Abstract
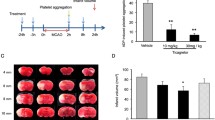
Hemorrhagic transformation (HT) is a major complication of ischemic stroke and further deteriorates neurological outcomes. Bradykinin 1 receptor (B1R) has been proven to mediate vasculo-toxicity in various experimental models. However, its role in the development of HT after stroke remains unclear. We detected the B1R expression in brain tissues with or without HT in a rat model of cerebral ischemia/reperfusion (I/R) with type 1 diabetes, showing higher B1R expression in the hemorrhagic areas than the ischemic tissues. Then, B1R agonist or antagonist was administrated intravenously just before reperfusion to investigate its effect on HT and the underlying molecular mechanism. Administration of low (300 nmol/kg) or high (1 μmol/kg) dose of B1R antagonist mitigated hemorrhage, improved neurobehavioral deficits, and preserved blood-brain-barrier (BBB) integrity after reperfusion for 8 h whereas the 300 nmol/kg of B1R agonist aggravated these outcomes, though only the high does of B1R antagonist affected the infarction volume. Extracellular signal-regulated kinase 1/2 (ERK1/2) phosphorylation was increased by B1R activation but decreased by B1R inhibition, which mediated B1R toxicity on BBB disruption and ischemia-related HT. Furthermore, B1R activation facilitated the mRNA and protein expressions of MMP-9 in the hemorrhagic tissues, and these increases were blocked by both ERK inhibitor U0126 and NF-κB inhibitor PDTC. U0126 also remarkably decreased the B1R-induced NF-κB/p65 activation. We concluded that upregulated B1R may contribute to early HT after I/R in type 1 diabetic rats via ERK1/2/NF-κB/MMP-9 pathway. B1R inhibition could be an encouraging therapeutic strategy to withstand HT after ischemic stroke in diabetic patients.







Similar content being viewed by others
References
Poppe AY, Majumdar SR, Jeerakathil T, Ghali W, Buchan AM, Hill MD. Admission hyperglycemia predicts a worse outcome in stroke patients treated with intravenous thrombolysis. Diabetes Care. 2009;32:617–22.
Paciaroni M, Agnelli G, Caso V, Corea F, Ageno W, Alberti A, et al. Acute hyperglycemia and early hemorrhagic transformation in ischemic stroke. Cerebrovasc Dis. 2009;28:119–23.
Kamada H, Yu F, Nito C, Chan PH. Influence of hyperglycemia on oxidative stress and matrix metalloproteinase-9 activation after focal cerebral ischemia/reperfusion in rats: relation to blood-brain barrier dysfunction. Stroke. 2007;38:1044–9.
Hu Q, Ma Q, Zhan Y, He Z, Tang J, Zhou C, et al. Isoflurane enhanced hemorrhagic transformation by impairing antioxidant enzymes in hyperglycemic rats with middle cerebral artery occlusion. Stroke. 2011;42:1750–6.
Regoli D, Plante GE. Impact of kinins in the treatment of cardiovascular diseases. Pharmacol Ther. 2012;135:94–111.
Cruwys SC, Garrett NE, Perkins MN, Blake DR, Kidd BL. The role of bradykinin B1 receptors in the maintenance of intra-articular plasma extravasation in chronic antigen-induced arthritis. Br J Pharmacol. 1994;113:940–4.
Abdouh M, Talbot S, Couture R, Hassessian HM. Retinal plasma extravasation in streptozotocin-diabetic rats mediated by kinin B(1) and B(2) receptors. Br J Pharmacol. 2008;154:136–43.
Austinat M, Braeuninger S, Pesquero JB, Brede M, Bader M, Stoll G, et al. Blockade of bradykinin receptor B1 but not bradykinin receptor B2 provides protection from cerebral infarction and brain edema. Stroke. 2009;40:285–93.
Raslan F, Schwarz T, Meuth SG, Austinat M, Bader M, Renné T, et al. Inhibition of bradykinin receptor B1 protects mice from focal brain injury by reducing blood–brain barrier leakage and inflammation. J Cereb Blood Flow Metab. 2010;30:1477–86.
Westermann D, Walther T, Savvatis K, Escher F, Sobirey M, Riad A, et al. Gene deletion of the kinin receptor B1 attenuates cardiac inflammation and fibrosis during the development of experimental diabetic cardiomyopathy. Diabetes. 2009;58:1373–81.
Sang H, Liu L, Wang L, Qiu Z, Li M, Yu L, et al. Opposite roles of bradykinin B1 and B2 receptors during cerebral ischaemia-reperfusion injury in experimental diabetic rats. Eur J Neurosci. 2016;43:53–65.
Sawe N, Steinberg G, Zhao H. Dual roles of the MAPK/ERK1/2 cell signaling pathway after stroke. J Neurosci Res. 2008;86:1659–69.
Mage M, Pécher C, Neau E, Cellier E, Dos Reiss ML, Schanstra JP, et al. Induction of B1 receptors in streptozotocin diabetic rats: possible involvement in the control of hyperglycemia-induced glomerular Erk 1 and 2 phosphorylation. Can J Physiol Pharmacol. 2002;80:328–33.
Potier L, Waeckel L, Vincent M-P, Chollet C, Gobeil F, Marre M, et al. Selective kinin receptor agonists as cardioprotective agents in myocardial ischemia and diabetes. J Pharmacol Exp Ther. 2013;346:23–30.
Seo JH, Guo S, Lok J, Navaratna D, Whalen MJ, Kim K-W, et al. Neurovascular matrix metalloproteinases and the blood-brain barrier. Curr Pharm Des. 2012;18:3645.
Arai K, Lee SR, Lo EH. Essential role for ERK mitogen-activated protein kinase in matrix metalloproteinase-9 regulation in rat cortical astrocytes. Glia. 2003;43:254–64.
Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91.
Cote J, Bovenzi V, Savard M, Dubuc C, Fortier A, Neugebauer W, et al. Induction of selective blood-tumor barrier permeability and macromolecular transport by a biostable kinin B1 receptor agonist in a glioma rat model. PLoS One. 2012;7:e37485.
Shi R, Yuan K, Hu B, Sang H, Zhou L, **e Y, et al. Tissue Kallikrein alleviates cerebral ischemia-reperfusion injury by activating the B2R-ERK1/2-CREB-Bcl-2 signaling pathway in diabetic rats. Oxidative Med Cell Longev. 2016;1843201
Couture R, Blaes N, Girolami JP. Kinin receptors in vascular biology and pathology. Curr Vasc Pharmacol. 2014;12:223–48.
Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–11.
Belayev L, Busto R, Zhao W, Ginsberg MD. Quantitative evaluation of blood-brain barrier permeability following middle cerebral artery occlusion in rats. Brain Res. 1996;739:88–96.
Muranyi M, Fujioka M, He Q, Han A, Yong G, Csiszar K, et al. Diabetes activates cell death pathway after transient focal cerebral ischemia. Diabetes. 2003;52:481–6.
Sengillo JD, Winkler EA, Walker CT, Sullivan JS, Johnson M, Zlokovic BV. Deficiency in mural vascular cells coincides with blood-brain barrier disruption inAlzheimer's disease. Brain Pathol. 2013;23:303–10.
Aijaz S, Balda MS, Matter K. Tight junctions: molecular architecture and function. Int Rev Cytol. 2006;248:261–98.
Moon SK, Cha BY, Kim CH. ERK1/2 mediates TNF-alpha-induced matrix metalloproteinase-9 expression in human vascular smooth muscle cells via the regulation of NF-kappaB and AP-1: involvement of the ras dependent pathway. J Cell Physiol. 2004;198:417–27.
Schanstra JP, Bataille E, Marin Castano ME, Barascud Y, Hirtz C, Pesquero JB, et al. The B1-agonist [des-Arg10]-kallidin activates transcription factor NF-kappaB and induces homologous upregulation of the bradykinin B1-receptor in cultured human lung fibroblasts. J Clin Invest. 1998;101:2080–91.
Campos MM, Souza GE, Calixto JB. In vivo B1 kinin-receptor upregulation. Evidence for involvement of protein kinases and nuclear factor kappaB pathways. Br J Pharmacol. 127:1851–9.
Larrue V, von Kummer RR, Muller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian acute stroke study (ECASS II). Stroke. 1999;32:438–41.
Chen C, Manaenko A, Zhan Y, Liu W, Ostrowki R, Tang J, et al. Hydrogen gas reduced acute hyperglycemia-enhanced hemorrhagic transformation in a focal ischemia rat model. Neuroscience. 2010;169:402–14.
Zhang F, Lin Y, Huang H, Sun J, Wen S, Lou M. Rosiglitazone attenuates hyperglycemia-enhanced hemorrhagic transformation after transient focal ischemia in rats. Neuroscience. 2013;250:651–7.
Paciaroni M, Agnelli G, Corea F, Ageno W, Alberti A, Lanari A, et al. Early hemorrhagic transformation of brain infarction: rate, predictive factors, and influence on clinical outcome results of a prospective multicenter study. Stroke. 2008;39:2249–56.
Bruno A, Levine S, Frankel M, Brott T, Lin Y, Tilley B, et al. Admission glucose level and clinical outcomes in the NINDS rt-PA stroke trial. Neurology. 2002;59:669–74.
Gloor SM, Wachtel M, Bolliger MF, Ishihara H, Landmann R, Frei K. Molecular and cellular permeability control at the blood–brain barrier. Brain Res Rev. 2001;36:258–64.
Schöller K, Feiler S, Anetsberger S, Kim SW, Plesnila N. Contribution of bradykinin receptors to the development of secondary brain damage after experimental subarachnoid hemorrhage. Neurosurgery. 2011;68:1118–23.
Cayla C, Todiras M, Iliescu R, Saul VV, Gross V, Pilz B, et al. Mice deficient for both kinin receptors are normotensive and protected from endotoxin-induced hypotension. FASEB J. 2007;21:1689–98.
Emanueli C, Chao J, Regoli D, Chao L, Ni A, Madeddu P. The bradykinin B1 receptor and the central regulation of blood pressure in spontaneously hypertensive rats. Br J Pharmacol. 1999;126:1769–76.
Mori MA, Araujo RC, Reis FC, Sgai DG, Fonseca RG, Barros CC, et al. Kinin B1 receptor deficiency leads to leptin hypersensitivity and resistance to obesity. Diabetes. 2008;57:1491–500.
Araujo RC, Mori MA, Merino VF, Bascands JL, Schanstra JP, Zollner RL, et al. Role of the kinin B1 receptor in insulin homeostasis and pancreatic islet function. Biol Chem. 2006;387:431–6.
Barros CC, Haro A, Russo FJ, Schadock I, Almeida SS, Ribeiro RA, et al. Altered glucose homeostasis and hepatic function in obese mice deficient for both kinin receptor genes. PLoS One. 2012;7:e40573.
Noshita N, Sugawara T, Hayashi T, Lewen A, Omar G, Chan PH. Copper/zinc superoxide dismutase attenuates neuronal cell death by preventing extracellular signal-regulated kinase activation after transient focal cerebral ischemia in mice. J Neurosci. 2002;22:7923–30.
Wang ZQ, Wu DC, Huang FP, Yang GY. Inhibition of MEK/ERK 1/2 pathway reduces pro-inflammatory cytokine interleukin-1 expression in focal cerebral ischemia. Brain Res. 2004;996:55–66.
Kilic E, Kilic U, Soliz J, Bassetti CL, Gassmann M, Hermann DM. Brain-derived erythropoietin protects from focal cerebral ischemia by dual activation of ERK-1/−2 and Akt pathways. FASEB J. 2005;19:2026–8.
Han BH, Holtzman DM. BDNF protects the neonatal brain from hypoxic-ischemic injury in vivo via the ERK pathway. J Neurosci. 2000;20:5775–81.
Chiu PS, Lai SC. Matrix metalloproteinase-9 leads to blood-brain barrier leakage in mice with eosinophilic meningoencephalitis caused by Angiostrongylus cantonensis. Acta Trop. 2014;140C:141–50.
Roe K, Kumar M, Lum S, Orillo B, Nerurkar VR, Verma S. West Nile virus-induced disruption of the blood-brain barrier in mice is characterized by the degradation of the junctional complex proteins and increase in multiple matrix metalloproteinases. J Gen Virol. 2012;93:1193–203.
Van Lint P, Libert C. Chemokine and cytokine processing by matrix metalloproteinases and its effect on leukocyte migration and inflammation. J Leukoc Biol. 2007;82:1375–81.
Cruise L, Ho LK, Veitch K, Fuller G, Morris BJ. Kainate receptors activate NF-kappaB via MAP kinase in striatal neurones. Neuroreport. 2000;11:395–8.
Jickling GC, Liu D, Stamova B, Ander BP, Zhan X, Lu A, et al. Hemorrhagic transformation after ischemic stroke in animals and humans. J Cereb Blood Flow Metab. 2014;34:185–99.
Rosell A, Cuadrado E, Ortega-Aznar A, Hernandez-Guillamon M, Lo EH, Montaner J. MMP-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke. 2008;39:1121–6.
Ehrenfeld P, Matus CE, Pavicic F, Toledo C, Nualart F, Gonzalez CB, et al. Kinin B1 receptor activation turns on exocytosis of matrix metalloprotease-9 and myeloperoxidase in human neutrophils: involvement of mitogen-activated protein kinase family. J Leukoc Biol. 2009;86:1179–89.
Kintsurashvili E, Duka A, Ignjacev I, Pattakos G, Gavras I, Gavras H. Age-related changes of bradykinin B1 and B2 receptors in rat heart. Am J Physiol Heart Circ Physiol. 2005;289:H202–5.
Fagan SC, Lapchak PA, Liebeskind DS, Ishrat T, Ergul A. Recommendations for preclinical research in hemorrhagic transformation. Transl Stroke Res. 2013;4:322–7.
Ifuku M, Farber K, Okuno Y, Yamakawa Y, Miyamoto T, Nolte C, et al. Bradykinin-induced microglial migration mediated by B1-bradykinin receptors depends on Ca2+ influx via reverse-mode activity of the Na+/Ca2+ exchanger. J Neurosci. 2007;27:13065–73.
Su J, Cui M, Tang Y, Zhou H, Liu L, Dong Q. Blockade of bradykinin B2 receptor more effectively reduces postischemic blood–brain barrier disruption and cytokines release than B1 receptor inhibition. Biochem Biophys Res Commun. 2009;388:205–11.
Ghebrehiwet B, Ji Y, Valentino A, Pednekar L, Ramadass M, Habiel D, et al. Soluble gC1qR is an autocrine signal that induces B1R expression on endothelial cells. J Immunol. 2014;192:377–84.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This study was supported by the National Natural Science Foundation of China (No. 81100870, 81400332) and Natural Science Foundation of Jiangsu Province (No. BK2011663).
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. Animal experimental procedures were conducted according to the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, revised 1996) and conformed to the Institutional Animal Care and Use Committee of the Nan**g University
Rights and permissions
About this article
Cite this article
Sang, H., Qiu, Z., Cai, J. et al. Early Increased Bradykinin 1 Receptor Contributes to Hemorrhagic Transformation After Ischemic Stroke in Type 1 Diabetic Rats. Transl. Stroke Res. 8, 597–611 (2017). https://doi.org/10.1007/s12975-017-0552-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-017-0552-4