Abstract
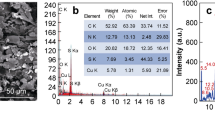
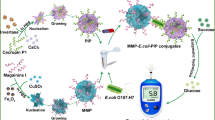
The rising cases of food poisoning and traveler’s diarrhea through Escherichia coli infection are a global concern. Magnetosome is biogenic magnetic nanoparticles extracted from magnetotactic bacteria and magnetosome-based biosensors offer simple and rapid detection of microbial pathogens. The current study demonstrates the application of a magnetosome-antibody complex-based biosensor for the detection of antigenic O-polysaccharide and E. coli from contaminated food samples. The magnetosome (1, 2 mg/mL)-antibody (0.8–200 µg/mL) complex was initially coupled with lipopolysaccharide (5 µg/mL) through antibody-antigen interaction. The magnetosome (1, 2 mg/mL)-antibody (0.8–200 µg/mL)-lipopolysaccharide (5 µg/mL) complexes were collected via external magnet and confirmed through spectroscopy studies. The magnetosome (1, 2 mg/mL)-antibody (0.8–200 µg/mL) complexes were applied to different concentration of lipopolysaccharide (0.01–50 µg/mL). The concentrated magnetosome (2 mg/ mL)-antibody (1.6 µg/mL) complex and lipopolysaccharide (0.1 µg/mL) were established in ELISA. Further, the magnetosome-antibody complex was applied on a screen-printed carbon electrode and stabilized through an external magnet. The least concentration of lipopolysaccharide (0.5 µg/mL) was determined in impedance. Further, the magnetosome-based biosensor was applied to various contaminated food samples- milk, water, and pineapple juice and the extracted lipopolysaccharide were selectively detected. The biosensor did not display any cross-reactivity and therefore exhibits specificity. The active E. coli (101 CFU/mL) from milk and water samples were also rapidly detected within 30 min by the developed biosensor. Furthermore, the field emission scanning electron microscope images confirmed the efficient detection of E. coli from the food sample.
Similar content being viewed by others
References
Desvaux, M., G. Dalmasso, R. Beyrouthy, N. Barnich, J. Delmas, and R. Bonnet (2020) Pathogenicity factors of genomic islands in intestinal and extraintestinal Escherichia coli. Front. Microbiol. 11: 2065.
World Health Organization, E. coli.https://www.who.int/news-room/fact-sheets/detail/e-coli.
Gill, A. and G. Huszczynski (2016) Enumeration of Escherichia coli O157:H7 in outbreak-associated beef patties. J. Food Prot. 79: 1266–1268.
Wu, E. L., O. Engström, S. Jo, D. Stuhlsatz, M. S. Yeom, J. B. Klauda, G. Widmalm, and W. Im (2013) Molecular dynamics and NMR spectroscopy studies of E. coli lipopolysaccharide structure and dynamics. Biophys. J. 105: 1444–1455.
Ameer, M. A., A. Wasey, and P. Salen (2022) Escherichia coli (E coli 0157 H7). [Updated 2022 Jul 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507845/.
Centers for Disease Control and Prevention, E. coli (Escherichia coli).https://www.cdc.gov/ecoli/index.html.
Das, S., R. Jayaratne, and K. E. Barrett (2018) The role of ion transporters in the pathophysiology of infectious diarrhea. Cell. Mol. Gastroenterol. Hepatol. 6: 33–45.
Akarsu, E. S. and S. Mamuk (2007) Escherichia coli lipopoly-saccharides produce serotype-specific hypothermic response in biotelemetered rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292: R1846–R1850.
Dogan, M. D., H. Ataoglu, and E. S. Akarsu (2000) Effects of different serotypes of Escherichia coli lipopolysaccharides on body temperature in rats. Life Sci. 67: 2319–2329.
Al-Gallas, N., R. Ben Aissa R, T. A. Annabi, O. Bahri, and A. Boudabous (2002) Isolation and characterization of shiga toxin-producing Escherichia coli from meat and dairy products. Food Microbiol. 19: 389–398.
Boukef, I., M. El Bour, N. Al Gallas, O. El Bahri, S. Mejri, R. Mraouna, R. Ben Aissa, A. Boudabous, P. Got, and M. Troussellier (2010) Survival of Escherichia coli strains in Mediterranean brackish water in the Bizerte lagoon in northern Tunisia. Water Environ. Res. 82: 2249–2257.
Guerrant, R. L., M. D. DeBoer, S. R. Moore, R. J. Scharf, and A. A. Lima (2013) The impoverished gut—a triple burden of diarrhoea, stunting and chronic disease. Nat. Rev. Gastroenterol. Hepatol. 10: 220–229.
Chowdhury, F., I. A. Khan, S. Patel, A. U. Siddiq, N. C. Saha, A. I. Khan, A. Saha, A. Cravioto, J. Clemens, F. Qadri, and M. Ali (2015) Diarrheal illness and healthcare seeking behavior among a population at high risk for diarrhea in Dhaka, Bangladesh. PLoS One 10: e0130105.
Bourgeois, A. L., T. F. Wierzba, and R. I. Walker (2016) Status of vaccine research and development for enterotoxigenic Escherichia coli. Vaccine 34: 2880–2886.
Yang, S. C., C. H. Lin, I. A. Aljuffali, and J. Y. Fang (2017) Current pathogenic Escherichia coli foodborne outbreak cases and therapy development. Arch. Microbiol. 199: 811–825.
Erridge, C., E. Bennett-Guerrero, and I. R. Poxton (2002) Structure and function of lipopolysaccharides. Microbes Infect. 4: 837–851.
Coats, S. R., C. T. Do, L. M. Karimi-Naser, P. H. Braham, and R. P. Darveau (2007) Antagonistic lipopolysaccharides block E. coli lipopolysaccharide function at human TLR4 via interaction with the human MD-2 lipopolysaccharide binding site. Cell. Microbiol. 9: 1191–1202.
Bertani, B. and N. Ruiz (2018) Function and biogenesis of lipopolysaccharides. EcoSal Plus 8: https://doi.org/10.1128/ecosalplus.ESP-0001-2018.
Bergstrand, A., C. Svanberg, M. Langton, and M. Nydén (2006) Aggregation behavior and size of lipopolysaccharide from Escherichia coli O55:B5. Colloids Surf. B Biointerfaces 53: 9–14.
Perdomo, R. and V. Montero (2006) Purification of E. coli 055:B5 lipopolysaccharides by size exclusion chromatography. Biotecnol. Apl. 23: 124–129.
Ivnitski, D., I. Abdel-Hamid, P. Atanasov, E. Wilkins, and S. Stricker (2000) Application of electrochemical biosensors for detection of food pathogenic bacteria. Electroanalysis 12: 317–325.
Wu, W., S. Zhao, Y. Mao, Z. Fang, X. Lu, and L. Zeng (2015) A sensitive lateral flow biosensor for Escherichia coli O157:H7 detection based on aptamer mediated strand displacement amplification. Anal. Chim. Acta 861: 62–68.
Lazcka, O., F. J. Del Campo, and F. X. Muñoz (2007) Pathogen detection: a perspective of traditional methods and biosensors. Biosens. Bioelectron. 22: 1205–1217.
Borghol, N., L. Mora, T. Jouenne, N. Jaffézic-Renault, N. Sakly, A. C. Duncan, Y. Chevalier, P. Lejeune, and A. Othmane (2010) Monitoring of E. coli immobilization on modified gold electrode: a new bacteria-based glucose sensor. Biotechnol. Bioprocess Eng. 15: 220–228.
Mehrotra, P. (2016) Biosensors and their applications — a review. J. Oral Biol. Craniofac. Res. 6: 153–159.
Lee, D., J. Hwang, Y. Seo, A. A. Gilad, and J. Choi (2018) Optical immunosensors for the efficient detection of target biomolecules. Biotechnol. Bioprocess Eng. 23: 123–133.
Cho, C. H., T. J. Park, and J. P. Park (2022) Affinity peptide-based electrochemical biosensor for the highly sensitive detection of bovine rotavirus. Biotechnol. Bioprocess Eng. 27: 607–614.
Pérez-López, B. and A. Merkoçi (2011) Nanomaterials based biosensors for food analysis applications. Trends Food Sci. Technol. 22: 625–639.
Sharma, R., K. V. Ragavan, M. S. Thakur, and K. S. M. S. Raghavarao (2015) Recent advances in nanoparticle based aptasensors for food contaminants. Biosens. Bioelectron. 74: 612–627.
Lim, S. H., Y. C. Ryu, and B. H. Hwang (2021) Aptamer-immobilized gold nanoparticles enable facile and on-site detection of Staphylococcus aureus. Biotechnol. Bioprocess Eng. 26: 107–113.
Ding, L., A. M. Bond, J. Zhai, and J. Zhang (2013) Utilization of nanoparticle labels for signal amplification in ultrasensitive electrochemical affinity biosensors: a review. Anal. Chim. Acta 797: 1–12.
Usov, N. A. and E. M. Gubanova (2020) Application of magnetosomes in magnetic hyperthermia. Nanomaterials (Basel) 10: 1320.
Nudelman, H., Y. Z. Lee, Y. L. Hung, S. Kolusheva, A. Upcher, Y. C. Chen, J. Y. Chen, S. C. Sue, and R. Zarivach (2018) Understanding the biomineralization role of magnetite-interacting components (MICs) from magnetotactic bacteria. Front. Microbiol. 9: 2480.
Wacker, R., B. Ceyhan, P. Alhorn, D. Schueler, C. Lang, and C. M. Niemeyer (2007) Magneto immuno-PCR: a novel immunoassay based on biogenic magnetosome nanoparticles. Biochem. Biophys. Res. Commun. 357: 391–396.
Revathy, T., M. A. Jayasri, and K. Suthindhiran (2016) Antimicrobial magnetosomes for topical antimicrobial therapy. pp. 67–101. In: A. M. Grumezescu (ed.). Nanobiomaterials in Antimicrobial Therapy: Applications of Nanobiomaterials. William Andrew Publishing, Kidlington, UK.
Uebe, R. and D. Schüler (2016) Magnetosome biogenesis in magnetotactic bacteria. Nat. Rev. Microbiol. 14: 621–637.
Jacob, J. J. and K. Suthindhiran (2020) Immobilisation of lipase enzyme onto bacterial magnetosomes for stain removal. Biotechnol. Rep. (Amst.) 25: e00422.
Jacob, J. J. and K. Suthindhiran (2021) Efficiency of immobilized enzymes on bacterial magnetosomes. Appl. Biochem. Microbiol. 57: 603–610.
Raguraman, V., M. A. Jayasri, and K. Suthindhiran (2020) Magnetosome mediated oral Insulin delivery and its possible use in diabetes management. J. Mater. Sci. Mater. Med. 31: 75.
Raguraman, V. and K. Suthindhiran (2020) Comparative studies on functionalization of bacterial magnetic nanoparticles for drug delivery. J. Clust. Sci. 31: 1275–1284.
Sannigrahi, S., S. K. Arumugasamy, J. Mathiyarasu, and K. Suthindhiran (2020) Magnetosome-anti-Salmonella antibody complex based biosensor for the detection of Salmonella typhimurium. Mater. Sci. Eng. C Mater. Biol. Appl. 114: 111071.
Varshney, M., L. Yang, X. L. Su, and Y. Li (2005) Magnetic nanoparticle-antibody conjugates for the separation of Escherichia coli O157:H7 in ground beef. J. Food Prot. 68: 1804–1811.
Alphandéry, E. (2014) Applications of magnetosomes synthesized by magnetotactic bacteria in medicine. Front. Bioeng. Biotechnol. 2: 5.
Fung, F., H. S. Wang, and S. Menon (2018) Food safety in the 21st century. Biomed. J. 41: 88–95.
Zhang, X., P. Geng, H. Liu, Y. Teng, Y. Liu, Q. Wang, W. Zhang, L. **, and L. Jiang (2009) Development of an electrochemical immunoassay for rapid detection of E. coli using anodic strip** voltammetry based on Cu@Au nanoparticles as antibody labels. Biosens. Bioelectron. 24: 2155–2159.
Ngo, V. K. T., H. P. U. Nguyen, T. P. Huynh, N. N. P. Tran, Q. V. Lam, and T. D. Huynh (2015) Preparation of gold nanoparticles by microwave heating and application of spectroscopy to study conjugate of gold nanoparticles with antibody E. coli O157:H7. Adv. Nat. Sci. Nanosci. Nanotechnol. 6: 035015.
Wang, Y. and E. C. Alocilja (2015) Gold nanoparticle-labeled biosensor for rapid and sensitive detection of bacterial pathogens. J. Biol. Eng. 9: 16.
Zheng, L., G. Cai, S. Wang, M. Liao, Y. Li, and J. Lin (2019) A microfluidic colorimetric biosensor for rapid detection of Escherichia coli O157:H7 using gold nanoparticle aggregation and smart phone imaging. Biosens. Bioelectron. 124–125: 143–149.
Das, M., K. H. Shim, S. S. A. An, and D. K. Yi (2011) Review on gold nanoparticles and their applications. Toxicol. Environ. Health Sci. 3: 193–205.
Narayanan, K. B. and N. Sakthivel (2008) Coriander leaf mediated biosynthesis of gold nanoparticles. Mater. Lett. 62: 4588–4590.
Jazayeri, M. H., H. Amani, A. A. Pourfatollah, H. Pazoki-Toroudi, and B. Sedighimoghaddam (2016) Various methods of gold nanoparticles (GNPs) conjugation to antibodies. Sens. Biosensing Res. 9: 17–22.
**ng, K. Y., J. Peng, D. F. Liu, L. M. Hu, C. Wang, G. Q. Li, G. G. Zhang, Z. Huang, S. Cheng, F. F. Zhu, N. M. Liu, and W. H. Lai (2018) Novel immunochromatographic assay based on Eu (III)-doped polystyrene nanoparticle-linker-monoclonal antibody for sensitive detection of Escherichia coli O157:H7. Anal. Chim. Acta 998: 52–59.
Yan, L., H. Da, S. Zhang, V. M. López, and W. Wang (2017) Bacterial magnetosome and its potential application. Microbiol. Res. 203: 19–28.
Li, X., H. Xu, Z.-S. Chen, and G. Chen (2011) Biosynthesis of nanoparticles by microorganisms and their applications. J. Nanomater. 2011: 270974.
Di Padova, F. E., H. Brade, G. R. Barclay, I. R. Poxton, E. Liehl, E. Schuetze, H. P. Kocher, G. Ramsay, M. H. Schreier, and D. B. McClelland (1993) A broadly cross-protective monoclonal antibody binding to Escherichia coli and Salmonella lipopolysaccharides. Infect. Immun. 61: 3863–3872.
Fernández, E., L. Vidal, A. Costa-García, and A. Canals (2016) Mercury determination in urine samples by gold nanostructured screen-printed carbon electrodes after vortex-assisted ionic liquid dispersive liquid-liquid microextraction. Anal. Chim. Acta 915: 49–55.
Đurđić, S., V. Vukojević, M. Ognjanović, L. Švorc, J. Mutić, and D. M. Stanković (2019) Nanomolar quantification of polydatin at boron doped diamond electrode. Application in dietary supplements. Int. J. Electrochem. Sci. 14: 5086–5095.
Knežević, S., M. Ognjanović, N. Nedić, J. F. Mariano, Z. Milanović, B. Petković, B. Antić, S. V. Djurić, and D. Stanković (2020) A single drop histamine sensor based on AuNPs/MnO2 modified screen-printed electrode. Microchem. J. 155: 104778.
Medina-Plaza, C., C. García-Hernández, J. A. de Saja, J. A. Fernández-Escudero, E. Barajas, G. Medrano, C. García-Cabezón, F. Martin-Pedrosa, and M. L. Rodriguez-Mendez (2015) The advantages of disposable screen-printed biosensors in a bioelectronic tongue for the analysis of grapes. Lebensm. Wiss. Technol. 62: 940–947.
Wu, L., B. Gao, F. Zhang, X. Sun, Y. Zhang, and Z. Li (2013) A novel electrochemical immunosensor based on magnetosomes for detection of staphylococcal enterotoxin B in milk. Talanta 106: 360–366.
Yamada, K., C. T. Kim, J. H. Kim, J. H. Chung, H. G. Lee, and S. Jun (2014) Single walled carbon nanotube-based junction biosensor for detection of Escherichia coli. PLoS One 9: e105767.
Singh, R., M. D. Mukherjee, G. Sumana, R. K. Gupta, S. Sood, and B. D. Malhotra (2014) Biosensors for pathogen detection: a smart approach towards clinical diagnosis. Sens. Actuators B Chem. 197: 385–404.
Acknowledgements
This study has been funded by Grant-in-Aid from DBT-No. BT/PR10570/PFN/20/839/2013) and is greatly acknowledged.
Author information
Authors and Affiliations
Contributions
SS prepared the manuscript and carried out all the experiments. SKA carried out the electrochemical experiments. JM conceptualized and supervised the electrochemical experiments. KS conceptualized, supervised, provided resources, funding acquisition for the study. All authors have reviewed the manuscript.
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Neither ethical approval nor informed consent was required for this study.
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sannigrahi, S., Kumar A, S., Mathiyarasu, J. et al. Detection of Escherichia coli in Food Samples by Magnetosome-based Biosensor. Biotechnol Bioproc E 28, 152–161 (2023). https://doi.org/10.1007/s12257-022-0235-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-022-0235-1