Abstract
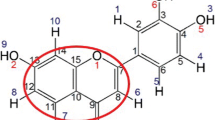
Ethanol is one of the most commonly used solvents to extract flavonoids from propolis. Hydrogen bonding interactions play an important role in the properties of liquid system. The main objective of the work is to study the hydrogen bonding interactions between flavonoid and ethanol. Luteolin is a very common flavonoid that has been found in different geographical and botanical propolis. In this work, it was selected as the representative flavonoid to do detailed research. The study was performed from a theoretical perspective using density functional theory (DFT) method. After careful optimization, there exist nine optimized geometries for the luteolin − CH3CH2OH complex. The binding distance of X − H···O, and the bond length, vibrational frequency, and electron density changes of X − H all indicate the formation of the hydrogen bond in the optimized geometries. In the optimized geometries, it is found that: (1) except for the H2’, H5’, and H6’, CH3CH2OH has formed hydrogen bonds with all the hydrogen and oxygen atoms in luteolin. The hydrogen atoms in the hydroxyl groups of luteolin form the strongest hydrogen bonds with CH3CH2OH; (2) all of the hydrogen bonds are closed-shell interactions; (3) the strongest hydrogen bond is the O3’ − H3’···O in structure A, while the weakest one is the C3 − H3···O in structure E; (4) the hydrogen bonds of O3’ − H3’···O, O − H···O4, O − H···O3’ and O − H···O7 are medium strength and covalent dominant in nature. While the other hydrogen bonds are weak strength and possess a dominant character of the electrostatic interactions in nature.




Similar content being viewed by others
References
Marcucci MC (1995) Apidologie 26:83
Burdock GA (1998) Food Chem. Toxicol. 36:347
Merino N, González R, González A, et al. (1996) Arch. Med. Res. 27:285
Sroka Z, Żbikowska B, Hładyszowski J (2015) J. Mol. Model. 21:1
Kumazawa S, Hamasak T, Nakayama T (2004) Food Chem. 84:329
Barbarić M, Mišković K, Bojić M, et al. (2011) J. Ethnopharmacol. 135:772
Scheller S, Wilczok T, Imielski S, et al. (1990) Int. J. Radiat. Biol. 57:461
Scheiner S (1997) Hydrogen bonding. Oxford University Press, New York
Jeffrey GA, Saenger W (2012) Hydrogen bonding in biological structures. Springer, Berlin
Kataoka K, Harada A, Nagasaki Y (2001) Adv. Drug Deliv. Rev. 47:113
Moulton B, Zaworotko MJ (2001) Chem. Rev. 101:1629
Yagai S, Nakajima T, Kishikawa K, et al. (2005) J. Am. Chem. Soc. 127:11134
Dong K, Zhang SJ, Wang JJ (2016) Chem. Commun. 52:6744
Lee SH, Doherty TV, Linhardt RJ, et al. (2009) Biotechnol. Bioeng. 102:1368
Kasiotis KM, Anastasiadou P, Papadopoulos A, et al. (2017) PLoS One 12:0170077
Dimkić I, Ristivojević P, Janakiev T, et al. (2016) Ind. Crop. Prod. 94:856
Cao Y, Wang Y, Yuan Q (2004) Chromatographia 59:135
Oliveira BG, Lima MCA, Pitta IR, et al. (2010) J. Mol. Model. 16:119
Clark T, Hennemann M, Murray JS, et al. (2007) J. Mol. Model. 13:291
Zheng YZ, Zhou Y, Liang Q, et al. (2016) J. Mol. Model. 22:257
Li QZ, Lin QQ, Li WZ, et al. (2008) ChemPhysChem 9:2265
Li QZ, Wu GS, Yu ZW (2006) J. Am. Chem. Soc. 128:1438
Dega-Szafran Z, Katrusiak A, Szafran M (2006) J. Mol. Struct. 785:160
Szafran M, Katrusiak A, Dega-Szafran Z (2007) J. Mol. Struct. 839:99
Ireta J, Neugebauer J, Scheffler M (2004) J. Phys. Chem. A 108:5692
Köddermann T, Wertz C, Heintz A, et al. (2006) ChemPhysChem 7:1944
Knorr A, Ludwig R (2015) Sci Rep 5:17505
Zheng YZ, Zhou Y, Liang Q, et al. (2017) Dyes Pigments 141:179
Zhao Y, Truhlar DG (2008) Theor Chem Account 120:215
Reed AE, Curtiss LA, Weinhold F (1988) Chem. Rev. 88:899
Bader RFW (1994) Atoms in molecules: a quantum theory. Clarendon, Oxford
Lu T, Chen F (2012) J. Comput. Chem. 33:580
Boys SF, Bernardi F (1970) Mol. Phys. 19:553
Espinosa E, Molins E, Lecomte C (1998) Chem. Phys. Lett. 285:170
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, VothGA SP, Dannenberg JJ, Dapprich S, DanielsAD FÖ, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian09. Revision B.01. Gaussian Inc, Wallingford
Pauling L (1960) The nature of the chemical bond. Cornell University Press, New York
Roohi H, Nowroozi AR, Anjomshoa E (2011) Comput. Theor. Chem. 965:211
Rozas I, Alkorta I, Elguero J (2000) J. Am. Chem. Soc. 122:11154
Pacios LF (2004) J. Phys. Chem. A 108:1177
Acknowledgements
This work was supported by the Foundation of Fujian Educational Committee (JZ160431) and earmarked fund for China Agriculture Research System (CARS-45-KXJ7).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zheng, YZ., Xu, J., Liang, Q. et al. A density functional theory study on the hydrogen bonding interactions between luteolin and ethanol. J Mol Model 23, 245 (2017). https://doi.org/10.1007/s00894-017-3409-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-017-3409-6