Abstract
Purpose
The optimal number of lymph nodes to be resected in patients with rectal cancer who undergo radical surgery after neoadjuvant therapy remains controversial. This study evaluated the prognostic variances between elderly and non-elderly patients and determined the ideal number of lymph nodes to be removed in these patients.
Methods
The Surveillance, Epidemiology, and End Results (SEER) datasets were used to gather information on 7894 patients diagnosed with stage T3-4/N+ rectal cancer who underwent neoadjuvant therapy from 2010 to 2019. Of these patients, 2787 were elderly and 5107 were non-elderly. A total of 152 patients from the Longyan First Affiliated Hospital of Fujian Medical University were used for external validation. Overall survival (OS) and cancer-specific survival (CSS) were evaluated to determine the optimal quantity of lymph nodes for surgical resection.
Results
The study found significant differences in OS and CSS between elderly and non-elderly patients, both before and after adjustment for confounders (P < 0.001). The removal of 14 lymph nodes may be considered a benchmark for patients with stage T3-4/N+ rectal cancer who undergo radical surgery following neoadjuvant therapy, as this number provides a more accurate foundation for the personalized treatment of rectal cancer. External data validated the differences in OS and CSS and supported the 14 lymph nodes as a new benchmark in these patients.
Conclusion
For patients with T3-4/N+ stage rectal cancer who undergo radical surgery following neoadjuvant therapy, the removal of 14 lymph nodes serves as a cutoff point that distinctly separates patients with a favorable prognosis from those with an unfavorable one.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal carcinoma (CRC) is one of the most common malignancies worldwide, characterized by high incidence and mortality. Up-to-date statistics from the USA show that, although the overall incidence of CRC is decreasing, an increasing number of cases are being diagnosed at an advanced stage. In addition, the incidence of CRC has increased in the younger population [1].
The management of advanced rectal cancer has changed dramatically over the past 40 years, thanks to new adjuvant and neoadjuvant therapies that complement surgical procedures. These therapies have transformed the approach to treatment, moving away from a traditional surgical approach to a more multifaceted and effective treatment method. Neoadjuvant therapy not only improves the success rate of radical surgery, but also effectively reduces local recurrence and increases the chance of sphincter preservation. Currently, the combination of neoadjuvant therapy and radical surgery is the standard treatment protocol for advanced rectal cancer [2].
In contrast to the declining incidence and mortality rates of CRC in patients over 50 years of age, an increase in incidence has been observed in the population under 50 years of age [3]. Five-year survival rates for CRC vary in patients of different ages [4], with patients over 60 years of age having lower contemporaneous survival rates than younger patients [5], highlighting the need for different management of CRC patients in different age groups.
Although the extent of local lymph node involvement is an important criterion in CRC staging and prognosis [6], there remains debate over the optimal number of lymph nodes to examine in patients with rectal cancer following neoadjuvant therapy. The American Joint Committee on Cancer (AJCC) [7] and the College of American Pathologists (CAP) recommend that at least 12 lymph nodes should be examined for precise staging of cancer [8]. For optimal lymph nodes examination, studies suggested that more examined lymph nodes are associated with better staging and survival [9]. Other research shows that retrieving fewer lymph nodes following preoperative chemoradiotherapy for rectal cancer does not necessarily mean that the surgery was incomplete [10]. It is clear that the accurate examination of lymph nodes plays a critical role in CRC staging. As a result, the determination of the ideal number of lymph nodes to be removed during radical surgery following neoadjuvant therapy is of significant clinical relevance.
This study was designed to investigate the optimal resection number of lymph nodes and to determine the risk factors affecting prognosis in patients with stage T3-4/N+ rectal cancer who received neoadjuvant therapy. We conducted extensive analyses of overall survival (OS) and cancer-specific survival (CSS) in elderly and non-elderly rectal cancer patients receiving neoadjuvant therapy. To verify the reliability of our findings, we validated these results with an external cohort. Our study utilized data from the Surveillance, Epidemiology and End Results (SEER) database.
Materials and methods
Included participants
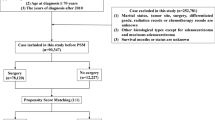
This study used a retrospective cohort design with information on patients with single primary tumor T3-4/N+ rectal cancer selected from the SEER database of data from 2010 to 2019. Data were screened and extracted by using SEER*Stat software version 8.4.2. Inclusion criteria were as follows: (1) T3-4/N+ rectal cancer diagnosis and staging based on the TNM staging system, with tumor type and histological classification based on the International Classification of Diseases of Oncology, Third Edition (ICD-O-3); (2) diagnosis was made between 2010 and 2019; (3) there were valid follow-up data, and the cause of death of the deceased patients was confirmed; and (4) they received radical surgical treatment. Exclusion criteria: non-primary tumor patients, uncertain pathological diagnosis, follow-up data invalid, tumors in the appendix or an unknown location, and unclear pathological grading and tumor size. The number of lymph nodes is unclear, as is the grading of the tumor according to the AJCC (8th edition) was excluded from the study. For the purposes of this study, we collected information on each patient. This included diagnostic year, age of patients, sex, tumor stage and grade, size of the tumor, the total number of lymph nodes removed, extent of the regional lymph nodes, marriage status, carcinoembryonic antigen CEA pretreatment level, marital status, perineural infiltration (PNI), whether they had received postoperative chemotherapy or radiotherapy, tumor deposit, and the number of months they survived. Patients missing data on any variable will be excluded from the study. The reporting recommendations (STROBE) are as required by the Guidelines for Reporting.
Data extraction
In this study, we analyzed the data of 7894 patients. The marriage status was categorized as married and unmarried. Unmarried included those who were widowed, divorced, separated, or single. The results from the X-tile procedure were used to categorize the number of lymph nodes removed (nLN) into two groups: ≥ 12 and < 12. Additionally, tumor size was classified as ≥ 5 cm and < 5 cm (Fig. 1). To validate the study model, data were collected from a group of patients with T3-4/N+ rectal cancer from the Longyan First Affiliated Hospital of Fujian Medical University. The study was conducted in accordance with the principles outlined in the Declaration of Helsinki and received ethical approval from the Ethics Committee of the Longyan First Affiliated Hospital of Fujian Medical University under approval number LYREC2024-k028-01.
To mitigate potential sources of bias in this paper, we conducted rigorous data screening in the SEER database during data collection to ensure that the data was diverse, comprehensive, and balanced. We also made efforts to avoid biases in data collection. During data preprocessing, we cleaned and screened the database to remove outliers and duplicate data, among other operations, to avoid interference in the analysis results. Finally, we validated the model through external verification to confirm its feasibility.
Statistical analysis
This study aimed to evaluate two primary survival endpoints: cancer-specific survival (CSS) and overall survival (OS). CSS was defined as the duration from the date of diagnosis to death caused by metastasis, recurrence, or related factors, or until the conclusion of the follow-up period. OS was defined as the duration from the patient’s date of diagnosis to death from any cause. The study focused on CSS, while OS served as a secondary outcome. The authors compared categorical variables using Fisher’s exact test and continuous variables using t-tests. Survival curves for OS and CSS were estimated using the Kaplan-Meier method, with between-group differences assessed using log-rank tests. Univariate and multivariate Cox proportional risk models were used to explore prognostic factors associated with OS and CSS. To address the issue of unequal baseline data, the authors employed propensity score matching (PSM), which allowed them to balance various important covariates, including age, race, tumor grade, tumor location, number of harvested lymph nodes, extent of regional lymph nodes examined, marital status, preoperative CEA level, tumor size, and marital status. All statistical analyses were conducted using R software (version 4.3.1).
Results
Patient characteristics
A total of 7894 patients diagnosed with T3-4/N+ rectal cancer who were receiving neoadjuvant therapy were divided into two groups: the elderly patient group (n = 2787) and the non-elderly patient group (n = 5107). The baseline characteristics of the two groups of patients are presented in Table 1. Compared to the non-elderly group, the elderly group had significantly higher (P < 0.05) tumor stage, pN, proportion receiving chemotherapy, pre-treatment CEA level, number of lymph nodes resected, proportions of tumor size < 5 cm, and peripheral nerve invasion (PNI). To adjust for potential confounders between patient groups, we applied a propensity score matching (PSM) method; Table 2 shows the baseline characteristics after matching. After matching, the two groups showed no significant differences (P > 0.05) in all the factors studied, suggesting that propensity score matching effectively balanced the baseline characteristics between the two groups.
Survival analysis in non-elderly and elderly T3-4/N+ rectal cancer patients
Before PSM, The non-elderly group had higher cancer-specific survival (CSS) and overall survival (OS) rates than the elderly group, (CSS 83.8% vs. 80.0%, respectively, P < 0.001; OS 80.2% vs. 68.1%, P < 0.001) (Fig. 2A, C). After PSM, CSS and OS rates remained; the elderly group had a lower percentage than the non-elderly group (CSS 80.3% vs. 83.8%, P < 0.001; OS 68.6% vs. 80.1%, P < 0.001) (Fig. 2B, D).
Analysis of optimal number of lymph nodes removed and survival rates
This study, which analyzed patients with T3-4/N+ rectal cancer after neoadjuvant therapy using the survminer R package, identified 14 lymph nodes as the optimal threshold for resection (Fig. 3A). The survival analysis clearly demonstrated that patients with 14 or more resected lymph nodes had a significantly higher CSS than those with fewer than 14 lymph nodes resected (86.0% vs. 80.7%, P < 0.005, Fig. 3B). The conventional threshold of 12 lymph nodes is clear: patients with 12 or more had a higher chance of survival than those with less than 12 (85.5% vs. 78.8%, P < 0.05, Fig. 3C). There was no significant difference observed in cancer-specific survival (CSS) between the group with 14 or more lymph nodes removed and the group with 12 or more lymph nodes removed (P = 0.41, Fig. 3D). To validate these findings, an additional 152 patients from the Longyan First Affiliated Hospital of Fujian Medical University were utilized for external validation. The validation analysis confirmed that ≥ 14 lymph nodes significantly improved CSS and OS compared with < 14 lymph nodes. In contrast, no significant difference was found in OS or CSS when comparing patients with ≥ 12 lymph nodes to those with < 12 lymph nodes (Fig. 3E–H). Therefore, we are certain that the 14 lymph node threshold is a better way of distinguishing patients with better prognoses from those with poor prognoses.
Optimal number of lymph nodes resected and associated cancer-specific and overall survival in T3-T4/N+ rectal cancer after neoadjuvant therapy. The optimal threshold for lymph node resection was as follows: The number of lymph nodes resected was 14. B, C Comparison of CSS: lymph node ≥ 14 vs. < 14 lymph nodes group and lymph node ≥ 12 vs. < 12 lymph nodes group, respectively. D Comparison of CSS for lymph node ≥ 12 and ≥ 14 groups. E, F Validation cohorts: comparison of CSS and OS in ≥ 14 lymph nodes vs. < 14 lymph nodes. G, H Validation cohort: CSS and OS comparison of ≥ 12 lymph nodes versus < 12 lymph nodes
Analyzed using univariate and multivariate Cox regression models
We conducted univariate and multivariate analyses to identify significant prognostic factors for cancer-specific survival (CSS) in rectal cancer patients. Our findings suggest that age of patients, sex, histopathology, pT stage, pN stage, extent of regional lymph nodes examined, presence of tumor deposits, pretreatment CEA level, total number of lymph nodes removed, receipt of chemotherapy, perineural invasion (PNI), tumor size ≥ 5 cm, and marriage status were all significantly correlated with worse CSS (each P < 0.05). These factors may serve as useful predictors for the prognosis of rectal cancer patients (Table 3). Furthermore, age of patients, sex, pN, chemotherapy, pretreatment CEA level, the total number of lymph nodes removed ≥ 14, PNI, tumor deposits, and marriage status were all found to be independent prognostic factors for CSS.
Subgroup analyses for resected lymph nodes and CSS in elderly and non-elderly groups
We further explored the influence of the resected number of lymph nodes on cancer-specific survival (CSS) in both the elderly and non-elderly groups. Our analyses revealed a significant association between the number of resected lymph nodes and CSS in both groups (Fig. 4A–H). In our external validation cohort, we explored the potential impact of the specific location of lymph nodes, chemotherapy regimen, radiotherapy course, and microsatellite status on reaching the optimal threshold of 14 resected lymph nodes by subgroup analysis. The results of the analyses showed a significant difference between resection of at least 14 lymph nodes and survival in the group of patients with negative N0.253 lymph nodes (P < 0.05). However, in other subgroups, no significant association was observed between reaching the threshold of 14 lymph nodes and survival, regardless of the specific location of the lymph nodes, chemotherapy regimen, course of radiotherapy, or microsatellite status. Thus, the removal of more lymph nodes appears to be associated with better survival. Our findings suggest that other factors, such as treatment regimen or pathological features, have insignificant impact on the resected lymph nodes reaching this threshold (Supplementary file 1A-N).
Subgroup analysis of harvested lymph nodes and cancer-specific survival (CSS) in elderly and non-elderly groups: A, B non-elderly: CSS for ≥ 12 vs. < 12 lymph nodes and ≥ 14 vs. < 14 lymph nodes. C, D Elderly: CSS for ≥ 12 vs. < 12 lymph nodes and ≥ 14 vs. < 14 lymph nodes. E, F Validation non-elderly: CSS for ≥ 12 vs. < 12 lymph nodes and ≥ 14 vs. < 14 lymph nodes. G, H Validation elderly: CSS for ≥ 12 vs. < 12 lymph nodes and ≥ 14 vs. < 14 lymph nodes
Discussion
Colorectal cancer (CRC) is a common gastrointestinal malignancy that represents a major health threat, given its high morbidity and mortality rates. It is increasingly impacting younger populations and often presents at advanced stages [1]. Surgery-based combination therapy including neoadjuvant radiotherapy is the standard treatment option for colorectal cancer, especially for locally progressive low and intermediate rectal cancers, which is of great significance for anus-preservation, R0 resection, and survival of locally progressive rectal cancer patients [11]. The number of examined lymph nodes is crucial for pathological staging and has been strongly associated with survival outcomes. In rectal cancer, the adequate removal of lymph nodes is an essential indicator of the quality of radical rectal cancer surgery [12]. While both the National Comprehensive Cancer Network (NCCN) and the American Joint Committee on Cancer (AJCC) recommend examining at least 12 lymph nodes after surgery for CRC [5, 21,22,23]. There is an absence of studies that examine the impact of lymph node count following neoadjuvant therapy on the prognosis of elderly versus non-elderly patients with CRC. In the current study, among patients with more than 14 resected lymph nodes who underwent radical surgery for rectal cancer after neoadjuvant therapy, those younger than 65 years had better OS and CSS outcomes than older patients. This may be related to decreased physical strength and weakness in older patients after surgery.
It is widely acknowledged that age negatively affects lymph node sampling [24,25,26]. The number of samples decreases by 9% for every 10 years of age [26]. Giovanni Li Destri hypothesized that in older patients, the number of samples would be affected due to physiological deterioration of the lymph nodes, a weakened immune response, and the presence of comorbidities [27]. This is consistent with Restivo A’s findings, which demonstrated that age is a risk factor for early distant recurrence in rectal cancer patients who received preoperative radiotherapy [28]. The results of our study indicated that age was an independent prognostic factor, as demonstrated by univariate and multivariate analyses.
The number of lymph nodes detected is indicative of the quality of surgical radical treatment in the case of rectal cancer and is also an essential factor for the assessment of risk stratification and guidance of subsequent adjuvant therapy for patients with stage II and stage III rectal cancer [29]. For patients undergoing neoadjuvant radiotherapy, the number of lymph nodes detected can provide a more intuitive reflection of the efficacy of the neoadjuvant therapy. In general, a reduction in tumor size was negatively correlated with the number of lymph nodes acquired. Overall, the number of lymph nodes obtained is typically less than 12, which has been the subject of debate regarding the rate of local recurrence and distant metastasis [8, 30]. Chase J Wehrle et al. have concluded that the criteria for detecting 12 lymph nodes after neoadjuvant chemoradiation therapy (nCRT) and neoadjuvant therapy are not consistent in resectable stage III rectal cancer. The requirement for lymph node harvest (LNH) for pathological lymph node staging may vary depending on the tumor’s response to neoadjuvant treatment [31]. Matthew D. Hall et al. suggested that at least eight lymph nodes should be examined in patients with rectal cancer undergoing nCRT, noting that eight lymph nodes represent the threshold for adequate lymph node dissection after nCRT [32]. Xu Guan et al. [33] demonstrated that an increased number of LNH was associated with more accurate lymph node staging and a higher survival rate. Another study concluded that 15 LNH represented the optimal threshold for the assessment of the quality of lymph node examination and prognostic stratification [10]. Our study indicated that individuals with greater than 14 lymph nodes excised demonstrated superior OS and CSS outcomes. These findings are warranted to be further validated.
Several limitations of this study should be noted: Firstly, the lack of comprehensive data on the specific location and pathological examination methods in the SEER database precluded their inclusion in our multifactorial analysis. Secondly, the lack of information on adjuvant therapy indications, treatment regimens, and chemotherapy cycles precludes any assessment of the relationship between lymph node count and adjuvant therapy. Thirdly, the external validation sample employed in this study was derived from a Chinese center, which represents a limited sample size. Finally, the study center and the SEER database did not provide specific data regarding the waiting period after radiotherapy. It is necessary to include this variable in future studies to more accurately evaluate its impact on treatment outcomes.
Conclusion
This study unequivocally demonstrates the importance of retrieving lymph nodes during radical surgery for rectal cancer following neoadjuvant therapy. For patients diagnosed with stage T3-4/N+ rectal cancer undergoing radical surgery after neoadjuvant therapy, 14 lymph nodes represent a new threshold for prognostic differentiation. Although the difference in lymph node retrieval between counts of 12 and 14 was not statistically significant, using a benchmark of 14 lymph nodes more accurately distinguished patients with favorable prognoses from those with less favorable outcomes compared to a benchmark of 12 lymph nodes.
Data availability
No datasets were generated or analyzed during the current study.
References
Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A (2023) Colorectal cancer statistics, 2023. CA Cancer J Clin 73(3):233–254. https://doi.org/10.3322/caac.21772
Benson AB, Venook AP, Al-Hawary MM (2022) Rectal cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 20(10):1139–1167. https://doi.org/10.6004/jnccn.2022.0051
Clark Gavin Rc, Anderson Annie S (2020) Variation in changes in the incidence of colorectal cancer by age and association with screening uptake: an observational study. BMJ Open 10(9):e037925
Saraste D, Järås J, Martling A (2020) Population-based analysis of outcomes with early-age colorectal cancer. Br J Surg 107:301–309
Masot O, Cox A, Mold F et al (2022) Decision support-tools for early detection of infection in older people (aged> 65 years): a sco** review. BMC Geriatr 22(1):552. https://doi.org/10.1186/s12877-022-03218-w. PMID: 35778707
Anele CC, Askari A, Navaratne L, Patel K, Jenkin JT, Faiz OD et al (2020) The association of age with the clinicopathological characteristics and prognosis of colorectal cancer: a UK single-centre retrospective study. Colorectal Dis 22:289–297
Gunderson LL, Jessup JM, Sargent DJ, Greene FL, Stewart AK (2010) Revised TN categorization for colon cancer based on national survival outcomes data. J Clin Oncol 28:264–271. https://doi.org/10.1200/JCO.2009.24.0952
Nelson H, Petrelli N, Carlin A, Couture J, Fleshman J, Guillem J, Miedema B, Ota D, Sargent D, National cancer institute expert panel (2001) Guidelines (2000) for colon and rectal cancer surgery. J Natl Cancer Inst 93(8):583–596. https://doi.org/10.1093/jnci/93.8.583. PMID: 11309435
Compton CC, Greene FL (2004) The staging of colorectal cancer: 2004 and beyond. CA Cancer J Clin 54:295–308
Guan X, Jiao S, Wen R, Yu G, Liu J, Miao D, Wei R, Zhang W, Hao L, Zhou L, Lou Z, Liu S, Zhao E, Wang G, Zhang W, Wang X (2023) Optimal examined lymph node number for accurate staging and long-term survival in rectal cancer: a population-based study. Int J Surg 109(8):2241–2248. https://doi.org/10.1097/JS9.0000000000000320.PMID:37428195
Hun ** Kim (2015) Low lymph node retrieval after preoperative chemoradiation for rectal cancer is associated with improved prognosis in patients with a good tumor response. Ann Surg Oncol. 22(6):2075–81. https://doi.org/10.1245/s10434-014-4235-z
Sun Z, Adam MA, Kim J, Turner MC (2017) Association between neoadjuvant chemoradiation and survival for patients with locally advanced rectal cancer. Colorectal Dis 19(12):1058–1066. https://doi.org/10.1111/codi.13754
Huang X, Liu H, Liao X, **ao Z, Huang Z, Li G (2021) Prognostic factors for T1–2 colorectal cancer after radical resection: lymph node distribution is a valuable predictor of its survival. Asian J Surg 44(1):241–246. https://doi.org/10.1016/j.asjsur.2020.06.013
Tran Christopher, Howlett Christopher, Driman David K (2020) Evaluating the impact of lymph node resampling on colorectal cancer nodal stage. Histopathology 77(6):974–983. https://doi.org/10.1111/his.14209
Rullier A, Laurent C, Capdepont M et al (2008) Lymph nodes after preoperative chemoradiotherapy for rectal carcinoma: number, status, and impact on survival. Am J Surg Pathol 32(1):45–50. https://doi.org/10.1097/PAS.0b013e3180dc92ab
Chotard G, Capdepont M, Denost Q (2021) Effects of neoadjuvant chemotherapy plus chemoradiotherapy on lymph nodes in rectal adenocarcinoma. Virchows Arch 479(4):657–666. https://doi.org/10.1007/s00428-021-03108-3
Luna-Perez P, Rodrı́guez-Ramı́rez S, Alvarado I, de la Barrera MG, Labastida S (2003) Prognostic significance of retrieved lymph nodes per specimen in resected rectal adenocarcinoma after preoperative chemoradiation therapy. Arch Med Res 34:281–286
Scott N, Thorne C, Jayne D (2004) Lymph node retrieval after neoadjuvant radiotherapy for rectal adenocarcinoma. J Clin Pathol 57(335–336):4
Qiu L, Junjiao Hu, Weng Z, Liu S (2021) A prospective study of dual-energy computed tomography for differentiating metastatic and non-metastatic lymph nodes of colorectal cancer. Quant Imaging Med Surg 11(8):3448–3459. https://doi.org/10.21037/qims-20-3
Stocchi L, Fazio VW, Lavery I et al (2011) Individual surgeon, pathologist, and other factors affecting lymph node harvest in stage II colon carcinoma. Is a minimum of 12 examined lymph nodes sufficient? Ann Surg Oncol 18(2):405–412
Hong Yang, Jiadi **ng, Chenghai Zhang (2022) Lymph node yield less than 12 is not a poor predictor of survival in locally advanced rectal cancer after laparoscopic TME following neoadjuvant chemoradiotherapy. Front Oncol 12:1080475. https://doi.org/10.3389/fonc.2022.1080475. eCollection 2022
Liu Ao, Zheng Y, Yang P, Chu H, Hou X (2023) Change in onset age of first primary colorectal cancer in the USA. Int J Colorectal Dis 38(1):45. https://doi.org/10.1007/s00384-023-04336-6
Suwanabol PA, Li Y, Abrahamse Paul (2022) Functional and cognitive decline among older adults after high-risk surgery. Ann Surg 275(1):e132–e139. https://doi.org/10.1097/SLA.0000000000003950
De Roo Ana C, Li Yun, Abrahamse Paul H (2020) Long-term functional decline after high-risk elective colorectal surgery in older adults. Dis Colon Rectum 63(1):75–83. https://doi.org/10.1097/DCR.0000000000001541
Torre C, Paliogiannis P, Pulighe F, Scognamillo F, Castiglia P, Trignano M (2013) Impact of age on the quality of lymphadenectomy for colorectal cancer. Cancer Invest 31:39–42
Steele SR, Chen SL, Stojadinovic A, Nissan A, Zhu K, Peoples GE, Bilchik A (2011) The impact of age on quality measure adherence in colon cancer. J Am Coll Surg 213:95–103; discussion 104–105
Chou JF, Row D, Gonen M, Liu YH, Schrag D, Weiser MR (2010) Clinical and pathologic factors that predict lymph node yield from surgical specimens in colorectal cancer: a population-based study. Cancer 116:2560–2570
Li Destri G, Di Carlo I, Scilletta R, Scilletta B, Puleo S (2014) Colorectal cancer and lymph nodes: the obsession with the number 12. World J Gastroenterol 20(8):1951–1960. https://doi.org/10.3748/wjg.v20.i8.1951.PMID:24587671;PMCID:PMC3934465
Restivo A, Delrio P, Deidda S et al (2020) Predictors of early distant relapse in rectal cancer patients submitted to preoperative chemoradiotherapy. Oncol Res Treat 43:146–152
Isik A, Peker K, Firat D, Yilmaz B, Sayar I, Idiz O, Cakir C, Demiryilmaz I, Yilmaz I (2014) Importance of metastatic lymph node ratio in non-metastatic, lymph node-invaded colon cancer: a clinical trial. Med Sci Monit 20:1369–1375
Chang GJ, Rodriguez-Bigas MA, Eng C, Skibber JM (2009) Lymph node status after neoadjuvant radiotherapy for rectal cancer is a biologic predictor of outcome. Cancer 115:5432–5440
Wehrle CJ, Woo K, Chang J, Gamaleldin M, DeHaan R, Dahdaleh F, Felder S, Rosen DR, Champagne B, Steele SR, Naffouje SA (2024) Impact of neoadjuvant therapy on nodal harvest in clinical stage III rectal cancer: establishing optimum cut-offs by disease response. J Surg Oncol. https://doi.org/10.1002/jso.27586. Epub ahead of print. PMID: 38221655
Hall MD, Schultheiss TE, Smith DD, Fakih MG, Kim J, Wong JY, Chen YJ (2015) Impact of total lymph node count on staging and survival after neoadjuvant chemoradiation therapy for rectal cancer. Ann Surg Oncol 22(Suppl 3):S580–S587. https://doi.org/10.1245/s10434-015-4585-1. Epub 2015 May 9 PMID: 25956577
Acknowledgements
We would like to thank Dr. Sisi **e for her constructive comments on the content of the article during the revision process, as well as her invaluable assistance in data interpretation and manuscript writing, which were crucial to the completion of this study.
Funding
This work was supported by Longyan City Joint Funding (FLY2020CWS020046), Natural Science Foundation of Fujian Province (2021J01122670), and Longyan City Joint Funding (2022LYF17094), Longyan City Joint Funding (2021LYF17042).
Author information
Authors and Affiliations
Contributions
Study concepts: **aojie Wang, Shuangming Lin. Study design: **aojie Wang, Shuangming Lin, Baofeng Liang. Data acquisition: Baofeng Liang, Nong Yu, Xueyi Xue. Quality control of data and algorithms: Baofeng Liang, Hao Zeng, Nong Yu. Data analysis and interpretation: Sisi **e, Baofeng Liang, Hao Zeng, Nong Yu. Statistical analysis: Baofeng Liang, Nong Yu. Manuscript preparation: Sisi **e, Baofeng Liang, Xueyi Xue, Zhipeng Que. Manuscript editing: Sisi **e, Baofeng Liang, Nong Yu, Zhipeng Que. Manuscript review: Sisi **e, **aojie Wang, Shuangming Lin, Dongbo Xu. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
The study was approved by the Longyan First Affiliated Hospital of Fujian Medical University Ethical Review Board (approval number: LYREC2024-k028-01). We strictly adhered to the Declaration of Helsinki and relevant national ethical guidelines to ensure participant privacy and data confidentiality. The SEER database is an open public database, and the release of data from the SEER database does not require informed patient consent.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
384_2024_4655_MOESM1_ESM.pdf
Supplementary file1: A hierarchical analysis was performed on T3-4/N+ rectal cancers based on the optimal number of 14 lymph nodes removed. The analysis was stratified by the location of the lymph nodes (A-H), the chemotherapy regimen (I, J), the course of radiotherapy (K, L), and the microsatellite status (M, N). (PDF 248 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liang, B., **e, S., Yu, N. et al. Impact of lymph node retrieval on prognosis in elderly and non-elderly patients with T3-4/N+ rectal cancer following neoadjuvant therapy: a retrospective cohort study. Int J Colorectal Dis 39, 86 (2024). https://doi.org/10.1007/s00384-024-04655-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s00384-024-04655-2