Abstract
Volumetric absorptive microsampling (VAMS) techniques have gained popularity these last years as innovative tool for collection of blood pharmacokinetic (PK) samples in clinical trials as they offer many advantages over dried blood spot and conventional venous blood sampling. The use of Mitra®, a blood collection device based on volumetric absorptive microsampling (VAMS) technology, was implemented during clinical development of padsevonil (PSL), an anti-seizure medication (ASM) candidate. The present study describes the approach used to bridge plasma (obtained from conventional venous blood sampling) and blood exposures (obtained with Mitra®) to support the use of Mitra as sole blood PK sampling method in clinical trials. Paired blood (using Mitra®) and plasma samples (using conventional venous blood sampling) were collected in healthy volunteers as well as in patients with epilepsy. PSL concentration in plasma and blood were analyzed using different approaches which included evaluation of blood-to-plasma ratios (B/P) over time, linear regression, Bland-Altman analysis as well as development of a linear-mixed effect model based on clinical pharmacology studies. Results showed that the observed in vivo B/P and the measured bias between the 2 collection methods were consistent with the measured in vitro B/P. Graphical analysis demonstrated a clear time effect on the B/P which was confirmed in the linear mixed effect model with sampling time identified as significant covariate. Finally, the built-in model was validated using independent datasets and was shown to adequately predict plasma concentration based on blood concentration with a mean bias of less than 9% (predicted versus observed plasma concentration).
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
PSL was an ASM candidate that was rationally designed for its interaction at the presynaptic and postsynaptic levels. The mechanism of action of PSL combines the synaptic vesicle protein 2 [SV2] isoforms (equally high affinity to SV2A, SV2B, and SV2C) at the presynaptic level and the GABAA receptors (postsynaptic level) for enhancement of GABAergic inhibition (moderate affinity at the benzodiazepine site of the γ-aminobutyric acid type A [GABAA] receptor) (1,2,3). PSL binds SV2A with an affinity that is approximately 2000- and 100-fold greater than that of levetiracetam and brivaracetam, respectively (2, 3). Like benzodiazepines, PSL binds the GABA-A receptor at the α/γ subunit interface (3, 4).
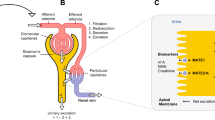
During its drug development, the PK of PSL in adults was characterized in phase 1 and phase 2a/2b studies by collection of plasma PK samples (rich or sparse sampling approaches). In parallel to the adult program, pediatric investigations were also planned according to an agreed strategy with Food and Drug Administration (FDA) and European Medicines Agency (EMA). However, clinical studies involving PK analysis in pediatric populations are especially difficult to conduct because invasive sampling techniques (i.e., venipuncture, large blood volumes, …) are often required. In recent years, many new technologies have emerged for the collection of blood samples in clinical trials (5, 6). Dried blood spot (DBS) has been the frontrunner for many years (7, 8), but issues primarily related to hematocrit, and the consequent effect on assay reproducibility, have hindered general acceptance of this technique as an effective alternative blood sampling method. Another alternative blood sampling method, VAMS, seems to be very promising since it is not vulnerable to hematocrit bias (9,10,11). VAMS technology allows the sampling of a very small, reproducible, and precise volume of liquid (10, 20, 30 µL) by means of a hydrophilic polymer tip. With the Mitra® device (see Fig. 1), the tip is applied to a blood bead obtained from a simple finger prick to collect a whole-blood PK sample (12). VAMS technology has several additional advantages over traditional venous sampling, i.e., decreased patient burden (less invasive technique, lower blood volume, the possibility for home sampling); more flexibility in collecting PK samples (sampling is not limited to time window during a clinical visit which provides opportunity to assess properly exposure-responses in late stages trials) (5); reduced operational burden as samples can be stored and shipped at room temperature and freeze-thaw cycles and plasma preparations steps are not required (13,14,15).
Mitra® device based on VAMS technology (source: https://www.neoteryx.com/microsampling-news/media-assets)
Considering the added benefits of the VAMS technology, especially for its use in pediatric populations, collection of PK blood samples with Mitra® device (using VAMS technology) was implemented in the clinical program of PSL.
The present work describes a global bridging approach based on pooled analysis from multiple clinical studies that was put in place within the PSL clinical development program to implement Mitra® as sole PK sampling technique in future clinical trials. Our bridging study significantly differs from the approach described in the paper from Li et al. (16) which mainly summarized the comparison of PSL concentration between Mitra® and plasma obtained from one single PK study performed in healthy Chinese participants (also included in our analysis – study UP0075).
A robust characterization of the relationship between plasma and blood concentrations was critical to allow changing the plasma assay to a blood assay (Mitra®). The strategy was based on rich sampling of paired blood samples collected using Mitra® device and by conventional venipuncture (for plasma exposure) in healthy volunteers as well as in patients with epilepsy. Different types of analysis were performed and included evaluation of B/P over time, linear regression, Bland-Altmann analysis as well as development of a linear-mixed effect model to predict plasma from blood (Mitra) based on phase 1 clinical pharmacology studies. This model was then subsequently validated using independent data (internal and external datasets).
Material and Methods
Bioanalytical Methods
Two different analytical methods were used for the quantification of PSL in plasma and dried blood Mitra® samples (see supplementary (supp) Table 1 for more details on each method).
Plasma Samples
An analytical method for the determination of PSL in human plasma using solid phase extraction followed by liquid chromatography with tandem mass spectrometry using electrospray ionization in positive ion with multiple reaction monitoring mode was used. A 50 µL aliquot of plasma sample was pipetted into polypropylene tubes and spiked with 50 µL of a deuterated internal standard solution (500 ng/mL in water). After vortexing for 30 s, 400 µL of trifluoroacetic acid (0.5%) were added and the total volume of the mixture was loaded on an Oasis HLB 30-mg adsorbent material in a 96-well plate format for the solid phase extraction. At the end of extraction procedure, the supernatant was transferred (200 µl) to a clean 96-well plate sealed before subsequent LC/MS-MS analysis The method was validated according to FDA and EMA guidelines (17) and covers the concentration range of 0.5 to 500 ng/mL.
Mitra® Samples
Freshly prepared standards and quality control (QC) samples in human whole blood were aliquoted into the wells of a 2-mL 96-well plate (80 µL). Mitra 96-Autorack tips was used to adsorb the standards, QCs and blanks on Mitra tips. Standards and QCs loaded onto Mitra® tips were placed in a plastic 96-well drying rack provided by Neoteryx and allowed to dry overnight (at least 12 h, in closed box with desiccant) at room temperature.
The dried VAMS tips (Mitra® 10 µL) on the plastic holder were removed and placed into wells of a clean 96-well plate for sample extraction. The deuterated internal standard solution for PSL was added to the samples (150 µL, 80.0 ng/mL, formic acid (0.1%) in H20). The 96-well plate was sonicated for 10 min, vortexed (1 h at 600 r.p.m.). The Mitra tips were carefully removed from the 96-well plate and 950 µL of water with trifluoroacetic acid (0.5%) was added to further proceed with solid phase extraction (Thermo SOLA cartridges 10mg-96well plate).
At the end of extraction procedure, the supernatant was transferred (200 µl) to a clean 96-well plate sealed before subsequent LC/MS-MS analysis. The method was validated according to FDA and EMA guidelines (17) and covers the concentration range of 2.00 to 2000 ng/mL.
Collection of Paired Samples in Clinical Studies
Paired PK samples are time-matched PK samples collected in parallel using the two collection methods, i.e, conventional venous blood sampling and blood collection by finger-prick using Mitra® device. The exact date and time of sampling for both methods was recorded in the source document. Actual sampling times were used for calculation of PK parameters and nominal sampling times were used in plots.
Conventional Venous Blood Sampling
Blood obtained from venipuncture was collected in a labeled-vacuum tube containing Li-Heparin. Immediately following collection, the tubes were inverted 8–10 times. The blood was stored for maximum 1 h at room temperature until centrifugation (10 min at 1500 g at room temperature) to collect plasma for PK analysis.
Blood Sampling with Mitra® Device
Blood collection (finger prick) using Mitra was performed by clinical staff. The fingertip of the study participants is first cleaned with alcohol swab and then is allowed to dry. With the help of the lancet, a finger prick is made, and the Mitra® tip is applied on the surface of the blood drop to allow an accurate collection of a 10 µL blood sample (see Fig. 1). Once the tip turned fully red, the tip was allowed to air-dry and was then shipped to bioanalytical lab in a foil bag containing a desiccant.
As a control, in the first clinical study (UP0039) using Mitra®, a Mitra® tip was also dipped into the blood samples collected from venipuncture (before it was centrifuged to collect plasma).
Clinical Studies
Table I summarizes the key characteristics of the clinical studies from which the plasma and blood concentration data were obtained to establish the relationship between PSL plasma and blood exposure.
Four phase 1 clinical trials were used to develop a statistical model to describe and predict PSL plasma concentrations from blood concentrations. Two datasets (one from phase 1 study and one from phase 2b clinical trial) were used to validate the statistical model.
UP0039 (n=40) was a placebo-controlled, phase 1, ethno-bridging clinical trial to evaluate the PK in Japanese and white healthy volunteers after a single (SD) and multiple doses (MD) of PSL (EudraCT no. 2017-000385-30). Three oral PSL dose levels (50, 100, and 200 mg) were studied in Asian participants after a single oral dose and one oral dose (200 mg) to White participants. Ten participants were treated per dose group. Conventional plasma PK samples were collected following single and multiple doses. Mitra® samples were only collected following single dose administration, and paired plasma/Mitra samples were obtained at 0.5, 1, 1.5, 2, 3, 4, 6, 12, 24, 36, and 48 h post-dose.
UP0057 (n=28) was a fixed sequence, phase 1 study in healthy adults, to evaluate the effect of coadministration of erythromycin on the PK of 100 mg Bis In Die (BID) PSL at steady state (EudraCT no. 2017-004694-13). Following single and multiple dose administration, paired PK samples (venous plasma and Mitra®) were collected at pre-dose and at 0.25, 0.5, 0.75, 1, 1.25, 1.5, 2, 3, 4, 6, 8, 12, 24, 36, 48, 72, 96, and 120 h post-dose. Data from both treatment arms were included in the statistical analyses (i.e., samples containing PSL alone or PSL and erythromycin).
UP0070 (n=14/group) was an open label, parallel-group phase 1 trial to evaluate the effect of oxcarbazepine on the PK of PSL in patients with epilepsy (EudraCT no. 2018-001941-16). Control group included patients with epilepsy under stable therapy with levetiracetam (or brivaracetam) and/or lamotrigine. Following attainment of PSL steady state on a multiple oral dose regimen of 400 mg BID (3 days up titration followed by 4.5 days 400 mg BID dosing regimen), PK samples were collected on day 8 at pre-dose and at 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8 and 12h post-dose from both control and oxcarbazepine groups following both venipuncture and Mitra® techniques.
UP0075 (n=12) was a randomized, double-blind, placebo controlled, single dose, phase 1 study to evaluate the PK of PSL in healthy Chinese participants following a single oral dose of 200 mg (Investigational New Drug (IND) no. 135622). PK samples were collected using both venipuncture and Mitra® techniques at 0.25, 0.5, 0.75, 1, 1.25, 1.5, 2, 3, 4, 6, 8, 12, 24, 36, and 48 h post-dose. Additional details on this clinical study have been published elsewhere (16).
EP0091 (n=323 (maintenance period)) was a randomized, double-blind, placebo-controlled, dose-finding (50, 100, 200, 400 mg bid), phase 2b study to evaluate the efficacy and safety of PSL as adjunctive treatment of focal onset seizures in adult subjects with drug resistant epilepsy (NCT03373383, EudraCT no. 2017-003200-48). One of the exploratory objectives was evaluation of two blood sampling techniques (venipuncture and Mitra®). At prespecified visits during the trial, a Mitra® sample was collected, and contemporaneous venipuncture samples were collected whenever possible. The time of sampling relative to the dose was recorded, with 269 paired samples (obtained from 220 patients) collected in total. Additional Mitra® samples were collected across different clinical visits as primary source of PK data for population PK modeling and exposure-response analysis.
Data
For global overview and initial analysis of the data (linear regression and Bland-Altman), individual study datasets and a pooled dataset including all paired Mitra® blood and venipuncture plasma samples collected in the four clinical pharmacology studies (UP0039, UP0057, UP0070, and UP0075) were used. For statistical modeling using linear mixed-effect, paired Mitra® and plasma samples collected between 0 to 12 h post-dose (pre-dose and 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, and 12 h) have been included since PSL is used for chronic treatment (BID dosing regimen).
Concentration-Time Data and B/P Ratio Over Time
For each study, blood concentration-time and plasma concentration-time profiles were graphically displayed. For each time point, a B/P was calculated and its change over time was displayed on a linear scale, for each study.
Statistical Analysis
Statistical analysis was performed using SAS Software version 9.4. Drug concentration-time data collected from both methods, Mitra® and venous sampling, were summarized using descriptive statistics and graphical displays. Per individual study and pooled over all studies, blood-to-plasma linear regression plots were made, to reflect the correlation between blood and plasma concentrations. Additionally, Bland‐Altman plots were generated to display the level of agreement between plasma concentrations obtained using Mitra® versus those obtained using conventional venous sampling.
Bland‐Altman plots were developed by obtaining the mean of the two sampling methods (venipuncture and Mitra®) for each participant and plotting the mean concentration of PSL against the percent difference of the Mitra® concentration from the venipuncture concentration for each participant and each sampling time point. Corresponding Bland‐Altman percent agreement estimates were computed, and 95% confidence intervals (CI) were calculated overall. Presenting the differences in the Bland-Altman plot enabled an average discrepancy or bias to be estimated and confirmed if it was constant across the range of values observed.
A statistical model was developed based on the pooled data from the 4 clinical pharmacology studies, excluding the white study participants of UP0039 (n=10 white) who were used as independent dataset to validate the model. A mixed model was fitted to the log-transformed plasma PSL concentration, with (venipuncture sampling), log transformed blood PSL concentration (Mitra® sampling) as a fixed effect, and study participant as a random effect. Due to differences in study designs, parameters such as PSL dose levels (50 mg/100 mg/200 mg/400 mg), dosing schedule (Single/Multiple PSL dosing), sampling time in hour (0, 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, and 12 h), race (white/black or African American/Asian), study population (healthy/patient), baseline hematocrit level (0–100%), gender (female, male), and age (numeric) potentially affected the blood-to-plasma relationship. Variance components, covariance structure and containment degrees of freedom were used to assess the effects of the sampling times, dosing schedule, PSL dose level, race and population. Covariates with backward elimination method at 0.10 significance level were excluded from the model one by one. In this approach, time-matched blood and plasma concentration data pairs were fitted to a mixed-effects model (e.g., log10(blood) = log10 (plasma)+ covariates). Inter- and intra-individual variability terms were evaluated as well as the influence of different covariates. The final model parameters were selected based on the Akaike Information Criterion (AIC) score (smaller is better) and complexity of the model (simpler is better) (Table II).
The selected statistical model was validated using data from the white participants from UP0039. With the statistical model, the plasma concentrations of white participants (UP0039) were predicted from the blood Mitra® data. Subsequently, plasma PK parameters (area under the curve (AUC) and Cmax), were calculated by non-compartmental analysis (NCA) (using SAS 9.4) using the predicted plasma concentrations. Least-square (LS) geometric mean ratio of predicted plasma PK parameters over actual plasma PK parameters of UP0039 (White cohort) as well as 90% confidence intervals was calculated. Similar to the approach used for logarithmically transformed data in bioequivalence study, an acceptance criterion for confidence intervals of 80–125% was applied to ensure that systemic exposure determined with the 2 approaches (prediction based on model and observed data) were not different using a range of exposure of ±20%.
Following the evaluation of the statistical model, an external validation of the model was conducted using data from EP0091, the pivotal phase 2b efficacy trial. During the trial, at some pre-selected sites, a limited number of patients were sampled to collect time-matched blood and plasma samples at steady state. Predicted plasma results vs actual plasma results were compared with regression and Bland-Altman analysis.
Results
The first step in the implementation of Mitra® device as PK sampling method in the clinical program of PSL was the development and validation of bioanalytical method to allow the quantification of PSL in Mitra® samples. The bioanalytical method has been validated according to FDA and EMA guidelines (17) and was shown to be accurate and precise over the range from 2.00 to 2000 ng/mL (Supp Table 2). One of the main challenges associated with quantification of dried blood sample is the effect of hematocrit. As shown in Supp Table 3, quantification of PSL in Mitra® tips was not affected by the hematocrit (tested within a clinically relevant range from 30 to 70%). As mentioned in “Material and Methods”, the analytical method for Mitra® samples is different from the one used for quantification of PSL in plasma samples (see supp Table 1 for more information). This is explained by different reasons: (i) matrix were different (plasma versus dried blood), (ii) the plasma method was developed and validated for PSL and its metabolites while the Mitra method was validated only for PSL, and (iii) Mitra method was developed and validated years after the plasma method and used up-to-date state of the art technology.
Opportunity was taken in planned clinical studies to collect paired Mitra® and venous plasma samples where collection of Mitra samples was added as exploratory objective. In total, 5 different clinical studies (summarized in Table I) have provided paired samples (obtained from conventional venous plasma and Mitra®) that were used to establish a bridge between plasma and blood exposure. For statistical model development and validation, considering that PSL is used as chronic treatment (BID dosing regimen), only paired samples collected between 0 and 12 h post-dose were included in the model. They represented a total of 3048 paired samples and were obtained from either single or multiple dosing of PSL at dose levels ranging from 50 m to 400 mg (BID dosing regimen for multiple dose) as well as from both healthy volunteers and patients with epilepsy. Within this large dataset, 4 subsets (UP0039 Japanese cohort, UP0057, UP0070, and UP0075) were selected for model development (2699 paired samples) and 2 other subsets (UP0039 White cohort (80 paired samples) and EP0091 (269 paired samples)) were used as independent datasets for model validation.
Before development of the statistical model, results obtained from paired samples were initially assessed using different graphical (profiles over time) and statistical analyses (linear regression and Bland-Altman). For these initial statistical analyses, the whole dataset of paired samples (including samples collected beyond 12 h post-dose as described in “Material and Methods”) from the four phase 1 clinical pharmacology studies was used to explore and analyze the relationship between plasma and blood concentrations.
Figure 2 presents the PSL mean concentration-time profiles from 2 representative clinical studies (UP0057 and UP0070 – Fig. 2a and c) as well as a representative individual profile obtained from the same studies (Fig. 2b and d). After oral administration of PSL, the mean PSL concentration increases similarly in blood (obtained with Mitra®) and plasma with plasma concentration being either similar or higher than blood concentrations, with no major differences observed between the 2 populations (healthy volunteers in UP0057 and epilepsy patients in UP0070). Individual profiles showed similar and consistent patterns to the mean profiles. Similar blood and plasma concentration-time profiles were observed for the 2 other clinical pharmacology studies (UP0075 and UP0039 – see Supp Fig. 1).
Mean (±SD) PSL concentration-time profiles are presented following multiple oral dosing of PSL at 100 mg BID in healthy volunteers (a) and 400 mg BID in patients with epilepsy (c). A typical PSL concentration-time profile for one individual is presented in (b) and (d). Data are from UP0057 (a, b) and from UP0070 (c, d). Plasma PSL concentrations (square symbols) are shown in blue and blood PSL concentrations (diamond symbols) in black
To explore further the relationship between blood and plasma concentration, the mean B/P over time was plotted as shown in Fig. 3 for studies UP0057 (Fig. 3a) and UP0070 (Fig. 3b). These results highlighted that the B/P varied over time and increased up to ~0.9 from dosing to 0.5–2 h post-dose. Then the B/P decreased to reach its steady-state value (0.6 to 0.7) from 4 h post-dose onwards. This time effect on the B/P was observed across a wide range of different conditions (populations, ethnicities, dose levels, gender, …). Indeed, similar patterns (change of B/P over time) were observed in the 2 other clinical studies, UP0039 and UP0075 (see suppl Fig. 2) as well as on individual profiles (data not shown). This observed time-effect on B/P was unlikely to be due to an operational bias because >99% of paired samples were collected within less than 3 min difference. In UP0039 only, B/P was also determined using blood concentration obtained from venous circulation (Mitra tip dipped into blood samples collected from venipuncture). Interestingly, using venous blood concentration, the B/P did not show a time effect and remained relatively constant over time as shown in Suppl Fig. 2A. In addition, the time effect was not observed in an in vitro assay aiming at determining the in vitro B/P (see sup Table 3). Indeed, equilibrium between blood and plasma was achieved within 30 min (first timepoint measured) suggesting no evidence of slow Kon/Koff for binding to red blood cells and/or plasma protein. Therefore, the observed time-dependency on B/P in clinical studies was more likely related to specific in vivo conditions. Of note, in multiple dose studies (UP0057 and UP0070) where a sample was collected prior to dosing, pre-dose B/P was approximately 0.6 and similar to the B/P observed at later timepoints post-dose (>4 h post-dose) indicating that the apparent change in B/P is related to new drug absorbed in the systemic circulation and might be related to a distribution phenomenon.
Initial statistical analyses including linear regression and Bland-Altman plots were performed on the whole dataset of paired samples collected during the study (see “Material and Methods”) and are presented in Fig. 4. The blood and plasma PSL concentrations are highly correlated as indicated by the R2 of ≥0.9 for each study (see supp Fig. 3 for UP0039 and UP0075 studies). The slope of the regression of the pooled data (4 clinical studies) was 0.70 (Fig. 4a), and were 0.65 for UP0057 (Fig. 4c), and 0.75 for UP0070 (Fig. 4e), which is in line with the B/P of PSL measured in vitro (0.65 – Supp Table 4). The Bland Altman plots showed that a mean bias of −42.4% was observed between blood and plasma concentration data based on the pooled dataset (Fig. 4b - 4 clinical studies). At the study level, the mean bias ranged from −36.7 % (UP0070 – Fig. 4f) to −46.4 % (UP0075 – see Supp Fig. 3), consistent with the known in vitro B/P of PSL (see supp Table 4).
Linear regressions between blood and plasma concentrations of PSL (a, c, e) and Bland Altman plots (b, d, f) are presented. The pooled dataset (obtained as described in “Material and Methods” from UP0057, UP0039, UP0070, and UP0075 – n=3377 paired samples) was used in a and b. Data from UP0057 (n=2529 paired samples) were used in c and d and from UP0070 (n=307 paired samples) in e and f
Considering the time-effect observed on B/P, sub-group analyses based on sampling timepoint (<4 h and ≥4 post-dose) were performed using the pooled dataset (4 clinical studies). As expected, these additional analyses (see Supp Fig. 4) showed a trend for a difference in the slope of linear regression (0.7 and 0.6 for <4 h (Supp Fig. 4A) and ≥4 h (Supp Fig. 4B), respectively) but the slope remained aligned with the in vitro B/P. Regarding Bland-Altman analysis, similar findings were observed and were consistent with time effect observed. Indeed, bias was found to be higher for sampling time point ≥4 h (Supp Fig. 4C) where a B/P ratio of 0.6–0.7 was observed compared to sampling time point <4 h (Supp Fig. 4D) (B/P close to 0.9). Overall, these sub-group analyses confirm the original observation of the time effect on the B/P.
Figure 5 presents a box plot reflecting the distribution of the individual B/P per study and the overall pooled value. Medians of B/P ranged between 0.64 and 0.75 and distribution showed overlap** interquartile ranges.
Boxplot of the individual blood-to-plasma ratio of the pooled data and across different studies (median (circle in the box represents mean), Q1 and Q3, min (1.5 times Inter Quartile range below Q1) and max (1.5 times inter quartile range above Q3). Each dot reflects a paired sample for PSL in blood and plasma
In addition to the previous analyses, a linear mixed-effects modeling approach was developed to evaluate the blood and plasma concentration data. In this approach, time matched blood and plasma concentration data pairs were fitted to a mixed effects model. Inter- and intra-individual terms as well as the influence of different covariates were evaluated. For model development, a total of 2699 paired samples were used from 98 study participants and 4 clinical phase 1 pharmacology studies (see Table I). Fifty-seven percent of the study participants were white, and 71% were healthy volunteers. Participants received either single or repeated doses (BID dosing regimen) of PSL with dose levels ranging from 50 to 400 mg. Two sets of paired samples (one from the white cohort of UP0039) and one from the phase 2b study (EP0091) were not included in model development and were used for validation purposes.
Table II presents covariates that were tested in the statistical model developed for the pooled dataset. Using backward elimination method, race, age, gender, and population (healthy vs patient) were the least significant covariates and were excluded from the model. Sampling time, hematocrit, dosing (single/repeated doses), and dose (50 mg to 400 mg) were significant covariates based on a 10% significance level. Inclusion of hematocrit in model (model C) only had minor improvement compared to simpler model without hematocrit (model F). Considering that hematocrit is a physiological parameter that might not always be available and to keep the model as simple as possible, only timepoint and dosing (Single/Repeated doses) as covariates are kept in the final model (model F), resulting in the following model equation:
Model without covariates (Model I) was also checked but not selected as best model due to AIC score. The model fit statistics shown in the supplemental data indicates the adequacy of the model for further application (Supp Fig. 5)).
This model was applied to predict the PSL plasma concentrations from the blood PSL concentration using a set of data that was not used in the model development. This set of data included collection of paired blood and plasma samples from UP0039 (rich PK sampling) and EP0091 (sparse PK sampling) (see Table I – bottom part). The observed blood and plasma concentration of PSL is illustrated in Supp Fig. 6A (UP0039 – White cohort) and Supp Fig. 6B (EP0091). The scatter plot (Fig. 6a) shows strong agreement between predicted and actual plasma concentration (UP0039), as the data remain close to the identity line. This was further illustrated in Fig. 6b, where the predicted geometric mean plasma concentration-time profiles of PSL are presented in comparison to the observed geometric means blood and plasma concentrations. Overall, the predicted geometric mean plasma concentrations overlap with the actual plasma concentrations indicating reasonable performance of the model. As shown by the Bland-Altmann analysis (Fig. 6c), the mean bias of predicted plasma concentration compared to actual plasma concentration was 3.3%, further confirming the good performance of the model even though a trend of higher variability (high proportion of data with a positive bias) at the low range of concentrations can be observed. This is likely explained the tendency of the model for over-prediction in the low concentration range (see residuals in Supp Fig. 5C). Finally, predicted plasma PK parameters (Cmax, AUC(0-t) and AUC) were calculated based on the individual predicted plasma concentration and were compared to observed plasma PK parameters using analysis of variance (ANOVA) (Table III). The trend for over-prediction of the model in the low concentration range did not translate into significant impact on the predicted PK parameters as shown by the geometric mean ratios (predicted plasma PK parameters over actual plasma PK parameters) which were within the 80–125% acceptance range (Table III and Fig. 6). While the 90% CI were within the 80–125% acceptance range for AUC, the lower limit of 90% CI for the Cmax (0.795) was slightly outside the lower limit of the acceptance criteria (0.80). These results demonstrate that the statistical model can adequately predict PSL plasma concentrations from blood concentration.
Model-predicted PSL plasma exposure from blood concentration data from White participants cohort of UP0039. a Linear regression between predicted and actual PSL plasma concentration. b Mean model-predicted plasma concentration time profile (red) plus the 95% CI compared to geometric mean actual plasma (blue) and blood (black) concentration time profiles. c Bland-Altman plots comparing model-predicted and actual plasma concentration. d Bars graph representing distribution of geometric mean ratio (diamond (geometric mean)) between model-predicted plasma PK parameters and actual plasma PK parameters with lower and upper 90% CI
As a final step of model validation, the model was applied to the sparse data from the phase 2b study (EP0091 – Table I). As shown on the Bland-Altman plot (Fig. 7), the plasma concentration data could be predicted with a mean bias of 8.9%, further supporting the use of the current model to relate blood and plasma concentrations of PSL.
Discussion
VAMS is a recently developed technique allowing the collection of blood and other biological matrices for application to many different bioanalytical purposes. In addition, VAMS is minimally invasive, with only <50 µL collected conveniently from a finger prick which facilitates samples collection in pediatric populations, which is critical for development of new ASM. This permits remote specimen collection which can be performed by parents or another family member at home and makes the process less stressful and represents clear benefits for PK sampling in pediatric populations (18, 19). However, it would be fair to acknowledge that one of the main drawbacks of finger prick is the pain reported by some patients (20) which is highly dependent on the lancing device used. Indeed, lancets equipped with a blade allowed the collection of higher blood volume compared to lancets with needle, but they were associated with higher pain (21, 22).
The present paper describes the implementation of Mitra®, a blood collection device based on VAMS technology, in the clinical development of PSL, which was based on in vivo bridging between plasma exposure and blood exposure (obtained from Mitra®). Such bridging was key to support regulatory approval of the final population PK model and exposure-response analysis based on phase 2b/phase 3 data obtained solely with PK blood sampling collected with Mitra while the whole clinical pharmacology package was based on plasma PK. Though new drug application and marketing authorization application have never been filed with PSL because program prematurely stopped, the present bridging strategy was shared during a Type C interaction and was considered adequate by FDA to support use of Mitra as unique PK sampling approach in future clinical trials. Even though many different approaches and methodologies have been used in the literature to bridge data obtained with conventional venous blood sampling and new patient centric sampling methods (23,24,25), they all aim at understanding and describing the relationship between the exposures observed with the two sampling methods. Considering that these new patient centric sampling methods, like VAMS, often collect whole blood in contrast to plasma from traditional venipuncture, the bridging needs to characterize B/P which is also important parameter when interpreting PK or linking PK to PD. Indeed, as nicely reviewed by Rowland (26), B/P of 0.55 indicates minimal uptake by blood cell (variability mainly driven by binding to plasma protein) while with B/P >2, affinity for blood cell might be a significant source of variability.
In our present work, the bridging studies were clinical studies where paired plasma (using conventional method) and blood (using Mitra®) samples were collected at the same timepoints. These important paired samples were collected from 4 different clinical pharmacology studies that provided high degree of diversity regarding dosing regimen (single versus multiple dose), population (patients and healthy volunteers), race (white, Japanese, Chinese) ,and dose levels (50 to 400 mg). In addition, sparse paired plasma and blood samples from a phase 2b efficacy study were also collected and used for model verification.
Different approaches were undertaken to evaluate and characterize the relationship between blood and plasma concentration of PSL. A first set of graphical analyses was performed and showed consistent patterns of blood concentration-time and B/P-time profiles across the different phase 1 studies, regardless of the dose, dosing regimen, population, and race. Of note, a time-effect on B/P was clearly observed with B/P increasing from dosing to 0.5–2 h post-dose and then decreased to reach its steady-state value from ~4 h post-dose onwards. Another interesting observation is that, in multiple dose studies, pre-dose B/P was similar to B/P observed at 4 h post-dose and beyond. These steady-state data suggested that the apparent change in B/P is related to new drug absorbed to the systemic circulation and might be related to a distribution phenomenon. This can be explained by the difference in blood sampling sites. Indeed, plasma is obtained from blood collected in the venous circulation while Mitra® blood samples are obtained from a finger prick. Skin-puncture blood is often referred to as capillary blood, but it is in reality a mixture of arteriolar, venous, and capillary blood, with the arterial blood predominating more due to the higher hydrostatic pressures in arterioles and the arterial limb of capillaries than in venules and the venous limb of capillaries (27). These differences in blood origin (arterial versus venous) are important to consider in the interpretation of B/P when blood is collected from different sampling sites. Indeed, during the PK distribution phase of a drug, the arterial blood concentration can be higher than the venous blood concentration because of the efficient extraction of drug from arterioles to tissue, this being known as the arterio-venous difference (28). During the distribution phase of a drug, this difference can lead to an “apparent” higher B/P when blood and plasma are collected from different sites (blood from arterial and plasma from venous origin) compared to steady-state conditions (no arterio-venous difference). This arterio-venous difference during the distribution phase is the most plausible explanation for the observed time-effect on the B/P of PSL and is supported by our data demonstrating that no time effect is observed when B/P is determined using blood and plasma concentration from the same origin (venous circulation). Similar findings have already been described and published for other drugs like olanzapine, paliperidone, paracetamol, oseltamivir, and artemisinin (29,30,31,32).
In a second step, regression analysis demonstrated a high correlation (R2 of >0.9) between plasma and blood PSL concentrations, with a slope parameter of 0.70 that was in agreement with the bias (−42%) determined with Bland-Altman analysis. Both analyses confirmed that the measured in vivo B/P of PSL was in agreement with the in vitro assessment of the B/P (0.6–0.7; supp Table 4). Except for Bland-Altman analysis, our PSL blood and plasma concentrations measured in Chinese study participants (UP0075) were in accordance with those published by Li et al. (16) and showed that PSL blood concentration are generally lower than plasma concentration. In our paper, data presented in the Y-axis of Bland-Altman plots were transformed (difference between Mitra and plasma as percentage of average concentration) while Li et al. (16) plotted the absolute difference between plasma and Mitra. Considering that plasma concentration is the reference, the absolute difference should have been calculated between Mitra and plasma and not the other way around. But, most importantly, when range of data is large as in the present situation and also, given that the difference between the 2 methods is proportional, it is highly recommended to transform the data (log-transformation or percentage) to apply Bland-Altman analysis (33, 34). In our study, for data transformation, we decided to use percentage in contrast to log-transformation because numbers can be read directly from the plot without the need for back-transformation in case log-transformed data are plotted.
In our dataset, the paired plasma and blood samples were collected in 4 clinical trials with different study designs. To account for all these differences, a statistical model (linear mixed effect model) was developed to evaluate the impact of different covariates. Of note, race (white, Japanese or Chinese), gender, age, and population (patients versus healthy participants) were not found to be significant covariates. Given the observed time effect on the B/P, it is not surprising that timepoint was found to be a significant covariate and was, therefore, included in the model. Hematocrit, dose, and dosing (single or repeated doses) were also identified significant covariates, but only dosing was included in the final model. While dosing was shown to be statistically significant as covariate in the model, there was no clear scientific rationale explaining this finding. Overall, the inclusion of dosing as covariate in the model is unlikely to be of clinical relevance but provided an improvement of model from a statistical standpoint. As a last step, the predictive performance of the model was assessed using 2 independent datasets, both of which showed the adequacy of the model to predict plasma concentration from blood samples collected with VAMS. Indeed, the bias measured between predicted and observed plasma concentration was <10% for both datasets with a trend of higher bias with the phase 2b (EP0091) dataset. Such observation is not surprising given the inherent lower accuracy for data capture (dosing and sampling times) and higher rate of errors observed within a phase 2b setting compared to a phase 1 study. Finally, the geometric mean ratio of predicted versus observed plasma PK parameter falls within 80–125% acceptance range used in the present study. Although the lower 90% CI for Cmax (0.795) was just below the lower limit (0.80) of the 80–125% acceptance range, it remains within the intra-participant variability observed for Cmax (25% - Data on file) and predictions are still considered very good. The lower GMR for Cmax compared to AUC is most likely due to the time-dependency observed on B/P which mainly occurs around the Cmax and is suggesting that the current model does not seem to recover exactly the observed Cmax. There was no strong justification and rationale to try develo** a much more complex model better describing Cmax. Indeed, based on prior knowledge with another antiseizure drug sharing similar SV2A mechanism of action, it is very likely that AUC and/or Cav will be the most relevant PK metric driving the efficacy (35). Therefore, the current model was considered adequate and fit for purpose given the excellent performance for prediction of AUC (confidence intervals within 80–125% range).
Overall, despite one main limitation in the present bridging approach (some covariates were only evaluated in one study), the model developed to bridge blood and plasma concentrations was considered adequate for further use in clinical development and to further support the use of Mitra® as the sole PK sampling technique in future clinical trials. Unfortunately, the PSL clinical program was stopped due to unmet primary efficacy endpoint in phase 2B/phase 3 studies (36).
Conclusions
The present paper shows the value and the importance of conducting thorough in vivo bridging studies to correlate the performance of two PK sampling techniques providing different matrices (plasma and blood), and to ensure that the drug concentration measurements support the PK interpretation/comparison between the two methods. Such bridging was deemed critical in the PSL clinical development to support the change from a plasma assay to a VAMS assay. Considering the novelty of the approach at the time of its implementation, an extensive bridging was performed, including 4 different clinical pharmacology studies and more than 2500 paired samples. The approach used for bridging was considered adequate by FDA to support Mitra as sole PK sampling for future clinical trials. Nowadays, such huge dataset is most likely not required, and the same outcome could have been achieved with a much more targeted approach and a more limited number of paired samples. Nevertheless, the present work sets a foundation for the implementation of future successful bridging approaches in other clinical development programs, without forgetting that each development program has its own specificities that will need consideration in the development of a bridging strategy.
Data Availability
All supporting data for this study is securely stored at UCB and is available upon request.
References
Muglia P, Hannestad J, Brandt C, DeBruyn S, Germani M, Lacroix B, et al. Padsevonil randomized phase IIa trial in treatment-resistant focal epilepsy: a translational approach. Brain Commun. 2020;2(2):fcaa183. https://doi.org/10.1093/braincomms/fcaa183.
Wood M, Daniels V, Provins L, Wolff C, Kaminski RM, Gillard M. Pharmacological profile of the novel antiepileptic drug candidate padsevonil: interactions with synaptic vesicle 2 proteins and the GABA(A) receptor. J Pharmacol Exp Ther. 2020;372(1):1–10. https://doi.org/10.1124/jpet.119.261149.
Niespodziany I, Ghisdal P, Mullier B, Wood M, Provins L, Kaminski RM, et al. Functional characterization of the antiepileptic drug candidate, padsevonil, on GABA(A) receptors. Epilepsia. 2020;61(5):914–23. https://doi.org/10.1111/epi.16497.
Sigel E, Ernst M. The benzodiazepine binding sites of GABA(A) receptors. Trends Pharmacol Sci. 2018;39(7):659–71. https://doi.org/10.1016/j.tips.2018.03.006.
Maass KF, Barfield MD, Ito M, James CA, Kavetska O, Kozinn M, et al. Leveraging patient-centric sampling for clinical drug development and decentralized clinical trials: promise to reality. Clin Transl Sci. 2022;15(12):2785–95. https://doi.org/10.1111/cts.13411.
Baillargeon KR, Mace CR. Microsampling tools for collecting, processing, and storing blood at the point-of-care. Bioeng Transl Med. 2023;8(2): e10476. https://doi.org/10.1002/btm2.10476.
Evans C, Arnold M, Bryan P, Duggan J, James CA, Li W, et al. Implementing dried blood spot sampling for clinical pharmacokinetic determinations: considerations from the IQ Consortium Microsampling Working Group. AAPS J. 2015;17(2):292–300. https://doi.org/10.1208/s12248-014-9695-3.
Kothare PA, Bateman KP, Dockendorf M, Stone J, Xu Y, Woolf E, et al. An integrated strategy for implementation of dried blood spots in clinical development programs. AAPS J. 2016;18(2):519–27. https://doi.org/10.1208/s12248-015-9860-3.
De Kesel PM, Capiau S, Lambert WE, Stove CP. Current strategies for co** with the hematocrit problem in dried blood spot analysis. Bioanalysis. 2014;6(14):1871–4. https://doi.org/10.4155/bio.14.151.
Mano Y, Kita K, Kusano K. Hematocrit-independent recovery is a key for bioanalysis using volumetric absorptive microsampling devices, Mitra. Bioanalysis. 2015;7(15):1821–9. https://doi.org/10.4155/bio.15.111.
Spooner N, Denniff P, Michielsen L, De Vries R, Ji QC, Arnold ME, et al. A device for dried blood microsampling in quantitative bioanalysis: overcoming the issues associated blood hematocrit. Bioanalysis. 2015;7(6):653–9. https://doi.org/10.4155/bio.14.310.
Sciberras D, Otoul C, Lurquin F, Smeraglia J, Lappert A, De Bruyn S, et al. A pharmacokinetic study of radiprodil oral suspension in healthy adults comparing conventional venous blood sampling with two microsampling techniques. Pharmacol Res Perspect. 2019;7(1): e00459. https://doi.org/10.1002/prp2.459.
Protti M, Mandrioli R, Mercolini L. Tutorial: volumetric absorptive microsampling (VAMS). Anal Chim Acta. 2019;1046:32–47. https://doi.org/10.1016/j.aca.2018.09.004.
Kok MGM, Fillet M. Volumetric absorptive microsampling: current advances and applications. J Pharm Biomed Anal. 2018;147:288–96. https://doi.org/10.1016/j.jpba.2017.07.029.
Londhe V, Rajadhyaksha M. Opportunities and obstacles for microsampling techniques in bioanalysis: special focus on DBS and VAMS. J Pharm Biomed Anal. 2020;182: 113102. https://doi.org/10.1016/j.jpba.2020.113102.
Li XY, Hu C, Zhu XH, Wang Y, Shu SQ, Luo Z. Pharmacokinetics and safety of padsevonil in healthy Chinese subjects and comparison of two sampling methods for padsevonil quantification. Eur Rev Med Pharmacol Sci. 2023;27(10):4698–707. https://doi.org/10.26355/eurrev_202305_32482.
EMA (CHMP/EWP/192217/2009 Rev 1 Corr.2). Guideline on bioanalytical method validation 2012. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf
Neupane B, Pandya H, Pandya T, Austin R, Spooner N, Rudge J, et al. Inflammation and cardiovascular status impact midazolam pharmacokinetics in critically ill children: an observational, prospective, controlled study. Pharmacol Res Perspect. 2022;10(5): e01004. https://doi.org/10.1002/prp2.1004.
Guerra Valero Y, Dorofaeff T, Parker L, Coulthard MG, Sparkes L, Lipman J, et al. Microsampling to support pharmacokinetic clinical studies in pediatrics. Pediatr Res. 2022;91(6):1557–61. https://doi.org/10.1038/s41390-021-01586-4.
Heinemann L. Finger pricking and pain: a never ending story. J Diabetes Sci Technol. 2008;2(5):919–21. https://doi.org/10.1177/193229680800200526.
Fruhstorfer H, Schmelzeisen-Redeker G, Weiss T. Capillary blood sampling: relation between lancet diameter, lancing pain and blood volume. Eur J Pain. 1999;3(3):283–6. https://doi.org/10.1053/eujp.1999.0132.
Serafin A, Malinowski M, Prazmowska-Wilanowska A. Blood volume and pain perception during finger prick capillary blood sampling: are all safety lancets equal? Postgrad Med. 2020;132(3):288–95. https://doi.org/10.1080/00325481.2020.1717160.
Dockendorf MF, Jaworowicz D, Humphrey R, Anderson M, Breidinger S, Ma L, et al. A model-based approach to bridging plasma and dried blood spot concentration data for phase 3 verubecestat trials. AAPS J. 2022;24(3):53. https://doi.org/10.1208/s12248-022-00682-5.
Roadcap B, Hussain A, Dreyer D, Carter K, Dube N, Xu Y, et al. Clinical application of volumetric absorptive microsampling to the gefapixant development program. Bioanalysis. 2020;12(13):893–904. https://doi.org/10.4155/bio-2020-0074.
Iacuzzi V, Posocco B, Zanchetta M, Gagno S, Poetto AS, Guardascione M, et al. Dried blood spot technique applied in therapeutic drug monitoring of anticancer drugs: a review on conversion methods to correlate plasma and dried blood spot concentrations. Pharm Res. 2021;38(5):759–78. https://doi.org/10.1007/s11095-021-03036-6.
Rowland M, Emmons GT. Use of dried blood spots in drug development: pharmacokinetic considerations. AAPS J. 2010;12(3):290–3. https://doi.org/10.1208/s12248-010-9188-y.
Merton G, Jones K, Lee M, Johnston A, Holt DW. Accuracy of cyclosporin measurements made in capillary blood samples obtained by skin puncture. Ther Drug Monit. 2000;22(5):594–8. https://doi.org/10.1097/00007691-200010000-00015.
Chiou WL. The phenomenon and rationale of marked dependence of drug concentration on blood sampling site. Implications in pharmacokinetics, pharmacodynamics, toxicology and therapeutics (Part II). Clin Pharmacokinet. 1989;17(4):275–90. https://doi.org/10.2165/00003088-198917040-00005.
Remmerie B, De Meulder M, Weiner S, Savitz A. Comparison of capillary and venous drug concentrations after administration of a single dose of risperidone, paliperidone, quetiapine, olanzapine, or aripiprazole. Clin Pharmacol Drug Dev. 2016;5(6):528–37. https://doi.org/10.1002/cpdd.290.
Mohammed BS, Cameron GA, Cameron L, Hawksworth GH, Helms PJ, McLay JS. Can finger-prick sampling replace venous sampling to determine the pharmacokinetic profile of oral paracetamol? Br J Clin Pharmacol. 2010;70(1):52–6. https://doi.org/10.1111/j.1365-2125.2010.03668.x.
Instiaty I, Lindegardh N, Jittmala P, Hanpithakpong W, Blessborn D, Pukrittayakamee S, et al. Comparison of oseltamivir and oseltamivir carboxylate concentrations in venous plasma, venous blood, and capillary blood in healthy volunteers. Antimicrob Agents Chemother. 2013;57(6):2858–62. https://doi.org/10.1128/AAC.02408-12.
Gordi T, Hai TN, Hoai NM, Thyberg M, Ashton M. Use of saliva and capillary blood samples as substitutes for venous blood sampling in pharmacokinetic investigations of artemisinin. Eur J Clin Pharmacol. 2000;56(8):561–6. https://doi.org/10.1007/s002280000179.
Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135–60. https://doi.org/10.1177/096228029900800204.
Dewitte K, Fierens C, Stockl D, Thienpont LM. Application of the bland–altman plot for interpretation of method-comparison studies: a critical investigation of its practice. Clin Chem. 2002;48(5):799–801. https://doi.org/10.1093/clinchem/48.5.799.
Schoemaker R, Wade JR, Stockis A. Brivaracetam population pharmacokinetics and exposure-response modeling in adult subjects with partial-onset seizures. J Clin Pharmacol. 2016;56(12):1591–602. https://doi.org/10.1002/jcph.761.
Rademacher M, Toledo M, Van Paesschen W, Liow KK, Milanov IG, Esch ML, et al. Efficacy and safety of adjunctive padsevonil in adults with drug-resistant focal epilepsy: results from two double-blind, randomized, placebo-controlled trials. Epilepsia Open. 2022;7(4):758–70. https://doi.org/10.1002/epi4.12656.
Acknowledgements
The authors thank the volunteers, patients, and caregivers in addition to the investigators and their teams who contributed to the clinical studies. The authors also thank Irene Morelli (York Bioanalytical Solutions) for her contribution to the bioanalytical work. Publication coordination was provided by Tom Grant, PhD (UCB Pharma, Slough, UK). UCB Pharma was responsible for the study design and collection and analysis of data. The authors, some of whom are UCB Pharma employees, were responsible for data interpretation, manuscript revision for intellectual content, and manuscript approval for submission.
Funding
The study was funded by UCB Pharma.
Author information
Authors and Affiliations
Contributions
Participated in research design: HK, CB, MB, MM, MW, CO, DS, and HC
Conducted experiments: CB and CO
Contributed new reagents or analytic tools: CR
Performed data analysis: HK, CB, CO, and HC
Wrote or contributed to the writing of the manuscript: HK, CB, MB, MM, MW, CO, DS, and HC
Corresponding author
Ethics declarations
Ethics Approval
The clinical studies were conducted in compliance with the Declaration of Helsinki and with all International Conference on Harmonization Good Clinical Practice guidelines. In addition, all local regulatory requirements were followed, in particular, those affording greater protection to the safety of trial participants. The final protocol, amendments, and informed consent documentation were reviewed and approved by Institutional Review Boards and/or Independent Ethics Committees at each participating center. A signed and dated informed consent was required from each subject before any screening procedures were conducted.
Conflict of Interest
Hugues Chanteux, Merran MacPherson, Hester Kramer, and Chiara Rospo are employees of UCB Pharma and receive stock/stock options from their employment. Massimo Bani was an employee of UCB Pharma at the time the studies were conducted and is now an employee of Bergmapharm Consulting SRL, Brescia, Italy. Christian Otoul, David Sciberras, and Mark Watling were employees of UCB Pharma at the time the studies were conducted. Ceyhun Bicer is consultant contracted by UCB.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kramer, H., Bicer, C., Otoul, C. et al. Clinical Bridging Studies and Modeling Approach for Implementation of a Patient Centric Sampling Technique in Padsevonil Clinical Development. AAPS J 26, 1 (2024). https://doi.org/10.1208/s12248-023-00866-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-023-00866-7