Abstract
Purpose
Identifying the onset age of cancer is essential for its early intervention. The aim of this study was to characterize the features and investigate the variation tendency of first primary colorectal cancer (CRC) onset age in the USA.
Methods
For this retrospective population-based cohort analysis, data on patients diagnosed with first primary CRC (n = 330,977) between 1992 and 2017 were obtained from the Surveillance, Epidemiology, and End Results dataset. Annual percent changes (APC) and average APCs were calculated to examine the changes in average age at CRC diagnosis using the Joinpoint Regression Program.
Results
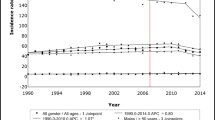
From 1992 to 2017, the average age at CRC diagnosis decreased from 67.0 to 61.2 years, declining by 0.22% and 0.45% annually before and after 2000. The age at diagnosis was lower in the distal than in the proximal CRC cases and the age has the downward trends in all subgroups of sex, race, and stage. Over one-fifth of CRC patients were initially diagnosed with distantly metastatic CRC, with the age lower than that in localized CRC cases (63.5 vs 64.8 years).
Conclusions
The first primary CRC onset age has decreased significantly in the USA over the last 25 years and the modern lifestyle may be responsible for the decline. Specifically, the age of proximal CRC is invariably higher than that of distal CRC. Moreover, the age of advanced stage is lower than that of the early stage. Clinicians should adopt earlier screening age and more effective screening techniques for CRC.



Similar content being viewed by others
Data availability
All the data used in this study can be accessed from the SEER program (https:// seer. cancer. gov/).
References
Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A (2020) Colorectal cancer statistics 2020. CA Cancer J Clin 70:145–164. https://doi.org/10.3322/caac.21601
Siegel RL, Miller KD, Fuchs HE, Jemal A (2022) Cancer statistics. CA Cancer J Clin 72(2022):7–33. https://doi.org/10.3322/caac.21708
Islami F, Goding Sauer A, Miller KD, Siegel RL, Fedewa SA, Jacobs EJ, McCullough ML, Patel AV, Ma J, Soerjomataram I, Flanders WD, Brawley OW, Gapstur SM, Jemal A (2018) Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin 68:31–54. https://doi.org/10.3322/caac.21440
Anand P, Kunnumakkara AB, Sundaram C, Harikumar KB, Tharakan ST, Lai OS, Sung B, Aggarwal BB (2008) Cancer is a preventable disease that requires major lifestyle changes. Pharm Res 25:2097–2116. https://doi.org/10.1007/s11095-008-9661-9
Mucci LA, Hjelmborg JB, Harris JR, Czene K, Havelick DJ, Scheike T, Graff RE, Holst K, Möller S, Unger RH, McIntosh C, Nuttall E, Brandt I, Penney KL, Hartman M, Kraft P, Parmigiani G, Christensen K, Koskenvuo M, Holm NV, Heikkila K, Pukkala E, Skytthe A, Adami HO, Kaprio J, Nordic C (2016) Twin Study of Cancer, Familial risk and heritability of cancer among twins in nordic countries. JAMA 315:68–76. https://doi.org/10.1001/jama.2015.17703
Winawer SJ, Zauber AG (2002) The advanced adenoma as the primary target of screening. Gastrointest Endosc Clin N Am 12:1–9, v. https://doi.org/10.1016/s1052-5157(03)00053-9
Araghi M, Soerjomataram I, Bardot A, Ferlay J, Cabasag CJ, Morrison DS, De P, Tervonen H, Walsh PM, Bucher O, Engholm G, Jackson C, McClure C, Woods RR, Saint-Jacques N, Morgan E, Ransom D, Thursfield V, Moller B, Leonfellner S, Guren MG, Bray F, Arnold M (2019) Changes in colorectal cancer incidence in seven high-income countries: a population-based study, Lancet. Gastroenterol Hepatol 4:511–518. https://doi.org/10.1016/S2468-1253(19)30147-5
Siegel RL, Torre LA, Soerjomataram I, Hayes RB, Bray F, Weber TK, Jemal A (2019) Global patterns and trends in colorectal cancer incidence in young adults. Gut 68:2179–2185. https://doi.org/10.1136/gutjnl-2019-319511
Vuik FE, Nieuwenburg SA, Bardou M, Lansdorp-Vogelaar I, Dinis-Ribeiro M, Bento MJ, Zadnik V, Pellise M, Esteban L, Kaminski MF, Suchanek S, Ngo O, Majek O, Leja M, Kuipers EJ, Spaander MC (2019) Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut 68:1820–1826. https://doi.org/10.1136/gutjnl-2018-317592
Doll R (1991) Progress against cancer: an epidemiologic assessment. The 1991 John C. Cassel Memorial Lecture. Am J Epidemiol 134:675–688. https://doi.org/10.1093/oxfordjournals.aje.a116143
Surveillance, Epidemiology, and End Results (SEER) Program SEER*Stat Database: Incidence - SEER Research Data, 13 Registries, Nov 2019 Sub (1992–2017) - Linked To County Attributes - Time Dependent (1990–2017) Income/Rurality, 1969–2018 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2020, based on the November 2019 submission. www.seer.cancer.gov
National Cancer Institute. SEER*Stat Databases: November 2019 Submission. 2020. Available online: https://seer.cancer.gov/data-software/documentation/seerstat/nov2019/. Accessed 21 Oct 2020
National Cancer Institute Surveillance Research Program. Surveillance, Epidemiology, and End Results (SEER). 2018. Available online: https://seer.cancer.gov/about/factsheets/SEER_Overview.pdf. Accessed 21 Oct 2020
Surveillance Research Program, National Cancer Institute. SEER*Stat software version 8.3.8. National Cancer Institute Web. www.seer.cancer.gov/seerstat. Accessed 1 Mar 2021
R Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing Web. https://www.R-project.org/. Accessed 21 June 2019
Cline BJ, Simpson MC, Gropler M, Bukatko AR, Adjei Boakye E, Mohammed KA, Osazuwa-Peters N (2020) Change in age at diagnosis of oropharyngeal cancer in the United States, 1975–2016. Cancers (Basel) 12. https://doi.org/10.3390/cancers12113191
Statistical Research and Applications Branch, National Cancer Institute (2019) Joinpoint Regression Program version 4.7.0.0. National Cancer Institute Web. https://surveillance.cancer.gov/joinpoint/. Accessed 22 June 2019
Kim HJ, Fay MP, Feuer EJ, Midthune DN (2000) Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 19:335–351. https://doi.org/10.1002/(sici)1097-0258(20000215)19:3%3c335::aid-sim336%3e3.0.co;2-z
National Cancer Institute. Linear or log-linear model. Available at https://surveillance.cancer.gov/joinpoint/faq/linear_model.html. Accessed 26 Oct 2020
National Center for Health Statistics (2021) Health, United States 2019. National Center for Health Statistics Web. https://doi.org/10.15620/cdc:100685. Accessed 10 Nov 2022
Peterse EFP, Meester RGS, de Jonge L, Omidvari AH, Alarid-Escudero F, Knudsen AB, Zauber AG, Lansdorp-Vogelaar I (2021) Comparing the cost-effectiveness of innovative colorectal cancer screening tests. J Natl Cancer Inst 113:154–161. https://doi.org/10.1093/jnci/djaa103
Siegel RL, Fedewa SA, Anderson WF, Miller KD, Ma J, Rosenberg PS, Jemal A (2017) Colorectal cancer incidence patterns in the United States, 1974–2013. J Natl Cancer Inst 109. https://doi.org/10.1093/jnci/djw322
Knudsen AB, Rutter CM, Peterse EFP, Lietz AP, Seguin CL, Meester RGS, Perdue LA, Lin JS, Siegel RL, Doria-Rose VP, Feuer EJ, Zauber AG, Kuntz KM, Lansdorp-Vogelaar I (2021) Colorectal cancer screening: an updated modeling study for the US Preventive Services Task Force. JAMA 325:1998–2011. https://doi.org/10.1001/jama.2021.5746
Bollart AS, Manfredi S, Piette C, Bretagne JF (2015) Frequency and efficacy of additional investigations following incomplete colonoscopies: a population-based analysis. Dig Liver Dis 47:720–725. https://doi.org/10.1016/j.dld.2015.05.007
Nogales Ó, García-Lledó J, Luján M, Nicolás D, Juanmartiñena JF, González-Suárez B, Sánchez-Ceballos F, Couto I, Olmedo J, Garfia C, Carretero C, Fernandez Urien I, Rodriguez S, Asteinza M, Olivencia P, Masedo A, Munoz-Navas M, Merino B, Gonzalez Asanza C (2017) Therapeutic impact of colon capsule endoscopy with PillCam COLON 2 after incomplete standard colonoscopy: a Spanish multicenter study. Rev Esp Enferm Dig 109:322–327. https://doi.org/10.17235/reed.2017.4369/2016
Triantafyllou K, Viazis N, Tsibouris P, Zacharakis G, Kalantzis C, Karamanolis DG, Ladas SD (2014) Colon capsule endoscopy is feasible to perform after incomplete colonoscopy and guides further workup in clinical practice. Gastrointest Endosc 79:307–316. https://doi.org/10.1016/j.gie.2013.07.061
Spada C, Hassan C, Barbaro B, Iafrate F, Cesaro P, Petruzziello L, Grazioli LM, Senore C, Brizi G, Costamagna I, Alvaro G (2015) Colon capsule versus CT colonography in patients with incomplete colonoscopy: a prospective, comparative trial. Gut 64:272–281. https://doi.org/10.1136/gutjnl-2013-306550
Ahmed M (2020) Colon cancer: a clinician’s perspective in 2019. Gastroenterol Res 13:1–10. https://doi.org/10.14740/gr1239
Singh KE, Taylor TH, Pan CG, Stamos MJ, Zell JA (2014) Colorectal cancer incidence among young adults in California. J Adolesc Young Adult Oncol 3:176–184. https://doi.org/10.1089/jayao.2014.0006
The American Cancer Society medical and editorial content team. Colorectal cancer early detection, diagnosis, and staging. Available at https://www.cancer.org/content/dam/CRC/PDF/Public/8606.00.pdf. Accessed 31 Dec 2020
Dougherty MK, Brenner AT, Crockett SD, Gupta S, Wheeler SB, Coker-Schwimmer M, Cubillos L, Malo T, Reuland DS (2018) Evaluation of interventions intended to increase colorectal cancer screening rates in the United States: a systematic review and meta-analysis. JAMA Intern Med 178:1645–1658. https://doi.org/10.1001/jamainternmed.2018.4637
Spada C, Hassan C, Galmiche JP, Neuhaus H, Adler SN, Epstein O, Gay G, Pennazio M, Benamouzig R, De Franchis R, Delvaux M, Deviere J, Eliakim R, Fraser C, Hagenmuller F, Herrerias JM, Keuchel M, Macrae F, Munoz-Navas M, Ponchon T, Quintero E, Riccioni ME, Rondonotti E, Marmo R, Sung JJ, Tajiri H, Toth E, Triantafyllou K, Van Gossum A, Costamagna G, European Society of Gastrointestinal (2012) Colon capsule endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 44:527–536. https://doi.org/10.1055/s-0031-1291717
Deding U, Herp J, Havshoei AL, Kobaek-Larsen M, Buijs MM, Nadimi ES, Baatrup G (2020) Colon capsule endoscopy versus CT colonography after incomplete colonoscopy. Application of artificial intelligence algorithms to identify complete colonic investigations. United European Gastroenterol J 8:782–789. https://doi.org/10.1177/2050640620937593
Horvat N, Raj A, Ward JM, Smith JJ, Markowitz AJ, Gollub MJ (2018) Clinical value of CT colonography versus preoperative colonoscopy in the surgical management of occlusive colorectal cancer. AJR Am J Roentgenol 210:333–340. https://doi.org/10.2214/AJR.17.18144
Luo H, Zhao Q, Wei W, Zheng L, Yi S, Li G, Wang W, Sheng H, Pu H, Mo H, Zuo Z, Liu Z, Li C, **e C, Zeng Z, Li W, Hao X, Liu Y, Cao S, Liu W, Gibson S, Zhang K, Xu G, Xu RH (2020) Circulating tumor DNA methylation profiles enable early diagnosis, prognosis prediction, and screening for colorectal cancer. Sci Transl Med 12. https://doi.org/10.1126/scitranslmed.aax7533
Chen B, Zhang RN, Fan X, Wang J, Xu C, An B, Wang Q, Wang J, Leung EL, Sui X, Wu Q (2020) Clinical diagnostic value of long non-coding RNAs in colorectal cancer: a systematic review and meta-analysis. J Cancer 11:5518–5526. https://doi.org/10.7150/jca.46358
Zanutto S, Ciniselli CM, Belfiore A, Lecchi M, Masci E, Delconte G, Primignani M, Tosetti G, Dal Fante M, Fazzini L, Airoldi A, Vangeli M, Turpini F, Rubis Passoni GG, Viaggi P, Arena M, Motta RIO, Cantu AM, Crosta C, De Roberto G, Iannuzzi F, Cassinotti A, Dall'Olio V, Tizzoni L, Sozzi G, Meroni E, Bisanti L, Pierotti MA, Verderio P, Gariboldi M (2020) Plasma miRNA-based signatures in CRC screening programs. Int J Cancer 146:1164–1173. https://doi.org/10.1002/ijc.32573
Sung JJY, Chiu HM, Lieberman D, Kuipers EJ, Rutter MD, Macrae F, Yeoh KG, Ang TL, Chong VH, John S, Li J, Wu K, Ng SSM, Makharia GK, Abdullah M, Kobayashi N, Sekiguchi M, Byeon JS, Kim HS, Parry S, Cabral-Prodigalidad PAI, Wu DC, Khomvilai S, Lui RN, Wong S, Lin YM, Dekker E (2022) Third Asia-Pacific consensus recommendations on colorectal cancer screening and postpolypectomy surveillance. Gut. https://doi.org/10.1136/gutjnl-2022-327377
Ng K, May FP, Schrag D (2021) US Preventive Services Task Force recommendations for colorectal cancer screening: forty-five is the new fifty. JAMA 325:1943–1945. https://doi.org/10.1001/jama.2021.4133
Park HS, Lloyd S, Decker RH, Wilson LD, Yu JB (2012) Overview of the Surveillance, Epidemiology, and End Results database: evolution, data variables, and quality assurance. Curr Probl Cancer 36:183–190. https://doi.org/10.1016/j.currproblcancer.2012.03.007
Funding
This research was supported by the National Natural Science Foundation of China (grant number 82000561 to H. Chu; grant number 81974062 and 81720108006 to X. Hou), Department of Science and Technology, Hubei Provincial People’s Government (grant number 2020FCA014 to X. Hou), and the Science Foundation of Union Hospital (grant number 2021xhyn005 to H. Chu).
Author information
Authors and Affiliations
Contributions
Conception and design: Huikuan Chu, **aohua Hou. Analysis and interpretation of data: Ao Liu, Yongqiang Zheng, Pengcheng Yang. Writing, review, and revision of the manuscript: Ao Liu, Yongqiang Zheng, Pengcheng Yang. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, A., Zheng, Y., Yang, P. et al. Change in onset age of first primary colorectal cancer in the USA. Int J Colorectal Dis 38, 45 (2023). https://doi.org/10.1007/s00384-023-04336-6
Accepted:
Published:
DOI: https://doi.org/10.1007/s00384-023-04336-6