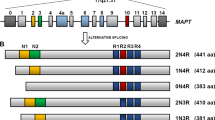
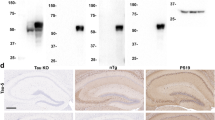
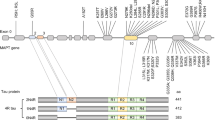
Abstract
Aggregation and cellular accumulation of tau protein is a defining feature of tauopathies, a class of histopathologically and clinically heterogeneous neurodegenerative diseases. Tauopathies include diseases as diverse as Alzheimer’s disease (AD), Pick’s disease, progressive supranuclear palsy, corticobasal degeneration, and chronic traumatic encephalopathy. Tau pathology affects different cell types in various tauopathies and strongly correlates with clinical symptoms. The complexity of tau pathology and the structural diversity of tau aggregates in different tauopathies represent an active area of research in neurodegeneration. The initiation, spreading, and cellular clearance of tau pathology play important roles in the disease process of tauopathies. Spreading of tau pathology throughout the brain may underlie the progressive nature of these diseases. Understanding of mechanisms underlying the spreading of tau pathology between neurons and glial cells is essential for the development and use of emerging therapeutics and tau biomarkers. Recent biomarkers have enabled identification of the earliest stages of AD pathology and tracking the anatomical progression of tau pathology over time. An increasing number of tau-targeting therapeutics are entering clinical trials and might lead to the development of a treatment for these devastating neurodegenerative disorders.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Vogels T, Leuzy A, Cicognola C et al (2020) Propagation of tau pathology: integrating insights from postmortem and in vivo studies. Biol Psychiatry 87(9):808–818. https://doi.org/10.1016/j.biopsych.2019.09.019
Prince M (2017) Progress on dementia-leaving no one behind. Lancet 390(10113):51–53. https://doi.org/10.1016/S0140-6736(17)31757-9
Scheltens P, Blennow K, Breteler MMB et al (2017) Alzheimer’s disease. Lancet 388(10043):505–517. https://doi.org/10.1016/S0140-6736(15)01124-1
Hoglinger GU, Respondek G, Kovacs GG (2018) New classification of tauopathies. Rev Neurol 174(9):664–668. https://doi.org/10.1016/j.neurol.2018.07.001
Vogels T, Murgoci A-N, Hromadka T (2019) Intersection of pathological tau and microglia at the synapse. Acta Neuropathol Comm 7(1):109. https://doi.org/10.1186/s40478-019-0754-y
Gomez-Isla T, Hollister R, West H et al (1997) Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer’s disease. Ann Neurol 41(1):17–24. https://doi.org/10.1002/ana.410410106
Arriagada PV, Growdon JH, Hedley-Whyte ET et al (1992) Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology 42(3):631–639
Wang Y, Mandelkow E (2016) Tau in physiology and pathology. Nat Rev Neurosci 17(1):5–21. https://doi.org/10.1038/nrn.2015.1
Zempel H, Mandelkow E (2014) Lost after translation: missorting of tau protein and consequences for Alzheimer disease. Trends Neurosci 37(12):721–732. https://doi.org/10.1016/j.tins.2014.08.004
Narasimhan S, Changolkar L, Riddle DM et al (2020) Human tau pathology transmits glial tau aggregates in the absence of neuronal tau. J Exp Med 217(2):e20190783. https://doi.org/10.1084/jem.20190783
Zhang Y, Chen K, Sloan SA et al (2014) An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 34(36):11929–11947. https://doi.org/10.1523/JNEUROSCI.1860-14.2014
Sotiropoulos I, Galas MC, Silva JM et al (2017) Atypical, non-standard functions of the microtubule associated Tau protein. Acta Neuropathol Comm 5(1):91. https://doi.org/10.1186/s40478-017-0489-6
Kent SA, Spires-Jones TL, Durrant CS (2020) The physiological roles of tau and Aβ: implications for Alzheimer’s disease pathology and therapeutics. Acta Neuropathol 140(4):417–447. https://doi.org/10.1007/s00401-020-02196-w
Gibbons GS, Lee VMY, Trojanowski JQ (2018) Mechanisms of cell-to-cell transmission of pathological tau: A Review. JAMA Neurol 76(1):101–108. https://doi.org/10.1001/jamaneurol.2018.2505
Jeganathan S, von Bergen M, Brutlach H et al (2006) Global hairpin folding of tau in solution. Biochemistry 45(7):2283–2293. https://doi.org/10.1021/bi0521543
Wesseling H, Mair W, Kumar M et al (2020) Tau PTM profiles identify patient heterogeneity and stages of Alzheimer’s disease. Cell 183(6):1699–1713. https://doi.org/10.1016/j.cell.2020.10.029
Guo T, Noble W, Hanger DP (2017) Roles of tau protein in health and disease. Acta Neuropathol 133(5):665–704. https://doi.org/10.1007/s00401-017-1707-9
von Bergen M, Friedhoff P, Biernat J et al (2000) Assembly of tau protein into Alzheimer paired helical filaments depends on a local sequence motif ((306)VQIVYK(311)) forming beta structure. PNAS 97(10):5129–5134
von Bergen M, Barghorn S, Li L et al (2001) Mutations of tau protein in frontotemporal dementia promote aggregation of paired helical filaments by enhancing local beta-structure. J Biol Chem 276(51):48165–48174. https://doi.org/10.1074/jbc.M105196200
Mirbaha H, Chen D, Morazova OA et al (2018) Inert and seed-competent tau monomers suggest structural origins of aggregation. eLife 7:e36584. https://doi.org/10.7554/eLife.36584
Kundel F, Hong L, Falcon B et al (2018) Measurement of tau filament fragmentation provides insights into prion-like spreading. ACS Chem Neurosci 9(6):1276–1282. https://doi.org/10.1021/acschemneuro.8b00094
Marreiro A, Van Kolen K, Sousa C et al (2020) Comparison of size distribution and (Pro249-Ser258) epitope exposure in in vitro and in vivo derived tau fibrils. BMC Mol and Cell Biol 21(1):81. https://doi.org/10.1186/s12860-020-00320-y
Falcon B, Cavallini A, Angers R et al (2015) Conformation determines the seeding potencies of native and recombinant Tau aggregates. J Biol Chem 290(2):1049–1065. https://doi.org/10.1074/jbc.M114.589309
Dujardin S, Commins C, Lathuiliere A et al (2020) Tau molecular diversity contributes to clinical heterogeneity in Alzheimer’s disease. Nat Med 26(8):1256–1263. https://doi.org/10.1038/s41591-020-0938-9
Crowther T, Goedert M, Wischik CM (1989) The repeat region of microtubule-associated protein tau forms part of the core of the paired helical filament of Alzheimer’s disease. Ann Med 21(2):127–132
Wischik CM, Novak M, Edwards PC et al (1988) Structural characterization of the core of the paired helical filament of Alzheimer disease. PNAS 85(13):4884–4888
Yagishita S, Itoh Y, Nan W et al (1981) Reappraisal of the fine structure of Alzheimer’s neurofibrillary tangles. Acta Neuropathol 54(3):239–246
Fitzpatrick AWP, Falcon B, He S et al (2017) Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature 547(7662):185–190. https://doi.org/10.1038/nature23002
Falcon B, Zhang W, Schweighauser M et al (2018) Tau filaments from multiple cases of sporadic and inherited Alzheimer’s disease adopt a common fold. Acta Neuropathol 136(5):699–708. https://doi.org/10.1007/s00401-018-1914-z
Falcon B, Zivanov J, Zhang W et al (2019) Novel tau filament fold in chronic traumatic encephalopathy encloses hydrophobic molecules. Nature 568(7752):420–423. https://doi.org/10.1038/s41586-019-1026-5
Falcon B, Zhang W, Murzin AG et al (2018) Structures of filaments from Pick’s disease reveal a novel tau protein fold. Nature 561(7721):137–140. https://doi.org/10.1038/s41586-018-0454-y
Zhang W, Tarutani A, Newell KL et al (2020) Novel tau filament fold in corticobasal degeneration. Nature 580(7802):283–287. https://doi.org/10.1038/s41586-020-2043-0
Arakhamia T, Lee CE, Carlomagno Y et al (2020) Posttranslational modifications mediate the structural diversity of tauopathy strains. Cell 180(4):633–644.e12. https://doi.org/10.1016/j.cell.2020.01.027
Zhang W, Falcon B, Murzin AG et al (2018) Heparin-induced tau filaments are polymorphic and differ from those in Alzheimer’s and Pick’s disease. BioRxiv:468892. https://doi.org/10.1101/468892
Sharma AM, Thomas TL, Woodard DR et al (2018) Tau monomer encodes strains. eLife 7:e37813. https://doi.org/10.7554/eLife.37813
Heckman MG, Brennan RR, Labbe C et al (2019) Association of MAPT subhaplotypes with risk of progressive supranuclear palsy and severity of tau pathology. JAMA Neurol 76(6):710–717. https://doi.org/10.1001/jamaneurol.2019.0250
Myer AJ, Pittman AM, Zhao AS et al (2007) The MAPT H1c risk haplotype is associated with increased expression of tau and especially of 4 repeat containing transcripts. Neurobiol Dis 25(3):561–570. https://doi.org/10.1016/j.nbd.2006.10.018
Rovelet-Lecrux A, Hannequin D, Guillin O et al (2010) Frontotemporal dementia phenotype associated with MAPT gene duplication. J Alzheimers Dis 21(3):897–902. https://doi.org/10.3233/JAD-2010-100441
Le Guennec K, Quenez O, Nicolas G et al (2017) 17q21.31 duplication causes prominent tau-related dementia with increased MAPT expression. Mol Psychiatry 22(8):1119–1125. https://doi.org/10.1038/mp.2016.226
Chen Z, Chen JA, Shatunov A et al (2019) Genome-wide survey of copy number variants finds MAPT duplications in progressive supranuclear palsy. Movement Disord 34(7):1049–1059. https://doi.org/10.1002/mds.27702
Huin V, Deramecourt V, Caparros-Lefebvre D et al (2016) The MAPT gene is differentially methylated in the progressive supranuclear palsy brain. Movement Disord 31(12):1883–1890. https://doi.org/10.1002/mds.26820
Andorfer C, Kress Y, Espinoza M et al (2003) Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J Neurochem 86(3):582–590
Strang KH, Golde TE, Giasson BI (2019) MAPT mutations, tauopathy, and mechanisms of neurodegeneration. Lab Investig 99(7):912–928. https://doi.org/10.1038/s41374-019-0197-x
Rizzu P, Van Swieten JC, Joosse M et al (1999) High prevalence of mutations in the microtubule-associated protein tau in a population study of frontotemporal dementia in the Netherlands. Am J Hum Genet 64(2):414–421. https://doi.org/10.1086/302256
Di Primio C, Quercioli V, Siano G et al (2017) The distance between N and C termini of tau and of FTDP-17 mutants Is modulated by microtubule interactions in living cells. Front Mol Neurosci 10:210. https://doi.org/10.3389/fnmol.2017.00210
Hutton M, Lendon CL, Rizzu P et al (1998) Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393(6686):702–705. https://doi.org/10.1038/31508
Hong M, Zhukareva V, Vogelsberg-Ragaglia V et al (1998) Mutation-specific functional impairments in distinct tau isoforms of hereditary FTDP-17. Science 282(5395):1914–1917
Gotz J, Bodea LG, Goedert M (2018) Rodent models for Alzheimer disease. Nat Rev Neurosci 19(10):583–598. https://doi.org/10.1038/s41583-018-0054-8
Daude N, Kim C, Kang SG et al (2020) Diverse, evolving conformer populations drive distinct phenotypes in frontotemporal lobar degeneration caused by the same MAPT-P301L mutation. Acta Neuropathol 139(6):1045–1070. https://doi.org/10.1007/s00401-020-02148-4
Drombosky KW, Chen D, Woodard D et al (2018) Native tau structure is disrupted by disease-associated mutations that promote aggregation. BioRxiv:330266. https://doi.org/10.1101/330266
Ambadipudi S, Biernat J, Riedel D et al (2017) Liquid-liquid phase separation of the microtubule-binding repeats of the Alzheimer-related protein tau. Nat Commun 8(1):275. https://doi.org/10.1038/s41467-017-00480-0
Wegmann S, Eftekharzadeh B, Tepper K et al (2018) Tau protein liquid-liquid phase separation can initiate tau aggregation. EMBO J 37(7):e98049. https://doi.org/10.15252/embj.201798049
Fichou Y, Lin Y, Rauch JN et al (2018) Cofactors are essential constituents of stable and seeding-active tau fibrils. PNAS 115(52):13234–13239. https://doi.org/10.1073/pnas.1810058115
Kametani F, Yoshida M, Matsubara T et al (2020) Comparison of common and disease-specific post-translational modifications of pathological tau associated with a wide range of tauopathies. Front Neurosci 14:1110. https://doi.org/10.3389/fnins.2020.581936
Li L, Jiang Y, Hu W, Tung YC et al (2019) Pathological alterations of tau in Alzheimer’s disease and 3xTg-AD mouse brains. Mol Neurobiol 56(9):6168–6183. https://doi.org/10.1007/s12035-019-1507-4
Courade JP, Angers R, Mairet-Coello G et al (2018) Epitope determines efficacy of therapeutic anti-tau antibodies in a functional assay with human Alzheimer tau. Acta Neuropathol 136(5):729–745. https://doi.org/10.1007/s00401-018-1911-2
Derisbourg M, Leghay C, Chiappetta G et al (2015) Role of the Tau N-terminal region in microtubule stabilization revealed by new endogenous truncated forms. Sci Rep 5:9659. https://doi.org/10.1038/srep09659
Novak M, Kabat J, Wischik CM (1993) Molecular characterization of the minimal protease resistant tau unit of the Alzheimer’s disease paired helical filament. EMBO J 12(1):365–370
Nicholls SB, DeVos SL, Commins C et al (2017) Characterization of TauC3 antibody and demonstration of its potential to block tau propagation. PLoS One 12(5):e0177914. https://doi.org/10.1371/journal.pone.0177914
Zilka N, Kovacech B, Barath P et al (2012) The self-perpetuating tau truncation circle. Biochem Soc T 40(4):681–686. https://doi.org/10.1042/BST20120015
Vogels T, Vargová G, Brezováková V et al (2020) Viral delivery of non-mutated human truncated tau to neurons recapitulates key features of human tauopathy in wild-type mice. J Alzheimers Dis 77(2):551–568. https://doi.org/10.3233/JAD-200047
de Calignon A, Fox LM, Pitstick R et al (2010) Caspase activation precedes and leads to tangles. Nature 464(7292):1201–1204. https://doi.org/10.1038/nature08890
Wang YP, Biernat J, Pickhardt M et al (2007) Stepwise proteolysis liberates tau fragments that nucleate the Alzheimer-like aggregation of full-length tau in a neuronal cell model. PNAS 104(24):10252–10257. https://doi.org/10.1073/pnas.0703676104
Bodea LG, Evans HT, Van der Jeugd A et al (2017) Accelerated aging exacerbates a pre-existing pathology in a tau transgenic mouse model. Aging Cell 16(2):377–386. https://doi.org/10.1111/acel.12565
Kundra R, Ciryam P, Morimoto RI et al (2017) Protein homeostasis of a metastable subproteome associated with Alzheimer’s disease. PNAS 114(28):5703–5711. https://doi.org/10.1073/pnas.1618417114
Wang P, Joberty G, Buist A et al (2017) Tau interactome map** based identification of Otub1 as Tau deubiquitinase involved in accumulation of pathological Tau forms in vitro and in vivo. Acta Neuropathol 133(5):731–749. https://doi.org/10.1007/s00401-016-1663-9
Zhang Y, Chen X, Zhao Y et al (2017) The role of ubiquitin proteasomal system and autophagy-lysosome pathway in Alzheimer’s disease. Rev Neurosci 28(8):861–868. https://doi.org/10.1515/revneuro-2017-0013
Kim E, Sakata K, Liao FF (2017) Bidirectional interplay of HSF1 degradation and UPR activation promotes tau hyperphosphorylation. PLoS Genet 13(7):e1006849. https://doi.org/10.1371/journal.pgen.1006849
Shelton LB, Baker JD, Zheng D et al (2017) Hsp90 activator Aha1 drives production of pathological tau aggregates. PNAS 114(36):9707–9712. https://doi.org/10.1073/pnas.1707039114
Darwich NF, Phan JM, Kim B et al (2020) Autosomal dominant VCP hypomorph mutation impairs disaggregation of PHF-tau. Science 370(6519):eaay8826. https://doi.org/10.1126/science.aay8826
Hoozemans JJM, van Haastert ES, Nijholt DAT et al (2009) The unfolded protein response is activated in pretangle neurons in Alzheimer’s disease hippocampus. Am J Pathol 174(4):1241–1251. https://doi.org/10.2353/ajpath.2009.080814
Liu YH, Wei W, Yin J et al (2009) Proteasome inhibition increases tau accumulation independent of phosphorylation. Neurobiol Aging 30(12):1949–1961. https://doi.org/10.1016/j.neurobiolaging.2008.02.012
Keck S, Nitsch R, Grune T et al (2003) Proteasome inhibition by paired helical filament-tau in brains of patients with Alzheimer’s disease. J Neurochem 85(1):115–122
Mori H, Kondo J, Ihara Y (1987) Ubiquitin is a component of paired helical filaments in Alzheimer’s disease. Science 235(4796):1641–1644
Tai HC, Serrano-Pozo A, Hashimoto T et al (2012) The synaptic accumulation of hyperphosphorylated tau oligomers in Alzheimer disease is associated with dysfunction of the ubiquitin-proteasome system. Am J Pathol 181(4):1426–1435. https://doi.org/10.1016/j.ajpath.2012.06.033
Chastagner P, Loria F, Vargas JY et al (2020) Fate and propagation of endogenously formed Tau aggregates in neuronal cells. EMBO Mol Med 12:e12025. https://doi.org/10.15252/emmm.202012025
Goo MS, Sancho L, Slepak N et al (2017) Activity-dependent trafficking of lysosomes in dendrites and dendritic spines. J Cell Biol 216(8):2499–2513. https://doi.org/10.1083/jcb.201704068
Inoue K, Rispoli J, Yang L et al (2013) Coordinate regulation of mature dopaminergic axon morphology by macroautophagy and the PTEN signaling pathway. PLoS Genet 9(10):e1003845. https://doi.org/10.1371/journal.pgen.1003845
Hernandez D, Torres CA, Setlik W et al (2012) Regulation of presynaptic neurotransmission by macroautophagy. Neuron 74(2):277–284. https://doi.org/10.1016/j.neuron.2012.02.020
Akwa Y, Gondard E, Mann A et al (2017) Synaptic activity protects against AD and FTD-like pathology via autophagic-lysosomal degradation. Mol Psychiatry 23(6):1530–1540. https://doi.org/10.1038/mp.2017.142
Kimura T, Suzuki M, Akagi T (2017) Age-dependent changes in synaptic plasticity enhance tau oligomerization in the mouse hippocampus. Acta Neuropathol Comm 5(1):67. https://doi.org/10.1186/s40478-017-0469-x
Jiang S, Bhaskar (2020) Degradation and transmission of tau by autophagic-endolysosomal networks and potential therapeutic targets for tauopathy. Front Mol Neurosci 13:586731. https://doi.org/10.3389/fnmol.2020.586731
Wiersma VI, van Ziel AM, Vazquez-Sanchez S et al (2019) Granulovacuolar degeneration bodies are neuron-selective lysosomal structures induced by intracellular tau pathology. Acta Neuropathol 138(6):943–970. https://doi.org/10.1007/s00401-019-02046-4
Puladi B, Dinekov M, Arzberger T et al (2020) The relation between tau pathology and granulovacuolar degeneration of neurons. Neurobiol Dis 147:105138. https://doi.org/10.1016/j.nbd.2020.105138
Wiersma VI, Hoozemans JJM, Scheper W (2020) Untangling the origin and function of granulovacuolar degeneration bodies in neurodegenerative proteinopathies. Acta Neuropathol Comm 8(1):153. https://doi.org/10.1186/s40478-020-00996-5
Thal DR, Del Tredici K, Ludolph AC et al (2011) Stages of granulovacuolar degeneration: their relation to Alzheimer’s disease and chronic stress response. Acta Neuropathol 122(5):577–589. https://doi.org/10.1007/s00401-011-0871-6
Koper MJ, Van Schoor E, Ospitalieri S et al (2020) Necrosome complex detected in granulovacuolar degeneration is associated with neuronal loss in Alzheimer’s disease. Acta Neuropathol 139(3):463–484. https://doi.org/10.1007/s00401-019-02103-y
Hou X, Watzlawik JO, Cook C et al (2020) Mitophagy alterations in Alzheimer’s disease are associated with granulovacuolar degeneration and early tau pathology. Alzheimers Dement. https://doi.org/10.1002/alz.12198
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82(4):239–259
Nelson PT, Alafuzoff I, Bigio EH et al (2012) Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropath Exp Neur 71(5):362–381. https://doi.org/10.1097/NEN.0b013e31825018f7
Scholl M, Lockhart SN, Schonhaut DR et al (2016) PET imaging of tau deposition in the aging human brain. Neuron 89(5):971–982. https://doi.org/10.1016/j.neuron.2016.01.028
Kovacs GG, Lukic MJ, Irwin DJ et al (2020) Distribution patterns of tau pathology in progressive supranuclear palsy. Acta Neuropathol 140(2):99–119. https://doi.org/10.1007/s00401-020-02158-2
Kovacs GG, **e SX, Robinson JL et al (2018) Sequential stages and distribution patterns of aging-related tau astrogliopathy (ARTAG) in the human brain. Acta Neuropathol Comm 6:50. https://doi.org/10.1186/s40478-018-0552-y
Alosco ML, Cherry JD, Huber BR et al (2020) Characterizing tau deposition in chronic traumatic encephalopathy (CTE): utility of the McKee CTE staging scheme. Acta Neuropathol 140(4):495–512. https://doi.org/10.1007/s00401-020-02197-9
Kim EJ, Hwang JHL, Gaus SE et al (2020) Evidence of corticofugal tau spreading in patients with frontotemporal dementia. Acta Neuropathol 139(1):27–43. https://doi.org/10.1007/s00401-019-02075-z
Schwarz AJ, Yu P, Miller BB et al (2016) Regional profiles of the candidate tau PET ligand 18F-AV-1451 recapitulate key features of Braak histopathological stages. Brain 139(Pt 5):1539–1550. https://doi.org/10.1093/brain/aww023
Lowe VJ, Wiste HJ, Senjem ML et al (2018) Widespread brain tau and its association with ageing, Braak stage and Alzheimer’s dementia. Brain 141(1):271–287. https://doi.org/10.1093/brain/awx320
Vogel JW, Iturria-Medina Y, Strandberg OT et al (2020) Spread of pathological tau proteins through communicating neurons in human Alzheimer’s disease. Nat Commun 11(1):2612. https://doi.org/10.1038/s41467-020-15701-2
Franzmeier N, Neitzel J, Rubinski A et al (2020) Functional brain architecture is associated with the rate of tau accumulation in Alzheimer’s disease. Nat Commun 11(1):347. https://doi.org/10.1038/s41467-019-14159-1
Franzmeier N, Dewenter A, Frontzkowski L et al (2020) Patient-centered connectivity-based prediction of tau pathology spread in Alzheimer’s disease. Sci Adv 6(48):eabd1327. https://doi.org/10.1126/sciadv.abd1327
Kaufman SK, Thomas TL, Del Tredici K et al (2017) Characterization of tau prion seeding activity and strains from formaldehyde-fixed tissue. Acta Neuropathol Comm 5(1):41. https://doi.org/10.1186/s40478-017-0442-8
Kaufman SK, Del Tredici K, Thomas TL et al (2018) Tau seeding activity begins in the transentorhinal/entorhinal regions and anticipates phospho-tau pathology in Alzheimer’s disease and PART. Acta Neuropathol 136(1):57–67. https://doi.org/10.1007/s00401-018-1855-6
DeVos SL, Corjuc BT, Oakley DH et al (2018) Synaptic tau seeding precedes tau pathology in human Alzheimer’s disease Brain. Front Neurosci 12:267. https://doi.org/10.3389/fnins.2018.00267
Frost B, Jacks RL, Diamond MI (2009) Propagation of Tau misfolding from the outside to the inside of a cell. J Biol Chem 284(19):12845–12852. https://doi.org/10.1074/jbc.M808759200
Guo JL, Lee VMY (2011) Seeding of normal tau by pathological tau conformers drives pathogenesis of Alzheimer-like tangles. J Biol Chem 286(17):15317–15331. https://doi.org/10.1074/jbc.M110.209296
Kfoury N, Holmes BB, Jiang H et al (2012) Trans-cellular propagation of Tau aggregation by fibrillar species. J Biol Chem 287(23):19440–19451. https://doi.org/10.1074/jbc.M112.346072
Santa-Maria I, Varghese M, Ksiezak-Reding H et al (2012) Paired helical filaments from Alzheimer disease brain induce intracellular accumulation of Tau protein in aggresomes. J Biol Chem 287(24):20522–20533. https://doi.org/10.1074/jbc.M111.323279
Clavaguera F, Bolmont T, Crowther RA et al (2009) Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol 11(7):909–913. https://doi.org/10.1038/ncb1901
Lasagna-Reeves CA, Castillo-Carranza DL, Sengupta U et al (2012) Alzheimer brain-derived tau oligomers propagate pathology from endogenous tau. Sci Rep 2:700. https://doi.org/10.1038/srep00700
Iba M, Guo JL, McBride JD et al (2013) Synthetic tau fibrils mediate transmission of neurofibrillary tangles in a transgenic mouse model of Alzheimer’s-like tauopathy. J Neurosci 33(3):1024–1037. https://doi.org/10.1523/JNEUROSCI.2642-12.2013
Albert M, Mairet-Coello G, Danis C et al (2019) Prevention of tau seeding and propagation by immunotherapy with a central tau epitope antibody. Brain 142(6):1736–1750. https://doi.org/10.1093/brain/awz100
de Calignon A, Polydoro M, Suarez-Calvet M et al (2012) Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron 73(4):685–697. https://doi.org/10.1016/j.neuron.2011.11.033
Harris JA, Koyama A, Maeda S et al (2012) Human P301L-mutant tau expression in mouse entorhinal-hippocampal network causes tau aggregation and presynaptic pathology but no cognitive deficits. PLoS One 7(9):e45881. https://doi.org/10.1371/journal.pone.0045881
Liu L, Drouet V, Wu JW et al (2012) Trans-synaptic spread of tau pathology in vivo. PLoS One 7(2):e31302. https://doi.org/10.1371/journal.pone.0031302
Wegmann S, Maury EA, Kirk MJ et al (2015) Removing endogenous tau does not prevent tau propagation yet reduces its neurotoxicity. EMBO J 34(24):3028–3041. https://doi.org/10.15252/embj.201592748
Wegmann S, Bennett RE, Delorme L et al (2019) Experimental evidence for the age dependence of tau protein spread in the brain. Sci Adv 5(6):eaaw6404. https://doi.org/10.1126/sciadv.aaw6404
Ahmed Z, Cooper J, Murray TK et al (2014) A novel in vivo model of tau propagation with rapid and progressive neurofibrillary tangle pathology: the pattern of spread is determined by connectivity, not proximity. Acta Neuropathol 127(5):667–683. https://doi.org/10.1007/s00401-014-1254-6
McAllister BB, Lacoursiere SG, Sutherland RJ et al (2020) Intracerebral seeding of amyloid-β and tau pathology in mice: factors underlying prion-like spreading and comparisons with α-synuclein. Neurosci Biobehav Rev 112:1–27. https://doi.org/10.1016/j.neubiorev.2020.01.026
Guo JL, Narasimhan S, Changolkar L et al (2016) Unique pathological tau conformers from Alzheimer’s brains transmit tau pathology in nontransgenic mice. J Exp Med 213(12):2635–2654. https://doi.org/10.1084/jem.20160833
Calafate S, Buist A, Miskiewicz K et al (2015) Synaptic contacts enhance cell-to-cell Tau pathology propagation. Cell Rep 11(8):1176–1183. https://doi.org/10.1016/j.celrep.2015.04.043
Takeda S, Wegmann S, Cho H et al (2015) Neuronal uptake and propagation of a rare phosphorylated high-molecular-weight tau derived from Alzheimer’s disease brain. Nat Commun 6:8490. https://doi.org/10.1038/ncomms9490
Wu JW, Herman M, Liu L et al (2013) Small misfolded tau species are internalized via bulk endocytosis and anterogradely and retrogradely transported in neurons. J Biol Chem 288(3):1856–1870. https://doi.org/10.1074/jbc.M112.394528
Dujardin S, Lécolle K, Caillierez R et al (2014) Neuron-to-neuron wild-type Tau protein transfer through a trans-synaptic mechanism: relevance to sporadic tauopathies. Acta Neuropathol Comm 2:14. https://doi.org/10.1186/2051-5960-2-14
Wu JW, Hussaini SA, Bastille IM et al (2016) Neuronal activity enhances tau propagation and tau pathology in vivo. Nat Neurosci 19(8):1085–1092. https://doi.org/10.1038/nn.4328
Wang Y, Balaji V, Kaniyappan S et al (2017) The release and trans-synaptic transmission of Tau via exosomes. Mol Neurodegener 12(1):5. https://doi.org/10.1186/s13024-016-0143-y
Nobuhara CK, DeVos SL, Commins C et al (2017) Tau antibody-targeting pathological species block neuronal uptake and interneuron propagation of tau in vitro. Am J Pathol 187(6):1399–1412. https://doi.org/10.1016/j.ajpath.2017.01.022
Katsikoudi A, Ficulle E, Cavallini A et al (2020) Quantitative propagation of assembled human tau from Alzheimer’s disease brain in microfluidic neuronal cultures. J Biol Chem 295(37):13079–13093. https://doi.org/10.1074/jbc.RA120.013325
Tai HC, Wang BY, Serrano-Pozo A et al (2014) Frequent and symmetric deposition of misfolded tau oligomers within presynaptic and postsynaptic terminals in Alzheimer’s disease. Acta Neuropathol Comm 2:146. https://doi.org/10.1186/s40478-014-0146-2
Hallinan GI, Vargas-Caballero M, West J et al (2019) Tau misfolding efficiently propagates between individual intact hippocampal neurons. J Neurosci 39(48):9623–9632. https://doi.org/10.1523/JNEUROSCI.1590-19.2019
Pooler AM, Phillips EC, Lau DHW et al (2013) Physiological release of endogenous tau is stimulated by neuronal activity. EMBO Rep 14(4):389–394. https://doi.org/10.1038/embor.2013.15
Yamada K, Holth JK, Liao F et al (2014) Neuronal activity regulates extracellular tau in vivo. J Exp Med 211(3):387–393. https://doi.org/10.1084/jem.20131685
Sokolow S, Henkins KM, Bilousova T et al (2015) Pre-synaptic C-terminal truncated tau is released from cortical synapses in Alzheimer’s disease. J Neurochem 133(3):368–379. https://doi.org/10.1111/jnc.12991
Chai X, Dage JL, Citron M (2012) Constitutive secretion of tau protein by an unconventional mechanism. Neurobiol Dis 48(3):356–366. https://doi.org/10.1016/j.nbd.2012.05.021
Merezhko M, Brunello CA, Yan X et al (2018) Secretion of tau via an unconventional non-vesicular mechanism. Cell Rep 25(8):2027–2035.e4. https://doi.org/10.1016/j.celrep.2018.10.078
Katsinelos T, Zeitler M, Dimou E et al (2018) Unconventional secretion mediates the trans-cellular spreading of tau. Cell Rep 23(7):2039–2055. https://doi.org/10.1016/j.celrep.2018.04.056
Pérez M, Avila J, Hernández F (2019) Propagation of tau via extracellular vesicles. Front Neurosci 13:698. https://doi.org/10.3389/fnins.2019.00698
Abounit S, Wu JW, Duff K et al (2016) Tunneling nanotubes: a possible highway in the spreading of tau and other prion-like proteins in neurodegenerative diseases. Prion 10(5):344–351. https://doi.org/10.1080/19336896.2016.1223003
Tardivel M, Begard S, Bousset L et al (2016) Tunneling nanotube (TNT)-mediated neuron-to neuron transfer of pathological tau protein assemblies. Acta Neuropathol Comm 4(1):117. https://doi.org/10.1186/s40478-016-0386-4
Alarcon-Martinez L, Villafranca-Baughman D, Quintero H et al (2020) Interpericyte tunnelling nanotubes regulate neurovascular coupling. Nature 585(7823):91–95. https://doi.org/10.1038/s41586-020-2589-x
Holmes BB, DeVos SL, Kfoury N et al (2013) Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds. PNAS 110(33):E3138–E3147. https://doi.org/10.1073/pnas.1301440110
Mirbaha H, Holmes BB, Sanders DW et al (2015) Tau trimers are the minimal propagation unit spontaneously internalized to seed intracellular aggregation. J Biol Chem 290(24):14893–14903. https://doi.org/10.1074/jbc.M115.652693
Funk KE, Mirbaha H, Jiang H et al (2015) Distinct therapeutic mechanisms of tau antibodies: promoting microglial clearance versus blocking neuronal uptake. J Biol Chem 290(35):21652–21662. https://doi.org/10.1074/jbc.M115.657924
Rauch JN, Chen JJ, Sorum AW et al (2018) Tau internalization is regulated by 6-O sulfation on heparan sulfate proteoglycans (HSPGs). Sci Rep 8(1):6382. https://doi.org/10.1038/s41598-018-24904-z
Stopschinski BE, Holmes BB, Miller GM et al (2018) Specific glycosaminoglycan chain length and sulfation patterns are required for cell uptake of tau vs. alpha-synuclein and beta-amyloid aggregates. J Biol Chem 293(27):10826–10840. https://doi.org/10.1074/jbc.RA117.000378
Weisová P, Cehlár O, Škrabana R et al (2019) Therapeutic antibody targeting microtubule-binding domain prevents neuronal internalization of extracellular tau via masking neuron surface proteoglycans. Acta Neuropathol Comm 7(1):129. https://doi.org/10.1186/s40478-019-0770-y
Hudák A, Kusz E, Domonkos I et al (2019) Contribution of syndecans to cellular uptake and fibrillation of α-synuclein and tau. Sci Rep 9(1):16543. https://doi.org/10.1038/s41598-019-53038-z
Zhao J, Zhu Y, Song X et al (2020) 3-O-sulfation of heparan sulfate enhances tau interaction and cellular uptake. Angew Chem Int Edit 59(5):1818–1827. https://doi.org/10.1002/anie.201913029
Puangmalai N, Bhatt N, Montalbano M et al (2020) Internalization mechanisms of brain-derived tau oligomers from patients with Alzheimer’s disease, progressive supranuclear palsy and dementia with Lewy bodies. Cell Death Dis 11(5):314. https://doi.org/10.1038/s41419-020-2503-3
Stopschinski BE, Thomas TL, Nadji S et al (2020) A synthetic heparinoid blocks Tau aggregate cell uptake and amplification. J Biol Chem 295(10):2974–2983. https://doi.org/10.1074/jbc.RA119.010353
Kanekiyo T, Zhang J, Liu Q et al (2011) Heparan sulphate proteoglycan and the low-density lipoprotein receptor-related protein 1 constitute major pathways for neuronal amyloid-beta uptake. J Neurosci 31(5):1644–1651. https://doi.org/10.1523/JNEUROSCI.5491-10.2011
Rauch JN, Luna G, Guzman E et al (2020) LRP1 is a master regulator of tau uptake and spread. Nature 580(7803):381–385. https://doi.org/10.1038/s41586-020-2156-5
Evans LD, Strano A, Campbell A et al (2020) Whole genome CRISPR screens identify LRRK2-regulated endocytosis as a major mechanism for extracellular tau uptake by human neurons. BioRxiv 2020.08.11.246363. https://doi.org/10.1101/2020.08.11.246363
Cooper JM, Lathuiliere A, Migliorini M et al (2020) LRP1 and SORL1 regulate tau internalization and degradation and enhance tau seeding. BioRxiv 2020.11.17.386581. https://doi.org/10.1101/2020.11.17.386581
De Cecco E, Celauro L, Vanni S et al (2020) The uptake of tau amyloid fibrils is facilitated by the cellular prion protein and hampers prion propagation in cultured cells. J Neurochem 155(5):577–591. https://doi.org/10.1111/jnc.15040
Takahashi M, Miyata H, Kametani F et al (2015) Extracellular association of APP and tau fibrils induces intracellular aggregate formation of tau. Acta Neuropathol 129(6):895–907. https://doi.org/10.1007/s00401-015-1415-2
Morozova V, Cohen LS, Makki AEH et al (2019) Normal and pathological tau uptake mediated by M1/M3 muscarinic receptors promotes opposite neuronal changes. Front Cell Neurosci 13:403. https://doi.org/10.3389/fncel.2019.00403
Corbett GT, Wang Z, Hong W et al (2020) PrP is a central player in toxicity mediated by soluble aggregates of neurodegeneration-causing proteins. Acta Neuropathol 139(3):503–526. https://doi.org/10.1007/s00401-019-02114-9
Zhong Z, Grasso L, Sibilla C et al (2018) Prion-like protein aggregates exploit the RHO GTPase to cofilin-1 signaling pathway to enter cells. EMBO J 37(6). https://doi.org/10.15252/embj.201797822
Evans LD, Wassmer T, Fraser G et al (2018) Extracellular monomeric and aggregated Tau efficiently enter human neurons through overlap** but distinct pathways. Cell Rep 22(13):3612–3624. https://doi.org/10.1016/j.celrep.2018.03.021
Calafate S, Flavin W, Verstreken P et al (2016) Loss of Bin1 promotes the propagation of tau pathology. Cell Rep 17(4):931–940. https://doi.org/10.1016/j.celrep.2016.09.063
Flavin WP, Bousset L, Green ZC et al (2017) Endocytic vesicle rupture is a conserved mechanism of cellular invasion by amyloid proteins. Acta Neuropathol 134(4):629–653. https://doi.org/10.1007/s00401-017-1722-x
Chen JJ, Nathaniel DL, Raghavan P et al (2019) Compromised function of the ESCRT pathway promotes endolysosomal escape of tau seeds and propagation of tau aggregation. J Biol Chem 294(50):18952–18966. https://doi.org/10.1074/jbc.RA119.009432
Ugbode C, Fort-Aznar L, Sweeney ST (2019) Leaky endosomes push tau over the seed limit. J Biol Chem 294(50):18967–18968. https://doi.org/10.1074/jbc.H119.011687
Falcon B, Noad J, McMahon H et al (2017) Galectin-8-mediated selective autophagy protects against seeded tau aggregation. J Biol Chem 293(7):2438–2451. https://doi.org/10.1074/jbc.M117.809293
Takeda S, Commins C, DeVos SL et al (2016) Seed-competent high-molecular-weight tau species accumulates in the cerebrospinal fluid of Alzheimer’s disease mouse model and human patients. Ann Neurol 80(3):355–367. https://doi.org/10.1002/ana.24716
Barini E, Plotzky G, Mordashova Y et al (2020) Tau in the brain interstitial fluid is fragmented and seeding-competent. BioRxiv 2020.07.15.205724. https://doi.org/10.1101/2020.07.15.205724
Asai H, Ikezu S, Tsunoda S et al (2015) Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci 18(11):1584–1593. https://doi.org/10.1038/nn.4132
Zilkova M, Nolle A, Kovacech B et al (2020) Humanized tau antibodies promote tau uptake by human microglia without any increase of inflammation. Acta Neuropathol Comm 8(1):74. https://doi.org/10.1186/s40478-020-00948-z
Hopp SC, Lin Y, Oakley D et al (2018) The role of microglia in processing and spreading of bioactive tau seeds in Alzheimer’s disease. J Neuroinflamm 15(1):269. https://doi.org/10.1186/s12974-018-1309-z
Ruan Z, Delpech JC, Venkatesan Kalavai S et al (2020) P2RX7 inhibitor suppresses exosome secretion and disease phenotype in P301S tau transgenic mice. Mol Neurodegener 15(1):47. https://doi.org/10.1186/s13024-020-00396-2
Crotti A, Sait HR, McAvoy KM et al (2019) BIN1 favors the spreading of Tau via extracellular vesicles. Sci Rep 9(1):9477. https://doi.org/10.1038/s41598-019-45676-0
Perea JR, Lopez E, Diez-Ballesteros JC et al (2019) Extracellular monomeric tau is internalized by astrocytes. Front Neurosci 13:442. https://doi.org/10.3389/fnins.2019.00442
Piacentini R, Li Puma DD, Mainardi M et al (2017) Reduced gliotransmitter release from astrocytes mediates tau-induced synaptic dysfunction in cultured hippocampal neurons. Glia 65(8):1302–1316. https://doi.org/10.1002/glia.23163
Martini-Stoica H, Cole AL, Swartzlander DB et al (2018) TFEB enhances astroglial uptake of extracellular tau species and reduces tau spreading. J Exp Med 215(9):2355–2377. https://doi.org/10.1084/jem.20172158
Nedergaard M, Goldman SA (2020) Glymphatic failure as a final common pathway to dementia. Science 370(6512):50–56. https://doi.org/10.1126/science.abb8739
Patel TK, Habimana-Griffin L, Gao X et al (2019) Dural lymphatics regulate clearance of extracellular tau from the CNS. Mol Neurodegener 14(1):11. https://doi.org/10.1186/s13024-019-0312-x
Harrison IF, Ismail O, Machhada A et al (2020) Impaired glymphatic function and clearance of tau in an Alzheimer’s disease model. Brain 143(8):2576–2593. https://doi.org/10.1093/brain/awaa179
Iliff JJ, Chen MJ, Plog BA et al (2014) Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci 34(49):16180–16193. https://doi.org/10.1523/JNEUROSCI.3020-14.2014
Pooler AM, Polydoro M, Maury EA et al (2015) Amyloid accelerates tau propagation and toxicity in a model of early Alzheimer’s disease. Acta Neuropathol Comm 3:14. https://doi.org/10.1186/s40478-015-0199-x
He Z, Guo JL, McBride JD et al (2018) Amyloid-β plaques enhance Alzheimer’s brain tau-seeded pathologies by facilitating neuritic plaque tau aggregation. Nat Med 24(1):29–38. https://doi.org/10.1038/nm.4443
Vergara C, Houben S, Suain V et al (2019) Amyloid-beta pathology enhances pathological fibrillary tau seeding induced by Alzheimer PHF in vivo. Acta Neuropathol 137(3):397–412. https://doi.org/10.1007/s00401-018-1953-5
Saito T, Mihira N, Matsuba Y et al (2019) Humanization of the entire murine Mapt gene provides a murine model of pathological human tau propagation. J Biol Chem 294(34):12754–12765. https://doi.org/10.1074/jbc.RA119.009487
Ehrenberg AJ, Khatun A, Coomans E et al (2020) Relevance of biomarkers across different neurodegenerative diseases. Alzheimers Res Ther 12(1):56. https://doi.org/10.1186/s13195-020-00601-w
Karikari TK, Benedet AL, Ashton NJ et al (2020) Diagnostic performance and prediction of clinical progression of plasma phospho-tau181 in the Alzheimer’s disease neuroimaging initiative. Mol Psychiatry. https://doi.org/10.1038/s41380-020-00923-z
Young PNE, Estarellas M, Coomans E et al (2020) Imaging biomarkers in neurodegeneration: current and future practices. Alzheimers Res Ther 12(1):49. https://doi.org/10.1186/s13195-020-00612-7
Mattay VS, Fotenos AF, Ganley CJ et al (2020) Brain tau imaging: Food and Drug Administration approval of (18)F-Flortaucipir injection. J Nucl Med 61:1411–1412. https://doi.org/10.2967/jnumed.120.252254
Brendel M, Barthel H, van Eimeren T et al (2020) Assessment of 18F-PI-2620 as a biomarker in progressive supranuclear palsy. JAMA Neurol 77(11):1408–1419. https://doi.org/10.1001/jamaneurol.2020.2526
Tagai K, Ono M, Kubota M et al (2020) High-contrast in vivo imaging of tau pathologies in Alzheimer’s and non-Alzheimer’s disease tauopathies. Neuron 16:S0896-6273(20)30766-2. https://doi.org/10.1016/j.neuron.2020.09.042
Karikari TK, Pascoal TA, Ashton NJ et al (2020) Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol 19(5):422–433. https://doi.org/10.1016/S1474-4422(20)30071-5
Thijssen EH, La Joie R, Wolf A et al (2020) Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat Med 26(3):387–397. https://doi.org/10.1038/s41591-020-0762-2
Janelidze S, Mattsson N, Palmqvist S et al (2020) Plasma P-tau181 in Alzheimer’s disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat Med 26(3):379–386. https://doi.org/10.1038/s41591-020-0755-1
Mielke MM, Hagen CE, Xu J et al (2018) Plasma phospho-tau181 increases with Alzheimer’s disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Alzheimers Dement 14(8):989–997. https://doi.org/10.1016/j.jalz.2018.02.013
Mattsson-Carlgren N, Janelidze S, Palmqvist S et al (2020) Longitudinal plasma p-tau217 is increased in early stages of Alzheimer’s disease. Brain 143(11):3234–3241. https://doi.org/10.1093/brain/awaa286
Palmqvist S, Janelidze S, Quiroz YT et al (2020) Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA 324(8):772–781. https://doi.org/10.1001/jama.2020.12134
Janelidze S, Berron D, Smith R et al (2020) Associations of plasma phospho-Tau217 levels with tau positron emission tomography in early Alzheimer disease. JAMA Neurol. https://doi.org/10.1001/jamaneurol.2020.4201
Hampel H, O’Bryant SE, Molinuevo JL et al (2018) Blood-based biomarkers for Alzheimer disease: map** the road to the clinic. Nat Rev Neurol 14(11):639–652. https://doi.org/10.1038/s41582-018-0079-7
Gauthier S, Therriault J, Pascoal T et al (2020) Impact of p-tau181 and p-tau217 levels on enrolment for randomized clinical trials and future use of anti-amyloid and anti-tau drugs. Expert Rev Neurother 20(12):1211–1213. https://doi.org/10.1080/14737175.2020.1841637
Horie K, Barthélemy NR, Sato C et al (2020) CSF tau microtubule binding region identifies tau tangle and clinical stages of Alzheimer’s disease. Brain awaa373. https://doi.org/10.1093/brain/awaa373
Ashton NJ, Hye A, Rajkumar AP et al (2020) An update on blood-based biomarkers for non-Alzheimer neurodegenerative disorders. Nat Rev Neurol 16(5):265–284. https://doi.org/10.1038/s41582-020-0348-0
Ashton NJ, Leuzy A, Lim YM et al (2019) Increased plasma neurofilament light chain concentration correlates with severity of post-mortem neurofibrillary tangle pathology and neurodegeneration. Acta Neuropathol Comm 7(1):5. https://doi.org/10.1186/s40478-018-0649-3
Congdon EE, Sigurdsson EM (2018) Tau-targeting therapies for Alzheimer disease. Nat Rev Neurol 14(7):399–415. https://doi.org/10.1038/s41582-018-0013-z
Panza F, Lozupone M, Seripa D et al (2020) Development of disease-modifying drugs for frontotemporal dementia spectrum disorders. Nat Rev Neurol 16(4):213–228. https://doi.org/10.1038/s41582-020-0330-x
Li C, Gotz J (2017) Tau-based therapies in neurodegeneration: opportunities and challenges. Nat Rev Drug Discov 16(12):863–883. https://doi.org/10.1038/nrd.2017.155
Jadhav S, Avila J, Scholl M et al (2019) A walk through tau therapeutic strategies. Acta Neuropathol Comm 7(1):22. https://doi.org/10.1186/s40478-019-0664-z
Gauthier S, Feldman HH, Schneider LS et al (2016) Efficacy and safety of tau-aggregation inhibitor therapy in patients with mild or moderate Alzheimer’s disease: a randomised, controlled, double-blind, parallel-arm, phase 3 trial. Lancet 388(10062):2873–2884. https://doi.org/10.1016/S0140-6736(16)31275-2
Novak P, Schmidt R, Kontsekova E et al (2016) Safety and immunogenicity of the tau vaccine AADvac1 in patients with Alzheimer’s disease: a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Neurol 16(2):123–134. https://doi.org/10.1016/S1474-4422(16)30331-3
Novak P, Schmidt R, Kontsekova E et al (2018) FUNDAMANT: an interventional 72-week phase 1 follow-up study of AADvac1, an active immunotherapy against tau protein pathology in Alzheimer’s disease. Alzheimers Res Ther 10(1):108. https://doi.org/10.1186/s13195-018-0436-1
Colin M, Dujardin S, Schraen-Maschke S et al (2020) From the prion-like propagation hypothesis to therapeutic strategies of anti-tau immunotherapy. Acta Neuropathol 139(1):3–25. https://doi.org/10.1007/s00401-019-02087-9
Mullard A (2020) Failure of first anti-tau antibody in Alzheimer disease highlights risks of history repeating. Nat Rev Drug Discov doi. https://doi.org/10.1038/d41573-020-00217-7
Pardridge WM (2020) Blood-brain barrier and delivery of protein and gene therapeutics to brain. Front Aging Neurosci 11:373
Kariolis MS, Wells RC, Getz JA et al (2020) Brain delivery of therapeutic proteins using an Fc fragment blood-brain barrier transport vehicle in mice and monkeys. Sci Transl Med 12(545):eaay1359. https://doi.org/10.1126/scitranslmed.aay1359
Ising C, Gallardo G, Leyns CEG et al (2017) AAV-mediated expression of anti-tau scFvs decreases tau accumulation in a mouse model of tauopathy. J Exp Med 214(5):1227–1238. https://doi.org/10.1084/jem.20162125
Liu W, Zhao L, Blackman B et al (2016) Vectored intracerebral immunization with the anti-tau monoclonal antibody PHF1 markedly reduces tau pathology in mutant tau transgenic mice. J Neurosci 36(49):12425–12435. https://doi.org/10.1523/JNEUROSCI.2016-16.2016
Quint WH, Matečko-Burmann I, Schilcher I et al (2020) Bispecific tau antibodies with additional binding to C1q or alpha-synuclein. BioRxiv 2020.11.10.376301. https://doi.org/10.1101/2020.11.10.376301
DeVos SL, Miller RL, Schoch KM et al (2017) Tau reduction prevents neuronal loss and reverses pathological tau deposition and seeding in mice with tauopathy. Sci Transl Med 9(374):eaag0481. https://doi.org/10.1126/scitranslmed.aag0481
Lee HJ, Boado RJ, Braasch DA et al (2002) Imaging gene expression in the brain in vivo in a transgenic mouse model of Huntington’s disease with an antisense radiopharmaceutical and drug-targeting technology. J Nucl Med 43(7):948–956
Pardridge WM (2016) Re-engineering therapeutic antibodies for Alzheimer’s disease as blood-brain barrier penetrating bi-specific antibodies. Exp Opin Biol Ther 16(12):1455–1468. https://doi.org/10.1080/14712598.2016.1230195
ACI-3024. Alzforum (nd) https://www.alzforum.org/therapeutics/aci-3024. Accessed 14 Dec 2020
LY3372689. Alzforum (nd) https://www.alzforum.org/therapeutics/ly3372689. Accessed 14 Dec 2020
Konstantinidou M, Li J, Zhang B et al (2019) PROTACs- a game-changing technology. Exp Opin Drug Disc 14(12):1255–1268. https://doi.org/10.1080/17460441.2019.1659242
Vargova G, Vogels T, Kostecka Z et al (2018) Inhibitory interneurons in Alzheimer’s disease. Bratisl Med J 119(4):205–209
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Vogels, T., Hromádka, T. (2022). Tau Pathology in Neurodegenerative Diseases . In: Peplow, P.V., Martinez, B., Gennarelli, T.A. (eds) Neurodegenerative Diseases Biomarkers. Neuromethods, vol 173. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-1712-0_4
Download citation
DOI: https://doi.org/10.1007/978-1-0716-1712-0_4
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-1711-3
Online ISBN: 978-1-0716-1712-0
eBook Packages: Springer Protocols