonov, Chem. Heterocycl. Compd. (Engl. Transl.), 1988, 24, 880 [Khim. Geterotsikl. Soedin., 1988, 1070].
V. G. Malkin, O. L. Malkina, M. E. Casida, D. R. Salahub, J. Am. Chem. Soc., 1994, 116, 5898.
A. M. Köster, R. Flores-Moreno, G. Geudtner, A. Goursot, T. Heine, J. U. Reveles, S. Patchkovskii, A. Vela, D. R. Salahub, DeMon2K 1.0.4. The deMon developers, NRC, Canada, 2004; http://www.demon-software.com.
A. A. Granovsky, http://classic.chem.msu.su/gran/gamess/index.html.
M. W. Schmidt, K. K. Baldridge, J. A. Boatz, S. T. Elbert, M. S. Gordon, J. H. Jensen, Sh. Koseki, N. Matsunaga, K. A. Nguyen, Sh. Su, T. L. Windus, M. Dupuis, J. A. Montgomery, J. Comp. Chem., 1993, 14, 1347.
K. K. Irikura, R. D. Johnson III, R. N. Kacker, J. Phys. Chem. A, 2005, 109, 8430.
SAINT. Version 6.02A, Bruker AXS, Inc., Madison (WI), 2001.
SHELXTL-Plus. Version 5.10, Bruker AXS, Inc., Madison (WI), 1997.
S. Yogisha, K. A. Raveesha, J. Nat. Prod., 2010, 3, 76.
L. J. Yu, Y. Chen, J. L. Treadway, J. Pharmacol. Exp. Ther., 2006, 3, 1230.
J. M. Hayes, A. L. Kantsadi, D. D. Leonidas, Phytochem. Rev., 2014, 13, 471.
Z. A. Gabbasov, E. G. Popov, I. Yu. Gavrilova, E. Ya. Pozin, R. A. Markosyan, Laboratornoe delo [Laboratory Practice], 1989, 10, 15 (in Russian).
B. Elya, K. Basah, A. Muńim, W. Yuliastuti, A. Bangun, E. K. Septiana, J. Biomed. Biotechnol., 2012; Article ID 281078; doi: 10.1155/2012/281078.
A. Jedsadayanmata, Naresuan Univ. J., 2005, 13, 35.
A. N. Mironov, Rukovodstvo po provedeniyu doklinicheskikh issledovanii lekarstvennykh sredstv [Guidance on Conducting Preclinical Studies of Pharmaceutical Agents], Part 1, Grif and K, Moscow, 2012, 944 pp. (in Russian).
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Academician of the Russian Academy of Sciences N. S. Zefirov on the occasion of his 80th birthday.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 11, pp. 2622—2631, November, 2015.
Rights and permissions
About this article
Cite this article
Morkovnik, A.S., Spasov, A.A., Kuz’menko, T.A. et al. Prototropic equilibrium in 1(11)H-2, 3, 4, 5-tetrahydro[1, 3]diazepino[1, 2-a]benzimidazole, synthesis and pharmacological properties of its N-substituted derivatives. Russ Chem Bull 64, 2622–2631 (2015). https://doi.org/10.1007/s11172-015-1200-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-015-1200-3