Abstract
We compiled an experimental database for the surface tension of binary mixtures containing a wide variety of fluids, from the chemical classes (water, alcohols, amines, ketones, linear and branched alkanes, naphthenes, aromatics, refrigerants, and cryogens). The resulting data set includes 65 pure fluids and 154 binary pairs with a total of 8205 points. We used this database to test the performance of a parachor model for the surface tension of binary mixtures. The model uses published correlations to determine the parachors of the pure fluids. The model has a single, constant binary interaction parameter for each pair that was found by fitting experimental mixture data. It can be also used in a predictive mode when the interaction parameters are set to zero. We present detailed comparisons on the performance of the model for both cases. In general, the parachor model in a predictive mode without fitted interaction parameters can predict the surface tension of binary mixtures of non-polar mixtures such as linear and branched alkanes, linear and branched alkanes with naphthenes, aromatics with aromatics, aromatics with naphthenes, and mixtures of linear alkanes of similar sizes with an average absolute percentage deviation of about 3 % or less. Polar mixtures of halocarbons with other halocarbons and also polar/nonpolar mixtures of alkanes with halocarbons could be modeled with an average absolute deviation of less than 0.35 mN·m−1 with the use of a binary interaction parameter. The parachor model even with a fitted binary interaction parameter performs poorly for mixtures of water and organic compounds and is not recommended.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Surface tension is an important physical property that has long had significance in the oil and gas industry, and is also of interest in applications as varied as pharmaceuticals [1, 2], heat transfer in low-global warming potential (GWP) refrigerants [3], ink-jet printing [4, 5] and diesel engine design [6]. Specific examples in the pharmaceutical industry include [2] the importance of controlling the surface tension of coating solutions of tablets to improve product appearance and control the rate of drug release, the effect of surface tension on the size of droplets in a nebulizer, and control of the size of eye drops. In addition, fluorocarbon based fluids with low surface tension are being investigated as blood substitutes for oxygen delivery [1]. In the refrigeration industry, new low-GWP refrigerant blends are being proposed. In order to evaluate the performance of heat exchangers, accurate knowledge of the surface tension is needed to model the bubble behavior in pool boiling [3]. In ink-jet printing, From [5] analyzed the fluid flow behavior of impulsively driven laminar jet flow in terms of dimensionless parameters involving the surface tension, density, viscosity, and a characteristic dimension, and made recommendations for when the fluid has stable drop formation. In order to optimize engine performance to reduce soot emissions, there is a need for surface tension data at high pressures and high temperatures [6]. Accurate property values for surface tension are necessary for successful analysis of all these processes.
In 1923, Macleod proposed a simple empirical relationship between surface tension σ and the density of the liquid and vapor phases ρL and ρV
where P is a temperature-independent parameter called the parachor by Sugden [7]. Other practical engineering methods for predicting surface tension can be found in handbooks such as Ref. [8]. In addition, there are numerous theoretically based approaches to predicting the surface tension such as density gradient theory [9, 10], density functional theory [11], hard-sphere fluid scaled particle theory [12], perturbation theory [13] and friction theory [14].
The parachor approach can also be applied to mixtures, as was demonstrated by Weinaug and Katz [15] and Hugill et al. [16]. Although the parachor method has been used for many years in the petrochemical industry, is in active use now [17], is the recommended approach in the API Technical Databook [18], and is discussed in reference books for engineers [8] there has not been a comprehensive evaluation of the performance of this type of model with respect to mixtures using a large database of binary data in the open literature. It is the goal of this work to provide an evaluation of the parachor model to a wide variety of binary mixtures, including not only common hydrocarbons involved in the petrochemical industry, but also recent low-GWP fluids of interest to the refrigeration industry and to indicate expected performance and limitations of this model for a wide variety of mixtures.
2 The Parachor Model
The parachor model that we will apply to mixtures was originally presented by Weinaug and Katz [15] and later modified by Hugill et al. [16] to allow for the use of binary interaction parameters. For a mixture,
with mixing and combining rules
where δij is an optional binary interaction parameter, and xi and yi are the molar compositions of the liquid and gas phases, respectively. Historically [15, 16, 19] the exponent m has been set to 4, but here we use m = 3.87 based on theoretical considerations as presented by Garrabos et al. [20]. In addition, it also is common to use a fixed value of the parachor obtained from compilations such as that of Quayle [21]. Zhelezny et al. [22] has studied the temperature dependence of the parachor. Mulero and coworkers [23,24,25,26,27,28,29,30,31] developed an extensive body of work on correlations for the surface tension of many important industrial fluids that can be used to compute the pure fluid parachors Pi as a function of temperature. These correlations are very accurate and can represent the data to within experimental uncertainty. We primarily use these correlations as implemented in the computer program REFPROP v10 [32] for pure fluid surface tension σi. The parachors are evaluated at the temperature of interest for the binary mixture, however for temperatures greater than or equal to 0.9Tc,i, where Tc,i is the pure fluid critical temperature, the parachor is calculated at 0.9Tc,i. It also is necessary to have the saturation densities and compositions xi and yi of the liquid and vapor phases. If the compositions and densities from the VLE calculations are inaccurate this will increase the uncertainty in the surface tension calculations, so care should be used in the selection of the VLE model. We obtain these compositions and densities from the default equations of state and models implemented in REFPROP v10 [32]; a description of these can be found in [33]. A few changes were made in the models of REFPROP v10 that enabled calculation for some mixtures not permitted in the original version, as well as some changes in mixture parameters that are summarized in the Supplementary Information in Appendix A.
3 Experimental Data
We extracted experimental data for the surface tension of binary mixtures for liquid–gas interfaces from the NIST TDE database [34] for which the pure fluid components are available, the composition of the liquid is explicitly specified, and also for which there are reliable models for the vapor–liquid equilibrium and thermodynamic properties in the REFPROP database [32]. We excluded HCl/water and benzene/water due to the lack of a good mixture model for thermodynamic properties in REFPROP. The resulting data set includes 65 pure fluids and 154 binary pairs with a total of 8205 points. Table 1 provides a list of the pure components along with information for compound identification, along with a reference for the pure fluid surface tension correlation implemented in REFPROP v10 [32] used to evaluate pure fluid surface tension in this work. A summary of the binary mixture data is given in Table 2 including a reference code (starting with the publication year), the experimental method, an uncertainty estimate, the fluids in the binary mixture, the number of data points, temperature range, and composition in terms of the mole fraction of the first component. The full data set is available in the supplementary information in the file InputData.txt. A discussion of experimental methods for obtaining surface tension can be found in Ref. [35]. The estimated uncertainties (at a k = 2 level) are those as assessed by the NIST TDE database and may not be the same as those stated by the original authors. As part of the data capture process, software [36] is used that assesses the uncertainty of the data taking into account factors such as the experimental method, the sample purity, property precision, precision of independent variables. However, the reader should consult the original data reference for complete details of the measurement technique and uncertainty analysis for assessment of the quality of an individual data set.
4 Results
Evaluations were first made with the interaction parameter in Eq. 4 set to zero (δij = 0) for all the mixtures. All properties such as the pure fluid surface tensions and the mixture densities and compositions required in Eqs. 1–4 were obtained using the REFPROP v10 [32] computer program, with additional changes that are detailed in the supplementary information, Appendix A. A second set of evaluations was made after fitting the binary interaction parameter δij to the experimental data with a trust-region reflective least squares algorithm in Python, scipy.optimize.curve_fit [198].Footnote 1 A single binary interaction parameter was fit for each fluid mixture pair, including all data sets for any given pair. For discussion of the results, we define AAPD as the average absolute percentage deviation, where PCTDEV = 100(σcalc − σexp)/σexp, and AAPD = (∑│PCTDEV│)/n, and the summation is over all n points. AAD is the average absolute deviation, AAD = (∑│σcalc − σexp │)/n, expressed in mN·m−1, and AADMAX is the maximum value of the AAD. We do not include in the statistics any points where the REFPROP program had convergence errors. Since the surface tension is zero at the critical point, some points near the critical region may have unusually large percentage deviations and it is more informative to examine the absolute deviation instead. Detailed results for each data set listed in Table 2 are presented in the supplemental information in Appendix B, Table B1. The data are also provided in the supplemental information. Here we will discuss the results in terms of chemical families.
4.1 Mixtures with n-Alkanes
Table 3 summarizes the results for mixtures with n-alkanes, presenting results both for binary interaction parameters set to zero and for fitted binary interaction parameters. Figure 1 displays these results graphically. The mixtures considered in this section contain n-alkanes mixed only with nonpolar fluids (branched alkanes, naphthenes, cryogens, and CO2) except for four mixtures with polar aprotic fluids dimethyl ether, acetone, dimethyl carbonate, and octamethylcyclotetrasiloxane (D4). Excluded from these results are mixtures of n-alkanes with hydrogen bonding fluids, aromatics, or halocarbons; these mixtures are treated separately in later sections. The results in Table 3 are arranged by mixture classes.
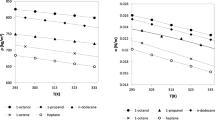
Overall, Fig. 1 and Table 3 show that without the use of binary interaction parameters, non-polar mixtures such as linear and branched alkanes, and linear and branched alkanes with naphthenes have average absolute percentage deviations of about 3 % or less. The propane/dimethyl ether mixture and the cyclohexane/acetone mixture also are represented very well without an interaction parameter. Mixtures of linear alkanes show increasing deviations as the mixtures become more asymmetric with respect to size, as has been discussed previously [199]. Figure 2 shows that the deviations of the parachor model for a series of mixtures of components of varying chain lengths (pentane, heptane, decane, and dodecane). Note that the full citations for the reference codes used in the figures are given in Table 2. The pentane/hexadecane mixture has the largest size difference, and the largest deviation, reaching 2.5 mN·m−1, and this deviation can be reduced with the use of a fitted binary interaction parameter to 1 mN·m−1 indicating that even mixtures of linear alkanes that only have size differences can benefit from the use of a binary interaction parameter. The temperatures of the data covered 293 K to 598 K, the details for each data set are given in Table 2. Although we used a simple constant binary interaction parameter, Hugill and Van Welsenes [16] and Gasem et al. [200] pointed out that the binary interaction parameters are temperature dependent, and introducing temperature dependence in the interaction parameters could further reduce the deviations.
Mixtures of n-alkanes with dimethyl carbonate and hexadecane with a siloxane have larger deviations with the maximum absolute deviation of approximately 2 mN·m−1, and although the use of an interaction parameter can reduce the deviations, the parachor model does not perform quite as well for these systems (with a max AD of ~ 1 mN·m−1) as it does for the n-alkane/n-alkane systems that often have max AD of 0.7 mN·m−1 or less with an interaction parameter. There are three mixtures of methane with cryogens; methane/argon and methane/krypton were represented to within 3 % without an interaction parameter, but methane/nitrogen required a binary interaction parameter to achieve an AAPD of less than 4 %.
Finally, the parachor model without interaction parameters does not adequately capture the mixture composition behavior of n-alkanes with carbon dioxide, and an interaction parameter is needed. This is illustrated in Fig. 3. The temperatures of the data covered 303 K to 378 K, the details for each data set are given in Table 2. Similar to what is indicated in Fig. 2, Fig. 3 shows the largest deviations occur for systems with the largest size differences, with decane/CO2 showing larger deviations than hexane/CO2 and butane/CO2. For the hexane/CO2 mixture without interaction parameters, the AAPD is near 30 % but can be reduced to less than 2 % (0.3 mN·m−1) with a binary interaction parameter. Note that the percentage deviations for decane/CO2 and butane/CO2 are still large even with a binary interaction parameter, but this is because the data sets contain points approaching the critical region where the values of the surface tensions are small and the resulting percentage deviations are very large.
4.2 Mixtures with Alcohols
Table 4 summarizes the results for mixtures with alcohols, and Fig. 4 displays these results graphically. We include only mixtures with methanol and ethanol; larger alcohols are not presently available in REFPROP. This group of mixtures includes alcohols with a variety of fluid types [alcohols, n-alkanes, branched alkanes, amines, aromatics, glycols, ketones, naphthenes, and a fatty acid methyl ester (FAME)]. Mixtures with water are excluded and treated in Sect. 4.3. For the binary mixture of methanol and ethanol, the parachor method represents the surface tension to essentially within experimental uncertainty, and an interaction parameter is unnecessary. Similarly, mixtures of methanol and ethanol with acetone are represented very well without an interaction parameter. Mixtures of alcohols with aromatics and alcohols with linear alkanes have AAPD’s without binary interaction parameters ranging from roughly 1 % to 5 %, which can be reduced to less than 3 % with binary interaction parameters. In Fig. 4, for mixtures without binary interaction parameters, mixtures of alcohols with the amines MEA and DEA, and with ethylene glycol show large deviations. Of the components in mixtures with methanol and ethanol, pure MEA, DEA, and ethylene glycol have the largest values of surface tension (approximately 45 mN·m−1 at 313 K) compared to less than about 27 mN·m−1 for the other fluids in Table 4, and approximately 21 mN·m−1 for pure methanol and ethanol. Maximum deviations can be as large as 7 mN·m−1 for the mixtures with these three fluids and the parachor model is not recommended without a binary interaction parameter. With a binary interaction parameter, the maximum deviations can be reduced to 1–2 mN·m−1.
4.3 Mixtures with Water
Table 5 summarizes the results for mixtures with water, and Fig. 5 displays these results graphically. The mixtures of water and heavy water with methanol and ethanol, and those with water and acetone show extremely large deviations, with a maximum AAD reaching 15–41 mN·m−1. The parachor model completely fails to represent the surface tension of these mixtures. The use of a single, constant binary interaction parameter somewhat reduces the magnitude of the deviations, but the model is still not very good with maximum deviations on the order of 5–13 mN·m−1. Without interaction parameters, all organic/aqueous mixtures investigated here show a common deviation pattern, where the deviations have an asymmetric shape with respect to composition, with a very rapid change as one nears the pure water end [8]. Water also has a very high surface tension (~ 70 mM·m−1 at 313 K) compared to other fluids. Figure 6, showing the percentage deviations of the acetone/water mixture as a function of composition, illustrates this pattern. The temperatures of the data covered 273 K to 343 K, the details for each data set are given in Table 2. One can see that although the use of an interaction parameter can somewhat reduce the size of the deviations, it cannot properly reproduce the composition behavior. A small amount of the organic can greatly change the surface tension, and the parachor model does not have the ability to model this composition behavior. It is possible that a more complex, composition and temperature dependent interaction parameter could capture this behavior, but it is beyond the scope of this work.
4.4 Mixtures with Aromatics
Table 6 summarizes the results for mixtures with aromatics, and Fig. 7 displays these results graphically. With a fitted binary interaction parameter all mixtures show an AAPD below 5 % except for the mixture toluene/octane that has an AAPD of 5 %. Without interaction parameters some of the points exceed 10 % deviation. However, these points occur at relatively high temperatures (380 K to 400 K) where the magnitude of the deviation is not excessively large (AAD of less than 0.82 mN·m−1) but the percentage deviations are larger due to the smaller value of the surface tension at higher temperatures. Other systems with deviations of approximately 10 % without interaction parameters are o-xylene/acetone, and some points in benzene/dodecane, toluene/pentane, and m-xylene/benzene. With interactions parameters the AADP in these systems, except m-xylene/benzene, can be reduced to 1 %. The m-xylene/benzene point with near 10 % deviation (for both fitted and non-fitted cases) is due to a pure fluid point for m-xylene (that we believe is flawed) from the 1929 data set of Hammick and Andrew [56]. In summary, when a fitted binary interaction parameter is used, the aromatic/alkane and aromatic/napthene mixtures have an AAPD of no greater than 1 %, however the deviations are a function of composition. With the use of a binary interaction parameter these mixtures can generally be represented to within 3 % over the entire composition range.
4.5 Mixtures with Halocarbons
Table 7 summarizes the results for mixtures with halocarbons, and Fig. 8 displays these results graphically. Included are mixtures containing some of the new low-GWP fluids such as R1234yf and R1234ze(E) in addition to HFC’s such as R32, R134a, R143a, R152a, and R125, and mixtures of polar halocarbons with nonpolar alkanes such as propane and butane. Without using an interaction parameter, almost all results are within 10 %, the AAPD’s are generally less than 5 %. Exceptions are visible in Fig. 8; the single point for R22/R115 has very large deviations; it is unclear why this mixture should deviate from the others. The mixture of R152a/propane also has deviations slightly greater than 10 % without an interaction parameter. It is unclear why R152a/propane should show this magnitude of deviation (AAPD 10.6 %), as a similar polar/nonpolar mixture of R32/propane displays smaller deviations (AAPD 2.6 %) without the use of an interaction parameter. The mixtures of halocarbons with other halocarbons without an interaction parameter have AAD of about 0.3 mN·m−1, while the mixtures of polar halocarbons with nonpolar alkanes have a higher AAD of up to 0.9 mN·m−1. The use of an interaction parameter improves the results, providing an AAD less than 0.35 mN·m−1 for both types of mixtures.
4.6 Mixtures with Miscellaneous Compounds
Table 8 summarizes the results for mixtures with miscellaneous compounds, and Fig. 9 displays these results graphically. The mixtures are either of cryogens with other cryogens, or siloxanes with siloxanes. All mixtures without interaction parameters except helium/argon show an AAPD of less than 10 %. As shown in Table 2, the helium/argon mixture data were obtained only for extremely dilute solutions of helium less than about a helium mole fraction of 0.01. Without more data over a larger composition range, it is difficult to assess the performance of the parachor model for the helium/argon system. In addition, there were convergence failures in REFPROP for the systems helium/argon, krypton/argon, and nitrogen/helium; points without convergence were not included in the statistics and binary interaction parameters were not determined for these systems. There also was an extremely limited composition range for neon/argon, hydrogen/argon, and nitrogen/helium so we cannot fully assess these systems either. The data for siloxane mixtures are very limited in the number of points, so it also is premature to assess these systems. For the cryogen/cryogen mixtures where there are a wide range of data, the parachor model appears to represent the data to within 10 % without interaction parameters, with AAPD’s of less than 5 %.
5 Conclusions
We compiled a database for the surface tension of binary mixtures by extracting data from the NIST TDE database [34]. It contains a wide variety of fluids, covering the chemical classes water, alcohols, amines, ketones, linear and branched alkanes, naphthenes, aromatics, refrigerants, and cryogens. The data set includes 65 pure fluids and 154 binary pairs with a total of 8205 points. We used this database to test the performance of a parachor model for mixtures, in both a predictive mode (no mixture data used) and with a single, constant binary interaction parameter found by fitting the mixture data. The parachor model is not new and variants of it have been used for many years, but a comprehensive summary of its performance on a wide variety of mixtures has not been available until now. The data are available in the supporting information to enable model comparisons for future research on binary mixtures with new models. In general, the parachor model in a predictive mode without fitted interaction parameters can predict the surface tension of binary mixtures of non-polar fluids such as linear and branched alkanes, linear and branched alkanes with naphthenes, aromatics with aromatics, aromatics with naphthenes, and mixtures of linear alkanes of similar sizes with an AAPD of about 3 % or less. For mixtures of linear alkanes of differing sizes, as the size difference increases it is necessary to use a fitted binary interaction parameter to reduce deviations. Similarly, in a predictive mode the model has large deviations for mixtures of n-alkanes with CO2, and an interaction parameter should be used. Mixtures of methanol and ethanol did not require an interaction parameter. Polar mixtures of halocarbons with other halocarbons and also polar/nonpolar mixtures of alkanes with halocarbons could be modeled with an AAD of less than 0.35 mN·m−1 with the use of a binary interaction parameter for each pair of fluids. Future work on develo** a predictive scheme for binary interaction parameters for classes of mixtures would make the parachor model more useful. Finally, the parachor model even with a fitted binary interaction parameter is not suitable for mixtures of water with organic compounds.
Notes
Certain equipment, instruments, software, or materials, commercial or non-commercial, are identified in this paper to specify the experimental procedure adequately. Such identification is not intended to imply recommendation or endorsement of any product or service by NIST, nor is it intended to imply that the materials or equipment identified are necessarily the best available for the purpose.
References
M.P. Krafft, Adv. Drug Deliv. Rev. 47, 209 (2001). https://doi.org/10.1016/S0169-409X(01)00107-7
A. Fathi Azarbayjani, A. Jouyban, S.Y. Chan, J. Pharm. Pharm. Sci. 12, 218 (2009). https://doi.org/10.18433/j32p40
R. Nagata, C. Kondou, S. Koyama, Int. J. Refrig. 63, 157 (2016). https://doi.org/10.1016/j.ijrefrig.2015.11.002
D. Jang, D. Kim, J. Moon, Langmuir 25, 2629 (2009). https://doi.org/10.1021/la900059m
J.E. Fromm, IBM J. Res. Dev. 28, 322 (1984). https://doi.org/10.1147/rd.283.0322
R.R. Mallepally, B.A. Bamgbade, A.J. Rowane, H.B. Rokni, M.S. Newkirk, M.A. McHugh, J. Supercrit. Fluids 134, 33 (2018). https://doi.org/10.1016/j.supflu.2017.12.003
S. Sugden, J. Chem. Soc. 125, 1177 (1924). https://doi.org/10.1039/ct9242501177
B.E. Poling, J.M. Prausnitz, J.P. O’Connell, The Properties of Gases and Liquids, 5th edn. (McGraw-HIll, New York, 2001)
J.W. Cahn, J.E. Hilliard, J. Chem. Phys. 28, 258 (1958). https://doi.org/10.1063/1.1744102
X.D. Liang, M.L. Michelsen, G.M. Kontogeorgis, Fluid Phase Equilib. 428, 153 (2016). https://doi.org/10.1016/j.fluid.2016.06.017
J.Z. Wu, AlChE J. 52, 1169 (2006). https://doi.org/10.1002/aic.10713
J.L. Li, J. Ma, C.J. Peng, H.L. Liu, Y. Hu, J. Jiang, Ind. Eng. Chem. Res. 46, 7267 (2007). https://doi.org/10.1021/ie070341m
S. Toxvaerd, J. Chem. Phys. 55, 3116 (1971). https://doi.org/10.1063/1.1676556
S.E. Quinones-Cisneros, U.K. Deiters, R.E. Rozas, T. Kraska, J. Phys. Chem. B 113, 3504 (2009). https://doi.org/10.1021/jp8073255
C.F. Weinaug, D.L. Katz, Ind. Eng. Chem. 35, 239 (1943). https://doi.org/10.1021/ie50398a028
J.A. Hugill, A.J. Van Welsenes, Fluid Phase Equilib. 29, 383 (1986). https://doi.org/10.1016/0378-3812(86)85038-5
T. Fechter, R. Villablanca, V. Leontijevic, A. Martin, P. Jaeger, M.J. Cocero, J. Supercrit. Fluids 196, 105899 (2023). https://doi.org/10.1016/j.supflu.2023.105899
T.E. Daubert, API Technical Databook (American Petroleum Institute, Washington, DC, 1997)
D.B. Macleod, Trans. Faraday Soc. 19, 38 (1923). https://doi.org/10.1039/TF9231900038
Y. Garrabos, F. Palencia, C. Lecioutre, D. Broseta, B. Le Neindre, Phys. Rev. E 76, 061109 (2007). https://doi.org/10.1103/PhysRevE.76.061109
O.R. Quayle, Chem. Rev. 53, 439 (1953). https://doi.org/10.1021/cr60166a003
V.P. Zhelezny, Y.V. Semenyuk, S.N. Ancherbak, N.V. Emel’yanenko, Russ. J. Phys. Chem. A 83, 182 (2009). https://doi.org/10.1134/s0036024409020071
I. Cachadiña, A. Mulero, J. Tian, J. Phys. Chem. Ref. Data 44, 023104 (2015). https://doi.org/10.1063/1.4921749
A. Mulero, I. Cachadiña, J. Phys. Chem. Ref. Data 43, 023104 (2014). https://doi.org/10.1063/1.4878755
A. Mulero, I. Cachadiña, D. Bautista, J. Phys. Chem. Ref. Data 50, 023104 (2021). https://doi.org/10.1063/5.0048675
A. Mulero, I. Cachadiña, M.I. Parra, J. Phys. Chem. Ref. Data 41, 043105 (2012). https://doi.org/10.1063/1.4768782
A. Mulero, I. Cachadiña, E.L. Sanjuán, J. Phys. Chem. Ref. Data 44, 033104 (2015). https://doi.org/10.1063/1.4927858
A. Mulero, I. Cachadiña, E.L. Sanjuán, J. Phys. Chem. Ref. Data 45, 033105 (2016). https://doi.org/10.1063/1.4959989
A. Mulero, I. Cachadiña, A. Vegas, J. Phys. Chem. Ref. Data 50, 033106 (2021). https://doi.org/10.1063/5.0061617
A. Mulero, I. Cachadiña, A. Vegas, J. Phys. Chem. Ref. Data 51, 023102 (2022). https://doi.org/10.1063/5.0092546
A. Mulero, I. Cachadiña, A. Becerra, J. Phys. Chem. Ref. Data 52, 013103 (2023). https://doi.org/10.1063/5.0139446
E.W. Lemmon, I.H. Bell, M.L. Huber, M.O. McLinden, REFPROP, NIST Standard Reference Database 23,Version 10.0 (NIST, Standard Reference Data Program, Gaithersburg, 2018). https://doi.org/10.18434/T4/1502528
M.L. Huber, E.W. Lemmon, I.H. Bell, M.O. McLinden, Ind. Eng. Chem. Res. 61, 15449 (2022). https://doi.org/10.1021/acs.iecr.2c01427
V. Diky, R.D. Chirico, M. Frenkel, A. Bazyleva, J.W. Magee, E. Paulechka, A. Kazakov, E.W. Lemmon, C.D. Muzny, A.Y. Smolyanitsky, S. Townsend, K. Kroenlein, NIST ThermoData Engine, NIST Standard Reference Database 103a/103b, Version 10.4 (NIST, Standard Reference Data Program, Gaithersburg, 2019). https://www.nist.gov/mml/acmd/trc/thermodata-engine/srd-nist-tde-103b
C. Tropea, A.L. Yarin, J.F. Foss, Springer Handbook of Experimental Fluid Mechanics (Springer, Berlin, 2007)
V. Diky, R.D. Chirico, R.C. Wilhoit, Q. Dong, M. Frenkel, J. Chem. Inf. Comput. Sci. 43, 15 (2003). https://doi.org/10.1021/ci025534t
M.L. Huber, Models for Viscosity, Thermal Conductivity, and Surface Tension of Selected Pure Fluids as Implemented in REFPROP v10.0 (National Institute of Standards and Technology, NISTIR 8209, 2018). https://nvlpubs.nist.gov/nistpubs/ir/2018/NIST.IR.8209.pdf
International Association for the Properties of Water and Steam, Release on Surface Tension of Heavy Water Substance, IAPWS R5-85 (1994). http://www.iapws.org/relguide/surfd2o.pdf
International Association for the Properties of Water and Steam, Revised Release on Surface Tension of Ordinary Water Substance, IAPWS R1-76 (2014). http://www.iapws.org/relguide/Surf-H2O.html
D.V.S. Jain, S. Singh, Indian J. Chem. 12, 714 (1974)
D.J.L. Prak, J.S. Cowart, A.M. McDaniel, P.C. Trulove, J. Chem. Eng. Data 59, 3571 (2014). https://doi.org/10.1021/je500498m
M.S. Dhillon, B.S. Mahl, Z. Phys. Chem. (Leipzig) 259, 249 (1978). https://doi.org/10.1515/zpch-1978-25933
B.S. Mahl, S.L. Chopra, P.P. Singh, Z. Phys. Chem. (Leipzig) 249, 337 (1972)
M. Domínguez-Peréz, E. Rilo, L. Segade, C. Franjo, O. Cabeza, J. Chem. Eng. Data 55, 1317 (2010). https://doi.org/10.1021/je900643r
A. Gayol, L.M. Casas, A.E. Andreatta, R.E. Martini, J.L. Legido, J. Chem. Eng. Data 58, 758 (2013). https://doi.org/10.1021/je301282p
L. Mosteiro, L.M. Casás, J.L. Legido, J. Chem. Thermodyn. 41, 695 (2009). https://doi.org/10.1016/j.jct.2008.12.010
G. Ouyang, G. Lu, Y. Yang, Z.Q. Huang, Acta Sci. Nat. Univ. Sunyatseni 43, 44 (2004)
R.K. Wanchoo, J. Narayan, Phys. Chem. Liq. 25, 15 (1992). https://doi.org/10.1080/00319109208027283
G. Ouyang, Y. Yang, S.S. Lu, Z.Q. Huang, B. Kang, J. Chem. Eng. Data 49, 330 (2004). https://doi.org/10.1021/je0341763
J.C.G. Calado, I.A. McLure, V.A.M. Soares, Fluid Phase Equilib. 2, 199 (1978). https://doi.org/10.1016/0378-3812(78)80009-0
J.J.-C. Hsu, N. Nagarajan, R.L. Robinson Jr., J. Chem. Eng. Data 30, 485 (1985). https://doi.org/10.1021/je00042a036
F. Gozalpour, A. Danesh, A.C. Todd, B. Tohidi, Fluid Phase Equilib. 233, 144 (2005). https://doi.org/10.1016/j.fluid.2005.03.032
R.P. Worley, J. Chem. Soc. 105, 273 (1914). https://doi.org/10.1039/CT9140500273
R. Tahery, J. Chem. Thermodyn. 106, 95 (2017). https://doi.org/10.1016/j.jct.2016.11.018
M. Dominguez-Perez, L. Segade, O. Cabeza, C. Franjo, E. Jimenez, J. Chem. Eng. Data 51, 294 (2006). https://doi.org/10.1021/je0504111
D.L. Hammick, L.W. Andrew, J. Chem. Soc. (1929). https://doi.org/10.1039/JR9290000754
J.L.R. Morgan, M.A. Griggs, J. Am. Chem. Soc. 39, 2261 (1917). https://doi.org/10.1021/ja02256a002
V.T. Lam, G.C. Benson, Can. J. Chem. 48, 3773 (1970). https://doi.org/10.1139/v70-637
T.D. Ling, M. Van Winkle, Ind. Eng. Chem. Chem. Eng. Data Ser. 3, 82 (1958). https://doi.org/10.1021/i460003a017
G.R. Vakili-Nezhaad, M. Al-Wadhahi, S. Al-Haddabi, A. Vakilinejad, W.E. Acree, J. Chem. Thermodyn. 152, 106292 (2021). https://doi.org/10.1016/j.jct.2020.106292
H. Kahl, T. Wadewitz, J. Winkelmann, J. Chem. Eng. Data 48, 580 (2003). https://doi.org/10.1021/je0201323
R.S. Myers, H.L. Clever, J. Chem. Thermodyn. 6, 949 (1974). https://doi.org/10.1016/0021-9614(74)90216-x
S.S. Shastri, A.K. Mukherjee, T.R. Das, J. Chem. Eng. Data 38, 399 (1993). https://doi.org/10.1021/je00011a017
S. Singh, B.S. Lark, S.K. Aggarwal, Indian J. Chem. Sect. A: Inorg. Phys. Theor. Anal. 21, 1116 (1982)
H. Kahl, T. Wadewitz, J. Winkelmann, J. Chem. Eng. Data 48, 1500 (2003). https://doi.org/10.1021/je034062r
S. Enders, H. Kahl, J. Winkelmann, J. Chem. Eng. Data 52, 1072 (2007). https://doi.org/10.1021/je7000182
B.I. Konobeev, V.V. Lyapin, Zh. Prikl. Khim. 43, 803 (1970)
I.M. Abdulagatov, A.P. Adamov, I.M. Abdurakhmanov, J. Eng. Phys. Thermophys. 63, 1193 (1992). https://doi.org/10.1007/BF00853519
M. Mohsen-Nia, H. Rasa, S.F. Naghibi, J. Chem. Thermodyn. 42, 110 (2010). https://doi.org/10.1016/j.jct.2009.07.018
M. Mohsen-Nia, Phys. Chem. Liq. 49, 608 (2011). https://doi.org/10.1080/00319104.2010.489189
J. Satherley, D.L. Cooper, D.J. Schiffrin, Fluid Phase Equilib. 456, 193 (2018). https://doi.org/10.1016/j.fluid.2017.10.023
H.L. Clever, W.E. Chase, J. Chem. Eng. Data 8, 291 (1963). https://doi.org/10.1021/je60018a004
K. Ridgway, P.A. Butler, J. Chem. Eng. Data 12, 509 (1967). https://doi.org/10.1021/je60035a012
R.L. Schmidt, H.L. Clever, J. Colloid Interface Sci. 26, 19 (1968). https://doi.org/10.1016/0021-9797(68)90266-X
T.M. Koller, S. Yan, C. Steininger, T. Klein, A.P. Fröba, Int. J. Thermophys. 40, 79 (2019). https://doi.org/10.1007/s10765-019-2544-y
D. Papaioannou, C.G. Panayiotou, J. Chem. Eng. Data 39, 457 (1994). https://doi.org/10.1021/je00015a012
E. Jimenez Cuesta, H. Casad, L. Segade, C. Franjo, J. Chem. Eng. Data 45, 862 (2000). https://doi.org/10.1021/je000060k
B. Giner, A. Villares, S. Martin, H. Artigas, C. Lafuente, J. Chem. Eng. Data 52, 1904 (2007). https://doi.org/10.1021/je700215z
H.G. Trieschmann, Z. Phys. Chem. Abt. B 29, 328 (1935)
V. Ramakrishna, M. Patel, Indian J. Chem. 8, 256 (1970)
R.L. Schmidt, J.C. Randall, H.L. Clever, J. Phys. Chem. 70, 3912 (1966). https://doi.org/10.1021/j100884a027
D. Gomez-Diaz, J.C. Mejuto, J.M. Navaza, A. Rodriguez-Alvarez, J. Chem. Eng. Data 47, 872 (2002). https://doi.org/10.1021/je010288n
D. Gomez-Diaz, J.C. Mejuto, J.M. Navaza, J. Chem. Eng. Data 46, 720 (2001). https://doi.org/10.1021/je000310x
A. Mejia, H. Segura, M. Cartes, C. Calvo, Fluid Phase Equilib. 270, 75 (2008). https://doi.org/10.1016/j.fluid.2008.06.006
S.K. Suri, V. Ramakrishna, J. Phys. Chem. 72, 3073 (1968). https://doi.org/10.1021/j100855a001
M. Abroodi, A. Bagheri, B.M. Razavizadeh, J. Mol. Liq. 287, 110924 (2019). https://doi.org/10.1016/j.molliq.2019.110924
E.B. Rinker, D.W. Oelschlager, A.T. Colussi, K.R. Henry, O.C. Sandall, J. Chem. Eng. Data 39, 392 (1994). https://doi.org/10.1021/je00014a046
G. Vazquez, E. Alvarez, R. Rendo, E. Romero, J.M. Navaza, J. Chem. Eng. Data 41, 806 (1996). https://doi.org/10.1021/je960012t
E. Alvarez, R. Rendo, B. Sanjurjo, M. Sanchez-Vilas, J.M. Navaza, J. Chem. Eng. Data 43, 1027 (1998). https://doi.org/10.1021/je980106y
J. Aguila-Hernandez, A. Trejo Rodriguez, J. Gracia-Fadrique, Fluid Phase Equilib. 185, 165 (2001). https://doi.org/10.1016/s0378-3812(01)00467-8
E. Alvarez, A. Cancela, R. Maceiras, J.M. Navaza, R. Taboas, J. Chem. Eng. Data 48, 32 (2003). https://doi.org/10.1021/je020048n
D. Fu, L. Du, H. Wang, J. Chem. Thermodyn. 69, 132 (2014). https://doi.org/10.1016/j.jct.2013.10.016
A. Dey, S.K. Dash, B. Mandal, Fluid Phase Equilib. 463, 91 (2018). https://doi.org/10.1016/j.fluid.2018.01.030
D. Fu, J. **e, F. Wang, S. Wang, J. Chem. Thermodyn. 116, 197 (2018). https://doi.org/10.1016/j.jct.2017.08.024
A. Shojaeian, J. Mol. Liq. 254, 26 (2018). https://doi.org/10.1016/j.molliq.2018.01.077
J. de los S. López-Lázaro, G.A. Iglesias-Silva, A. Estrada-Baltazar, J. Baraja-Fernandez, J. Chem. Eng. Data 60, 1823 (2015). https://doi.org/10.1021/acs.jced.5b00009
L. Segade, J.J. de Llano, M. Dominguez-Perez, O. Cabeza, M. Cabanas, E. Jimenez, J. Chem. Eng. Data 48, 1251 (2003). https://doi.org/10.1021/je034053i
A. Mejia, M. Cartes, H. Segura, J. Chem. Thermodyn. 43, 1395 (2011). https://doi.org/10.1016/j.jct.2011.04.005
A.E. Andreatta, R.E. Martini, J.L. Legido, L. Casas, Int. J. Eng. Res. Sci. 2, 51 (2016)
E. Onder, N. Sarier, Thermochim. Acta 690, 178698 (2020). https://doi.org/10.1016/j.tca.2020.178698
H.B. Evans, H.L. Clever, J. Phys. Chem. 68, 3433 (1964). https://doi.org/10.1021/j100793a507
J. Yang, J. Wu, J. Chem. Thermodyn. 170, 106782 (2022). https://doi.org/10.1016/j.jct.2022.106782
D.J.L. Prak, A.L. Mungan, J.S. Cowart, P.C. Trulove, J. Chem. Eng. Data 63, 1642 (2018). https://doi.org/10.1021/acs.jced.8b00008
S. Bi, X. Li, G. Zhao, J. Wu, Fluid Phase Equilib. 298, 150 (2010). https://doi.org/10.1016/j.fluid.2010.07.026
N. Nagarajan, R.L. Robinson Jr., J. Chem. Eng. Data 31, 168 (1986). https://doi.org/10.1021/je00044a012
R.D. Shaver, R.L. Robinson Jr., K.A.M. Gasem, Fluid Phase Equilib. 179, 43 (2001). https://doi.org/10.1016/s0378-3812(00)00475-1
L.I. Rolo, A.I. Caco, A.J. Queimada, I.M. Marrucho, J.A.P. Coutinho, J. Chem. Eng. Data 47, 1442 (2002). https://doi.org/10.1021/je025536+
A.J. Queimada, A.I. Caco, I.M. Marrucho, J.A.P. Coutinho, J. Chem. Eng. Data 50, 1043 (2005). https://doi.org/10.1021/je050024r
V.N. Grigor’ev, N.S. Rudenko, Zh. Eksp. Teor. Fiz. 47, 92 (1964)
Y.P. Blagoi, G.P. Kropachev, V.V. Pashkov, Ukr. Fiz. Zh. 12, 1300 (1967)
M. Abroodi, A. Bagheri, B.M. Razavizadeh, J. Chem. Eng. Data 65, 3173 (2020). https://doi.org/10.1021/acs.jced.0c00192
Anonymous, Chem. Eng. Oil Gas 3, 1 (1981). https://oversea.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&filename=STQG198103000&dbname=CJFD7984
G. Vazquez, E. Alvarez, J.M. Navaza, R. Rendo, E. Romero, J. Chem. Eng. Data 42, 57 (1997). https://doi.org/10.1021/je960238w
J. Han, J. **, D.A. Eimer, M.C. Melaaen, J. Chem. Eng. Data 57, 1095 (2012). https://doi.org/10.1021/je2010038
S.A. Jayarathna, C.K. Jayarathna, D.A. Kottage, S. Dayarathna, D.A. Eimer, M.C. Melaaen, J. Chem. Eng. Data 58, 343 (2013). https://doi.org/10.1021/je300920t
S.A. Jayarathna, A. Weerasooriya, S. Dayarathna, D.A. Eimer, M.C. Melaaen, J. Chem. Eng. Data 58, 986 (2013). https://doi.org/10.1021/je301279x
D.J. Luning Prak, J.S. Cowart, P.C. Trulove, J. Chem. Eng. Data 59, 3842 (2014). https://doi.org/10.1021/je5007532
J. Koefoed, J.V. Villadsen, Acta Chem. Scand. 12, 1124 (1958)
A.J. Queimada, F.A.E. Silva, A.I. Caco, I.M. Marrucho, J.A.P. Coutinho, Fluid Phase Equilib. 214, 211 (2003). https://doi.org/10.1016/s0378-3812(03)00354-6
H. Yue, Z. Liu, J. Chem. Eng. Data 61, 1270 (2016). https://doi.org/10.1021/acs.jced.5b00903
H. Zhou, W. Zhu, Huaxue Gongcheng 21, 61 (1993)
R. Heide, J. Schenk, in Bestimmung der der Transportgrößen von HFKW, Bericht zum AiF-Forschungsvorhaben Nr. 10044B, Heft 1: Viskosität und Oberflächenspannung (Forschungsrat Kältetechnik e.V., Frankfurt am Main, 1996)
M. Okada, T. Shibata, Y. Sato, Y. Higashi, Int. J. Thermophys. 20, 119 (1999). https://doi.org/10.1023/a:1021482231102
A.P. Fröba, S. Will, A. Leipertz, Int. J. Thermophys. 22, 1349 (2001). https://doi.org/10.1023/a:1012832701996
Y.Y. Duan, H. Lin, Fluid Phase Equilib. 213, 89 (2003). https://doi.org/10.1016/s0378-3812(03)00236-x
S. Bi, G. Zhao, J. Wu, Fluid Phase Equilib. 287, 23 (2009). https://doi.org/10.1016/j.fluid.2009.09.005
H. Lin, Y.Y. Duan, J. Chem. Eng. Data 49, 372 (2004). https://doi.org/10.1021/je0342163
H. Lin, Y.Y. Duan, Int. J. Thermophys. 24, 1495 (2003). https://doi.org/10.1023/b:ijot.0000004090.64922.63
C. Zhang, G. Li, L. Yue, Y. Guo, W. Fang, J. Chem. Eng. Data 60, 2541 (2015). https://doi.org/10.1021/acs.jced.5b00105
R.S. Myers, H.L. Clever, J. Chem. Eng. Data 14, 161 (1969). https://doi.org/10.1021/je60041a014
F.B. Sprow, J.M. Prausnitz, Trans. Am. Inst. Chem. Eng. 62, 1105 (1965). https://doi.org/10.1039/TF9666201105
W. Ramsay, E. Aston, Trans. R. Ir. Acad. 32, 93 (1902)
A. Ritzel, Z. Phys. Chem. Stoechiom. Verwandschaftsl. 60, 319 (1907)
J.L.R. Morgan, A.J. Scarlett, J. Am. Chem. Soc. 39, 2275 (1917). https://doi.org/10.1021/ja02256a003
J. Traube, J. Prakt. Chem. 31, 77 (1885)
M. Descude, J. Phys. Paris 2, 348 (1903)
J.L.R. Morgan, M. Neidle, J. Am. Chem. Soc. 35, 1856 (1913). https://doi.org/10.1021/ja02201a006
L.L. Bircumshaw, J. Chem. Soc. Trans. 121, 887 (1922). https://doi.org/10.1039/CT9222100887
R.C. Ernst, C.H. Watkins, H.H. Ruwe, J. Phys. Chem. 40, 627 (1936). https://doi.org/10.1021/j150374a008
S. Valentiner, H.W. Hohls, Z. Phys. 108, 101 (1937)
W.S. Bonnell, L. Byman, D.B. Keyes, Ind. Eng. Chem. 32, 532 (1940). https://doi.org/10.1021/ie50364a019
B. Stahlberger, A. Guyer, Helv. Chim. Acta 33, 243 (1950)
B.Y. Teitel’baum, T.A. Gortalova, E.E. Sidorova, Zh. Fiz. Khim. 25, 911 (1951)
Y.V. Efremov, Zh. Fiz. Khim. 42, 1906 (1968)
L.S.C. Wan, T. Jeyabalan, Chem. Pharm. Bull. 34, 4744 (1986). https://doi.org/10.1248/cpb.34.4744
M.A. Kalbassl, M.W. Biddulph, J. Chem. Eng. Data 33, 473 (1988). https://doi.org/10.1021/je00054a024
G. Vazquez, E. Alvarez, J.M. Navaza, J. Chem. Eng. Data 40, 611 (1995). https://doi.org/10.1021/je00019a016
R. Belda, J.V. Herraez, O. Diez, Phys. Chem. Liq. 43, 91 (2005). https://doi.org/10.1080/00319100512331327342
R.B. Maximino, Phys. Chem. Liq. 47, 475 (2009). https://doi.org/10.1080/00319100802241657
K. Ludzik, K. Kustrzepa, H. Piekarski, M. Jozwiak, J. Chem. Eng. Data 61, 1047 (2016). https://doi.org/10.1021/acs.jced.5b00485
D. Gonçalves, M.F. Paludetti, P.M. Florido, C. Tonetti, C.B. Gonçalves, C.E.C. Rodrigues, J. Chem. Eng. Data 63, 2718 (2018). https://doi.org/10.1021/acs.jced.8b00086
M.A. Raza, P.D. Hallett, X. Liu, M. He, W. Afzal, J. Chem. Eng. Data 64, 5049 (2019). https://doi.org/10.1021/acs.jced.9b00026
D. Gonçalves, C. Panzarin, C.B. Gonçalves, C.E.C. Rodrigues, Fluid Phase Equilib. 521, 112730 (2020). https://doi.org/10.1016/j.fluid.2020.112730
S. Khosharay, M. Rahmanzadeh, B. ZareNezhad, Int. J. Thermophys. 41, 166 (2020). https://doi.org/10.1007/s10765-020-02738-0
D. Gomez-Diaz, J.M. Navaza, J. Chem. Eng. Data 66, 2160 (2021). https://doi.org/10.1021/acs.jced.1c00061
A. Bagheri, A.H. Amiri-Majed, J. Chem. Thermodyn. 51, 45 (2012). https://doi.org/10.1016/j.jct.2012.02.017
S. Azizian, M. Hemmati, J. Chem. Eng. Data 48, 662 (2003). https://doi.org/10.1021/je025639s
R.H. Campbell, E.M. Kartzmark, J. Chem. Thermodyn. 5, 163 (1973). https://doi.org/10.1016/s0021-9614(73)80076-x
S.I. Uchida, K. Matsumoto, Kagaku Kogaku 22, 570 (1958)
W.E. Shipp, J. Chem. Eng. Data 15, 308 (1970). https://doi.org/10.1021/je60045a020
Z.M. Rong, Y. Lu, Huaxue Gongcheng 16, 56 (1988)
R.C. Ernst, E.E. Litkenhous, J.W. Spanyer, J. Phys. Chem. 36, 842 (1932). https://doi.org/10.1021/j150333a006
B.Y. Teitel’baum, S.G. Ganelina, T.A. Gortalova, Zh. Fiz. Khim. 25, 1043 (1951)
K.S. Howard, R.A. McAllister, AIChE J. 3, 325 (1957). https://doi.org/10.1002/aic.690030308
A.I. Toryanik, V.G. Pogrebnik, J. Struct. Chem. 17, 464 (1976). https://doi.org/10.1007/BF00746671
V.G. Baidakov, A.M. Kaverin, M.N. Khotienkova, Fluid Phase Equilib. 356, 90 (2013). https://doi.org/10.1016/j.fluid.2013.07.008
K.N. Seneviratne, T.J. Hughes, M.L. Johns, K.N. Marsh, E.F. May, J. Chem. Thermodyn. 111, 173 (2017). https://doi.org/10.1016/j.jct.2017.03.002
Y.P. Blagoi, Ukr. Fiz. Zh. 5, 109 (1960)
S. Fuks, A. Bellemans, Physica 32, 594 (1966). https://doi.org/10.1016/0031-8914(66)90049-8
K. Tanaka, Y. Higashi, J. Chem. Eng. Data 54, 1656 (2009). https://doi.org/10.1021/je800756r
G. Zhao, S. Bi, J. Wu, Z. Liu, J. Chem. Eng. Data 55, 3077 (2010). https://doi.org/10.1021/je901085t
Y.Y. Duan, L. Hong, J. Chem. Eng. Data 48, 1068 (2003). https://doi.org/10.1021/je030137f
Y. Liu, C. Kondou, C. Coquelet, C. Houriez, Int. J. Refrig. 132, 276 (2021). https://doi.org/10.1016/j.ijrefrig.2021.09.021
H. Lin, Y.Y. Duan, J. Chem. Eng. Data 50, 182 (2005). https://doi.org/10.1021/je049743g
J. Cui, S. Bi, X. Meng, J. Wu, J. Chem. Eng. Data 61, 950 (2016). https://doi.org/10.1021/acs.jced.5b00798
K. Tanaka, Y. Higashi, J. Chem. Eng. Jpn. 46, 371 (2013). https://doi.org/10.1252/jcej.13we021
Anonymous, Freon Fluorocarbons – Properties and Applications (E.I. Du Pont De Nemours & Co. (INC.), Wilmington, 1969)
Y.P. Blagoi, N.S. Rudenko, Izv. Vyssh. Uchebn. Zaved. Fiz. 2, 22 (1959)
V.B. Ostromukhov, M.G. Ostronov, Zh. Fiz. Khim. 68, 39 (1994)
V.G. Baidakov, A.M. Kaverin, V.N. Andbaeva, Fluid Phase Equilib. 270, 116 (2008). https://doi.org/10.1016/j.fluid.2008.06.016
A.M. Kaverin, V.N. Andbaeva, V.G. Baidakov, Zh. Fiz. Khim. 80, 495 (2006). https://doi.org/10.1134/s0036024406030174
V.G. Baidakov, A.M. Kaverin, Zh. Fiz. Khim. 78, 1150 (2004)
K.A. Clendenning, Can. J. Res. Sect. F 24, 249 (1946)
K. Nakanishi, T. Matsumoto, M. Hayatsu, J. Chem. Eng. Data 16, 44 (1971). https://doi.org/10.1021/je60048a010
Y.S. Won, D.K. Chung, A.F. Mills, J. Chem. Eng. Data 26, 140 (1981). https://doi.org/10.1021/je00024a011
B.C. Hoke, J.C. Chen, J. Chem. Eng. Data 36, 322 (1991). https://doi.org/10.1021/je00003a019
A. Horibe, S. Fukusako, M. Yamada, Int. J. Thermophys. 17, 483 (1996). https://doi.org/10.1007/bf01443405
N.G. Tsierkezos, I.E. Molinou, J. Chem. Eng. Data 43, 989 (1998). https://doi.org/10.1021/je9800914
K. Habrdova, S. Hovorka, L. Bartovska, J. Chem. Eng. Data 49, 1003 (2004). https://doi.org/10.1021/je049955d
J. Zhang, P. Zhang, F. Han, G. Chen, R. Deng, X.H. Wei, J. Chem. Eng. Data 53, 2372 (2008). https://doi.org/10.1021/je800271e
A.A. Rafati, A. Bagheri, M. Najafi, J. Chem. Thermodyn. 43, 248 (2011). https://doi.org/10.1016/j.jct.2010.09.003
A.K. Tiwari, Sonu, S.K. Saha, J. Chem. Thermodyn. 70, 24 (2014). https://doi.org/10.1016/j.jct.2013.10.010
Y. Saji, T. Okuda, Adv. Cryog. Eng. 10, 209 (1965)
S. Bi, J. Cui, G. Zhao, J. Wu, Fluid Phase Equilib. 414, 60 (2016). https://doi.org/10.1016/j.fluid.2016.01.013
H.I. Waterman, W.R.E. van Herwijnen, H.W. den Hartog, J. Appl. Chem. (Lond.) 8, 625 (1958). https://doi.org/10.1002/jctb.5010081001
K.C. Nadler, Ph.D. Thesis, Cornell University, 1987
I.I. Sulla, V.G. Baidakov, Zh. Fiz. Khim. 68, 63 (1994)
P. Virtanen, R. Gommers, T.E. Oliphant et al., Nat. Methods 17, 261 (2020). https://doi.org/10.1038/s41592-019-0686-2
A.S. Bezera, J.M.T. Santos, M.L.L. Paredes, Fluid Phase Equilib. 288, 55 (2010). https://doi.org/10.1016/j.fluid.2009.10.011
K.A.M. Gasem, P.B. Dulcamara, K.B. Dickson, R.L. Robinson, Fluid Phase Equilib. 53, 39 (1989). https://doi.org/10.1016/0378-3812(89)80071-8
Acknowledgements
We thank Dr. Allan Harvey for helpful discussions.
Funding
Open access funding provided by NTNU Norwegian University of Science and Technology (incl St. Olavs Hospital - Trondheim University Hospital). This work was partially funded by the National Institute of Standards and Technology. Additional support was provided by the NCCS Centre, performed under the Norwegian research programme Centres for Environment-friendly Energy Research (FME), funded by industry partners and the Research Council of Norway (257579).
Author information
Authors and Affiliations
Contributions
All authors participated in the writing and review of the manuscript. AML developed the python code used to perform the analysis and generate figures. VD provided the data.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Log, A.M., Diky, V. & Huber, M.L. Assessment of a Parachor Model for the Surface Tension of Binary Mixtures. Int J Thermophys 44, 110 (2023). https://doi.org/10.1007/s10765-023-03216-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-023-03216-z