Abstract
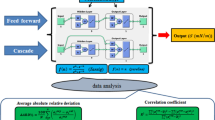
In this study, an artificial neural network (ANN) model was developed based on molecular descriptors to predict the surface tension of liquids. A dataset containing various features was constructed by collecting experimental data from 25 different fluids and extracting molecular structural descriptors. Feature selection was performed using the forward search wrapper method based on Random Forest, identifying 7 significant features (Temperature, MinAbsEStateIndex, LabuteASA, MolMR, Chi1v, qed and FpDensityMorgan3) for surface tension prediction. Subsequently, an ANN model was constructed with the selected features as inputs to predict the surface tension of liquids. The derived model demonstrates high accuracy with a correlation coefficient (R) exceeding 0.999 and a notably low mean square error (MSE = 1.843e−5). Moreover, the ANN model exhibited a total average absolute deviation (AAD) of 0.98 %, comparable to that of the REFPROP, which had a total AAD of 1.26 %. This quantitative model serves an easy tool for gaining insights into the molecular underpinnings of surface tension and predicting its value across various fluids.



Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
W.R. Smith, Q.X. Wang, The role of surface tension on the growth of bubbles by rectified diffusion. Ultrason. Sonochem. 98, 106473 (2023). https://doi.org/10.1016/j.ultsonch.2023.106473
B. He, S. Yang, Z. Qin, B. Wen, C. Zhang, Sci. Rep. 7, 1–7 (2017). https://doi.org/10.1038/s41598-017-12189-7
Y. Ding, L. Jia, L. Yin, C. Dang, X. Liu, J. Xu, Colloids Surf. A 658, 130670 (2023). https://doi.org/10.1016/j.colsurfa.2022.130670
C. Singh, A. Chaudhuri, Nat. Commun. 15, 3704 (2024). https://doi.org/10.1038/s41467-024-47727-1
R.W. Style, C. Hyland, R. Boltyanskiy, J.S. Wettlaufer, E.R. Dufresne, Nat. Commun. 4, 1–6 (2013). https://doi.org/10.1038/ncomms3728
M. Liu, S. Wang, L. Jiang, Nat. Rev. Mater. (2017). https://doi.org/10.1038/natrevmats.2017.36
S. Park, L. Liu, Ç. Demirkır et al., Nat. Chem. 15, 1532–1540 (2023). https://doi.org/10.1038/s41557-023-01294-y
H. Ragelle, M.W. Tibbitt, S.Y. Wu et al., Nat. Commun. 9, 1–10 (2018). https://doi.org/10.1038/s41467-018-03391-w
C. Miqueu, D. Broseta, J. Satherley, B. Mendiboure, J. Lachaise, A. Graciaa, Fluid Phase Equilib. 172, 169–182 (2000). https://doi.org/10.1016/S0378-3812(00)00384-8
S.M. Hosseini, M. Sadeghi, T. Zarei, J. Mol. Liq. 352, 118629 (2022). https://doi.org/10.1016/j.molliq.2022.118629
I. Cachadiña, J. Tian, A. Mulero, J. Chem. Thermodyn. 110, 201–210 (2017). https://doi.org/10.1016/j.jct.2017.03.001
A.M. Log, V. Diky, M.L. Huber, Int. J. Thermophys. 44, 110 (2023). https://doi.org/10.1007/s10765-023-03216-z
H. Moslehi, S.M. Hosseini, M.M. Alavianmehr, Fluid Phase Equilib. 568, 113751 (2023). https://doi.org/10.1016/j.fluid.2023.113751
V. Vinš, B. Planková, J. Hrubý, Int. J. Thermophys. 34, 792–812 (2013). https://doi.org/10.1007/s10765-012-1207-z
L.M.C. Pereira, A. Chapoy, R. Burgass, B. Tohidi, J. Chem. Thermodyn. 97, 55–69 (2016). https://doi.org/10.1016/j.jct.2015.12.036
A. Hernández, Int. J. Thermophys. 44, 1–20 (2023). https://doi.org/10.1007/s10765-022-03126-6
G. Di Nicola, M. Moglie, Int. J. Refrig. 34, 1098–1108 (2011). https://doi.org/10.1016/j.ijrefrig.2011.02.008
D. Elustondo, Chem. Phys. 545, 111145 (2021). https://doi.org/10.1016/j.chemphys.2021.111145
T.M. Koller, C. Steininger, M.H. Rausch, A.P. Fröba, Int. J. Thermophys. 38, 1–20 (2017). https://doi.org/10.1007/s10765-017-2301-z
K.A.G. Schmidt, G.K. Folas, B. Kvamme, Fluid Phase Equilib. 261, 230–237 (2007). https://doi.org/10.1016/j.fluid.2007.07.045
M. Nabipour, P. Keshavarz, Int. J. Refrig. 75, 217–227 (2017). https://doi.org/10.1016/j.ijrefrig.2016.12.011
Á. Mulero, I. Cachadiña, J.O. Valderrama, Fluid Phase Equilib. 451, 60–67 (2017). https://doi.org/10.1016/j.fluid.2017.07.022
A. Jabbarzadeh, H. Wu, Colloids Surf. A (2024). https://doi.org/10.1016/j.colsurfa.2024.133400
T. Kawahara, T. Imai, T. Okumura, C. Kondou, J. Mol. Liq. 386, 122532 (2023). https://doi.org/10.1016/j.molliq.2023.122532
C.J. Kankanamge, F.D. Lenahan, T. Klein, A.P. Fröba, Int. J. Thermophys. 43, 112 (2022). https://doi.org/10.1007/s10765-022-03038-5
D. Abooali, M.A. Sobati, Int. J. Refrig. 40, 282–293 (2014). https://doi.org/10.1016/j.ijrefrig.2013.12.007
A. Khajeh, H. Modarress, Int. J. Refrig. 35, 150–159 (2012). https://doi.org/10.1016/j.ijrefrig.2011.08.007
S. Sansare, T. Duran, H. Mohammadiarani, M. Goyal, G. Yenduri, A. Costa, X. Xu, T. O’Connor, D. Burgess, B. Chaudhuri, Int. J. Pharm. 603, 120713 (2021). https://doi.org/10.1016/j.ijpharm.2021.120713
A.M. Kaverin, V.N. Andbaeva, V.G. Baidakov, Russ. J. Phys. Chem. A 80, 413–417 (2006). https://doi.org/10.1134/S0036024406030174
C. Tegeler, R. Span, W. Wagner, J. Phys. Chem. Ref. Data 28, 779–850 (1999). https://doi.org/10.1063/1.556037
J.C.G. Calado, A.F.S.D.S. Mendonça, B.D.J.V. Saramago, V.A.M. Soares, J. Colloid Interface Sci. 189, 273–282 (1997). https://doi.org/10.1006/jcis.1997.4810
E.W. Lemmon, R. Span, J. Chem. Eng. Data 51, 785–850 (2006). https://doi.org/10.1021/je050186n
D. Stansfield, Proc. Phys. Soc. 72, 854 (1958). https://doi.org/10.1088/0370-1328/72/5/321
R. Span, E.W. Lemmon, R.T. Jacobsen, W. Wagner, A. Yokozeki, J. Phys. Chem. Ref. Data 29, 1361–1433 (2000). https://doi.org/10.1063/1.1349047
J.J. Jasper, J. Phys. Chem. Ref. Data 1, 841 (1972). https://doi.org/10.1063/1.3253106
R. Span, W. Wagner, J. Phys. Chem. Ref. Data 25, 1509–1596 (1996). https://doi.org/10.1063/1.555991
C. Wohlfarth, Landolt-Börnstein—Group IV Physical Chemistry 24 (2008). https://materials.springer.com/lb/docs/sm_lbs_978-3-540-75508-1_20
U. Setzmann, W. Wagner, J. Phys. Chem. Ref. Data 20, 1061–1155 (1991). https://doi.org/10.1063/1.555898
K. Tanaka, Y. Higashi, Int. J. Refrig. 30, 1368–1373 (2007). https://doi.org/10.1016/j.ijrefrig.2007.04.002
E.W. Lemmon, M.O. McLinden, W. Wagner, J. Chem. Eng. Data 54, 3141–3180 (2009). https://doi.org/10.1021/je900217v
H. Lin, Y.Y. Duan, J. Chem. Eng. Data 48, 1360–1363 (2003). https://doi.org/10.1021/je034093m
A.R. Dorokhov, A.A. Kiriyanenko, A.N. Solov’ev, J. Appl. Mech. Tech. Phys. 10, 89–92 (1969). https://doi.org/10.1007/BF00916257
R.T. Jacobsen, S.G. Penoncello, E.W. Lemmon, Fluid Phase Equilib. 80, 45–46 (1992). https://doi.org/10.1016/0378-3812(92)87054-Q
V. Marx, A. Pruss, W. Wagner, Duesseldorf: VDI Verlag, Series 19 (Waermetechnik/Kaeltetechnik), 57 (1992)
A.P. Fröba, S. Will, A. Leipertz, Int. J. Thermophys. 21, 1225–1253 (2000). https://doi.org/10.1023/A:1006689724974
B.A. Younglove, M.O. McLinden, J. Phys. Chem. Ref. Data 23, 731–779 (1994). https://doi.org/10.1063/1.555950
H.B. Chae, J.W. Schmidt, M.R. Moldover, J. Chem. Eng. Data 35, 6–8 (1990). https://doi.org/10.1021/je00059a002
C. Kondou, R. Nagata, N. Nii, S. Koyama, Y. Higashi, Refrig. Sci. Technol. 53, 538–545 (2015). https://doi.org/10.1016/j.ijrefrig.2015.01.005
M.E. Mondéjar, M.O. McLinden, E.W. Lemmon, J. Chem. Eng. Data 60, 2477–2489 (2015). https://doi.org/10.1021/acs.jced.5b00348
K. Tanaka, Y. Higashi, Int. J. Refrig. 33, 474–479 (2010). https://doi.org/10.1016/j.ijrefrig.2009.10.003
M. Richter, M.O. McLinden, E.W. Lemmon, J. Chem. Eng. Data 56, 3254–3264 (2011). https://doi.org/10.1021/je200369m
R. Akasaka, E.W. Lemmon, J. Chem. Eng. Data 64, 4679–4691 (2019). https://doi.org/10.1021/acs.jced.9b00007
M. Okada, Y. Higashi, Int. J. Thermophys. 16, 791–800 (1995). https://doi.org/10.1007/BF01438864
B. de Vries, R. Tillner-Roth, H. D. Baehr, 1995. 19th International Congress of Refrigeration, The Hague, The Netherlands, International Institute of Refrigeration, IVa:582–589. https://iifiir.org/en/fridoc/thermodynamic-properties-of-hcfc124-14011
E.W. Lemmon, R.T. Jacobsen, J. Phys. Chem. Ref. Data 34, 69–108 (2005). https://doi.org/10.1063/1.1797813
M.F. Liu, L.Z. Han, M.S. Zhu, Int. J. Thermophys. 15, 941–948 (1994). https://doi.org/10.1007/BF01447104
Y.Y. Duan, L. Shi, M.S. Zhu, L.Z. Han, X. Lei, Fluid Phase Equilib. 172, 237–244 (2000). https://doi.org/10.1016/S0378-3812(00)00375-7
R. Heide, Int. J. Refrig. 20, 496–503 (1997). https://doi.org/10.1016/S0140-7007(97)00044-3
R. Tillner-Roth, H.D. Baehr, J. Phys. Chem. Ref. Data 23, 657–729 (1994). https://doi.org/10.1063/1.555958
Y. Higashi, T. Shibata, J. Chem. Eng. Data 42, 438–440 (1997). https://doi.org/10.1021/je960274v
E.W. Lemmon, R.T. Jacobsen, J. Phys. Chem. Ref. Data 29, 521–552 (2000). https://doi.org/10.1063/1.1318909
H. Lin, Y.Y. Duan, Int. J. Thermophys. 24, 1495–1508 (2003). https://doi.org/10.1023/B:IJOT.0000004090.64922.63
S.L. Outcalt, M.O. McLinden, J. Phys. Chem. Ref. Data 25, 605–636 (1996). https://doi.org/10.1063/1.555979
S. Bi, G. Zhao, J. Wu, Fluid Phase Equilib. 287, 23–25 (2009). https://doi.org/10.1016/j.fluid.2009.09.005
S. Bi, J. Cui, X. Meng, J. Wu, Fluid Phase Equilib. 405, 25–30 (2015). https://doi.org/10.1016/j.fluid.2015.07.010
H. Qi, D. Fang, K. Gao, X. Meng, J. Wu, Int. J. Thermophys. 37, 55 (2016). https://doi.org/10.1007/s10765-016-2061-1
M. Okada, T. Arima, M. Hattori, K. Watanabe, J. Chem. Eng. Data 33, 399–401 (1988). https://doi.org/10.1021/je00054a003
A. Kamei, S.W. Beyerlein, R.T. Jacobsen, J. Phys. Chem. Ref. Data 16, 1155–1164 (1995). https://doi.org/10.1007/BF02081283
S.G. Penoncello, E.W. Lemmon, R.T. Jacobsen, Z. Shan, J. Phys. Chem. Ref. Data 32, 1473–1499 (2003). https://doi.org/10.1063/1.1559671
H. Lin, Y.Y. Duan, Z.W. Wang, Fluid Phase Equilib. 214, 79–86 (2003). https://doi.org/10.1016/S0378-3812(03)00316-9
J. Pan, X. Rui, X. Zhao, L. Qiu, Fluid Phase Equilib. 321, 10–16 (2012). https://doi.org/10.1016/j.fluid.2012.02.012
R. Akasaka, Y. Zhou, E.W. Lemmon, J. Phys. Chem. Ref. Data 44, 013104 (2015). https://doi.org/10.1063/1.4913493
R. Tillner-Roth, A. Yokozeki, J. Phys. Chem. Ref. Data 26, 1273–1328 (1997). https://doi.org/10.1063/1.556002
M.S. Zhu, C.X. Lu, J. Chem. Eng. Data 39, 205–206 (1994). https://doi.org/10.1021/je00014a003
D. Bücker, W. Wagner, J. Phys. Chem. Ref. Data 35, 929–1019 (2006). https://doi.org/10.1063/1.1901687
S. Kim, J. Chen, T. Cheng, A. Gindulyte, J. He, S. He, Q. Li, B.A. Shoemaker, P.A. Thiessen, B. Yu, Nucleic Acids Res. 47, D1102–D1109 (2019). https://doi.org/10.1093/nar/gky1033
G.Landrum, ACS fall national meeting and exposition aug sandiego. https://www.morressier.com/o/event/5fc6445603137aa5258c485b/article/5fc645232d78d1fec467580d
R. Winter, F. Montanari, F. Noé, D.A. Clevert, Chem. Sci. 10, 1692–1701 (2019). https://doi.org/10.1039/c8sc04175j
W. Qian et al., FirePhysChem 3, 339–349 (2023). https://doi.org/10.1016/j.fpc.2023.04.002
P. Labute, A widely applicable set of descriptors. J. Mol. Graph. 18, 464–477 (2000). https://doi.org/10.1016/S1093-3263(00)00068-1
S.A. Wildman, G.M. Crippen, J. Chem. Inf. Comput. Sci. 39, 868–873 (1999). https://doi.org/10.1021/ci990307l
H.H. Lowell, B.K. Lemont, Rev. Comput. Chem. (1991). https://doi.org/10.1002/9780470125793.ch9
J. Yang, Y. Cai, K. Zhao, H. **e, X. Chen, Drug Discov. Today 27, 103356 (2022). https://doi.org/10.1016/j.drudis.2022.103356
Y. Tian, S. Zhang, H. Yin, A. Yan, Chemom. Intell. Lab. Syst. 196, 103888 (2020). https://doi.org/10.1016/j.chemolab.2019.103888
B. Yang, C. Zeng, L. Wang, Y. Guo, G. Chen, Z. Guo, Y. Chen, D. Li, P. Cao, H. Shu, T. Yu, J. Zhu, Int. J. Hydrog. Energy 46, 22998–23012 (2021). https://doi.org/10.1016/j.ijhydene.2021.04.130
J. Lagrandeur, S. Poncet, M. Sorin, M. Khennich, Int. J. Therm. Sci. (2019). https://doi.org/10.1016/j.ijthermalsci.2019.106097
E.W. Lemmon, I.H. Bell, M.L. Huber, M.O. McLinden, National Institute of Standards and Technology, Standard Reference Data Program, Gaith. https://www.nist.gov/srd/refprop
Funding
This work is financially supported by the National Natural Science Foundation of China (Nos. 52276020, 52376212).
Author information
Authors and Affiliations
Contributions
N.G. designed research; N.L. performed research; N.G., N.L., X.H.W., and G.M.C. analyzed data; N.L., X.H.W., and N.G. wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
-
1.
The mapminmax struct for inputs
name
'mapminmax'
xrows
7
xmax
[423.1500;6;50.6279;21.5490;2.1649;0.4895;2]
xmin
[28.6300;0;8.7393;0;0;0.3516;0.8889]
xrange
[394.5200;6;41.88862;21.5490;2.1649;0.1379;1.1111]
yrows
7
ymax
1
ymin
0
yrange
1
gain
[0.0025;0.16667;0.0239;0.0464;0.4619;7.2502;0.9000]
xoffset
[28.6300;0;8.7393;0;0;0.3516;0.8889]
no_change
0
-
2.
The mapminmax struct for output
name
'mapminmax'
xrows
1
xmax
25.9000
xmin
0.3800
xrange
25.5200
yrows
1
ymax
1
ymin
0
yrange
1
gain
0.0392
xoffset
0.3800
no_change
0
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, N., Wang, X., Gao, N. et al. A Quantitative Structure–Property Relationship Model for Surface Tension Based on Artificial Neural Network. Int J Thermophys 45, 106 (2024). https://doi.org/10.1007/s10765-024-03398-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-024-03398-0