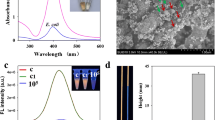
Abstract
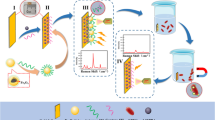
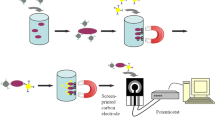
An intelligent colorimetric sensing platform integrated with in situ immunomagnetic separation function was developed for ultrasensitive detection of Escherichia coli O157: H7 (E. coli O157: H7) in food. Captured antibody modified magnetic nanoparticles (cMNPs) and detection antibody/horseradish peroxidase (HRP) co-functionalized AuNPs (dHAuNPs) were firstly synthesized for targeted enrichment and colorimetric assay of E. coli O157: H7, in which remarkable signal amplification was realized by loading large amounts of HRP on the surface of AuNPs. Coupling with the optical collimation attachments and embedded magnetic separation module, a highly integrated optical device was constructed, by which in situ magnetic separation and high-quality imaging of 96-well microplates containing E. coli O157: H7 was achieved with a smartphone. The concentration of E. coli O157: H7 could be achieved in one-step by performing digital image colorimetric analysis of the obtained image with a custom-designed app. This biosensor possesses high sensitivity (1.63 CFU/mL), short detecting time (3 h), and good anti-interference performance even in real-sample testing. Overall, the developed method is expected to be a novel field detection platform for foodborne pathogens in water and food as well as for the diagnosis of infections due to its portability, ease of operation, and high feasibility.
Graphical Abstract






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this article and its supplementary information files.
References
World Health Organization (2021) Estimating the burden of foodborne diseases: a practical handbook for countries: a guide for planning, implementing, and reporting country-level burden of foodborne disease. World Health Organization: Geneva, Switzerland. https://iris.who.int/bitstream/handle/10665/341634/9789240012264-eng.pdf?sequence=1
Rani A, Ravindran V, Surapaneni A et al (2021) Trends in point-of-care diagnosis for Escherichia coli O157: H7 in food and water. Int J Food Microbiol 349:109233. https://doi.org/10.1016/j.ijfoodmicro.2021.109233
Bai Z, Xu X, Wang C et al (2022) A comprehensive review of detection methods for Escherichia coli O157: H7. TrAC Trends Anal Chem 152:16646. https://doi.org/10.1016/j.trac.2022.116646
Han Z, Huang G, Liao J et al (2020) Disentangling survival of Escherichia coli O157: H7 in soils: from a subpopulation perspective. Sci Total Environ 749:141649. https://doi.org/10.1016/j.scitotenv.2020.141649
Oluwarinde B, Ajose D, Abolarinwa T et al (2023) Safety properties of Escherichia coli O157: H7 specific bacteriophages: recent advances for food safety. Foods 12(21):3989. https://doi.org/10.3390/foods12213989
Saxena T, Kaushik P, Mohan M (2015) Prevalence of E. coli O157: H7 in water sources: an overview on associated diseases, outbreaks and detection methods. Diagn Microbiol Infect Dis 82(3):249–264. https://doi.org/10.1016/j.diagmicrobio.2015.03.015
Ben-David A, Davidson C (2014) Estimation method for serial dilution experiments. J Microbiol Methods 107:214–221. https://doi.org/10.1016/j.mimet.2014.08.023
Silva D, Domingues L (2015) On the track for an efficient detection of Escherichia coli in water: a review on PCR-based methods. Ecotoxicol Environ Saf 113:400–411. https://doi.org/10.1016/j.ecoenv.2014.12.015
Wu L, Li G, Xu X et al (2019) Application of nano-ELISA in food analysis: recent advances and challenges. TrAC. Trends Anal Chem 113:140–156. https://doi.org/10.1016/j.trac.2019.02.002
Zhao Q, Lu D, Zhang G et al (2021) Recent improvements in enzyme-linked immunosorbent assays based on nanomaterials. Talanta 223:121722. https://doi.org/10.1016/j.talanta.2020.121722
Hameed S, **e L, Ying Y (2018) Conventional and emerging detection techniques for pathogenic bacteria in food science: a review. Trends Food Sci Technol 81:61–73. https://doi.org/10.1016/j.tifs.2018.05.020
Kim S, Kim S (2021) Bacterial pathogen detection by conventional culture-based and recent alternative (polymerase chain reaction, isothermal amplification, enzyme linked immunosorbent assay, bacteriophage amplification, and gold nanoparticle aggregation) methods in food samples: a review. J Food Saf 41(1):12870. https://doi.org/10.1111/jfs.12870
Ferone M, Gowen A, Fanning S et al (2020) Microbial detection and identification methods: Bench top assays to omics approaches. Compr Rev Food Sci Food Saf 19(6):3106–3129. https://doi.org/10.1111/1541-4337.12618
Ji Y, Huang Y, Cheng Z et al (2023) Lateral flow strip biosensors for foodborne pathogenic bacteria via direct and indirect sensing strategies: a review. J Agric Food Chem 71:10250–10268. https://doi.org/10.1021/acs.jafc.3c02067
Yang T, Luo Z, Bewal T et al (2022) When smartphone enters food safety: a review in on-site analysis for foodborne pathogens using smartphone-assisted biosensors. Food Chem 394:133534. https://doi.org/10.1016/j.foodchem.2022.133534
Lin L, Fang M, Liu W et al (2023) Recent advances and perspectives of functionalized carbon dots in bacteria sensing. Microchim Acta 190:363. https://doi.org/10.1007/s00604-023-05938-1
Mesas Gómez M, Molina-Moya B, de Araujo Souza B et al (2024) Improved biosensing of Legionella by integrating filtration and immunomagnetic separation of the bacteria retained in filters. Microchim Acta 191:82. https://doi.org/10.1007/s00604-023-06122-1
Zhang J, Nie C, Fu W et al (2022) Photoresponsive DNA-modified magnetic bead-assisted rolling circle amplification-driven visual photothermal sensing of Escherichia coli. Anal Chem 94(48):16796–16802. https://doi.org/10.1021/acs.analchem.2c03714
Liu Y, Wang X, Shi X et al (2022) A colorimetric sensor for Staphylococcus aureus detection based on controlled click chemical-induced aggregation of gold nanoparticles and immunomagnetic separation. Microchim Acta 189:104. https://doi.org/10.1007/s00604-022-05211-x
Wang Z, Cai R, Gao Z et al (2020) Immunomagnetic separation: an effective pretreatment technology for isolation and enrichment in food microorganisms detection. Compr Rev Food Sci Food Saf 19(6):3802–3824. https://doi.org/10.1111/1541-4337.12656
Kang Y, Shi S, Sun H et al (2022) Magnetic nanoseparation technology for efficient control of microorganisms and toxins in foods: a review. J Agric Food Chem 70:16050–16068. https://doi.org/10.1021/acs.jafc.2c07132
Shang Y, **ng G, Ai J et al (2023) Nanozyme-mdiated catalytic signal amplification for microfluidic biosensing of foodborne bacteria. Anal Chem 35:13368–13375. https://doi.org/10.1021/acs.analchem.2c03686
**ao F, Li W, Wang Z et al (2023) Smartphone-assisted biosensor based on broom-like bacteria-specific magnetic enrichment platform for colorimetric detection of Listeria monocytogenes. J Hazard Mater 459:132250. https://doi.org/10.1016/j.jhazmat.2023.132250
Zheng L, Cai G, Wang S et al (2019) A microfluidic colorimetric biosensor for rapid detection of Escherichia coli O157: H7 using gold nanoparticle aggregation and smart phone imaging. Biosens Bioelectron 124–125:143–149. https://doi.org/10.1016/j.bios.2018.10.006
Shang Y, **ng G, Liu X et al (2022) Fully integrated microfluidic biosensor with finger actuation for the ultrasensitive detection of Escherichia coli O157: H7. Anal Chem 94(48):16787–16795. https://doi.org/10.1021/acs.analchem.2c03686
Deng R, Chao X, Li H et al (2023) Smartphone-based microplate reader for high-throughput quantitation of disease markers in serum. Analyst 148(4):735–741. https://doi.org/10.1039/D2AN01571D
Liu Z, Wang Y, Deng R et al (2016) Fe3O4@graphene oxide@Ag particles for surface magnet solid-phase extraction surface-enhanced Raman scattering (SMSPE-SERS): from sample pretreatment to detection all-in-one. ACS Appl Mater Interfaces 8(22):14160–14168. https://doi.org/10.1021/acsami.6b02944
Wu P, Xue F, Zuo W et al (2022) A universal bacterial catcher Au–PMBA-nanocrab-based lateral flow immunoassay for rapid pathogens detection. Anal Chem 94(10):4277–4285. https://doi.org/10.1021/acs.analchem.1c04909
You S-M, Luo K, Jung J-Y et al (2021) Gold nanoparticle-coated starch magnetic beads for the separation, concentration, and SERS-based detection of E. coli O157: H7. ACS Appl Mater Interfaces 12(16):18292–18300. https://doi.org/10.1021/acsami.0c00418
**e Y, Huang Y, Li J et al (2021) A trigger-based aggregation of aptamer-functionalized gold nanoparticles for colorimetry: an example on detection of Escherichia coli O157: H7. Sens Actuat B-Chem 339:129865. https://doi.org/10.1016/j.snb.2021.129865
Zhang G, Hu H, Deng S et al (2023) An integrated colorimetric and photothermal lateral flow immunoassay based on bimetallic Ag–Au urchin-like hollow structures for the sensitive detection of E. coli O157: H7. Biosens Bioelectron 225:115090. https://doi.org/10.1016/j.bios.2023.115090
Chen B, Tao Q, OuYang S et al (2023) Biocathodes reducing oxygen in BPE-ECL system for rapid screening of E. coli O157: H7. Biosens Bioelectron 221:114940. https://doi.org/10.1016/j.bios.2022.114940
Wang Y, Bu T, Cao Y et al (2023) A versatile PdRu bimetallic nanoenzyme-integrated enzyme-linked immunosorbent assay for highly sensitive Escherichia coli O157: H7 detection. Anal Chem 95:9237–9243. https://doi.org/10.1021/acs.analchem.3c00743
Ge R, Lin X, Dai H et al (2022) Photoelectrochemical sensors with near-infrared-responsive reduced graphene oxide and MoS2 for quantification of Escherichia coli O157: H7. ACS Appl Mater Interfaces 14(36):41649–41658. https://doi.org/10.1021/acsami.2c13292
Funding
This work was supported by the Natural Science Foundation of China (Grant No. 21874098 and 81741095), Natural Science Foundation of Shanxi Province (Grant No. 20210302124683), Shanxi Province International Collaboration Project (Grant No. 201903D421053), and Research project of Taiyuan Liuweizhai Industrial Co., Ltd. (Taiyuan, China).
Author information
Authors and Affiliations
Contributions
Chunxin Wang: investigation, methodology, and writing—original draft. Rong Deng: investigation, methodology, and writing—review and editing. Haiqin Li: investigation and formal analysis. Zhigang Liu: methodology. **aofeng Niu: investigation and methodology. **aochun Li: writing—review and editing, supervision, and project administration.
Corresponding authors
Ethics declarations
Ethics approval
This research did not involve human or animal samples.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (MP4 13.7 MB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, C., Deng, R., Li, H. et al. An integrated magnetic separation enzyme-linked colorimetric sensing platform for field detection of Escherichia coli O157: H7 in food. Microchim Acta 191, 454 (2024). https://doi.org/10.1007/s00604-024-06497-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-024-06497-9