Abstract
Wolfram Syndrome (WS) is a fatal human inherited disease with symptoms of diabetes, vision decreasing, and neurodegeneration caused by mutations in the endoplasmic reticulum (ER)-resident protein WFS1. WFS1 has been reported to play an important role in glucose metabolism. However, the role of WFS1 in axonal regeneration in the central nervous system has so far remained elusive. Herein, we established a model of the wfs1b globally deficient zebrafish line. wfs1b deficiency severely impeded the Mauthner-cell (M-cell) axon regeneration, which was partly dependent on the ER stress response. The administration of ER stress inhibitor 4-Phenylbutyric acid (4-PBA) promoted M-cell axon regeneration in wfs1b−/− zebrafish larvae, while the ER stress activator Tunicamycin (TM) inhibited M-cell axon regeneration in wfs1b+/+ zebrafish larvae. Moreover, complementation of wfs1b at the single-cell level stimulated M-cell axon regeneration in the wfs1b−/− zebrafish larvae. Altogether, our results revealed that wfs1b promotes M-cell axon regeneration through the ER stress signal pathway and provide new evidence for a therapeutic target for WS and axon degeneration.
Similar content being viewed by others
Introduction
Wolfram syndrome (WS) is an autosomal recessive neurodegenerative disorder, generally characterized by childhood-onset diabetes mellitus and optic nerve atrophy accompanied by hearing loss and diabetes insipidus [1,2,3]. WS patients had the clinical symptoms of visual field defects, cerebellar ataxia, epilepsy, anxiety, and cognitive impairment from neurological aspects [4, 5]. Subsequently, neuroimaging results showed that WS patients developed generalized brain atrophy, particularly in the cerebellum, medulla, and pons [6,7,8,9,10,11]. However, there is no effective treatment for WS.
The WFS1 protein, encoded by the wfs1 gene, is an integral membrane protein located in the endoplasmic reticulum (ER), and the mutation of the wfs1 gene is identified as the leading causative factor of WS. The functions of WFS1 have been extensively investigated in beta cells due to their abundance in the pancreas [6, 12,13,14]. This protein is also plentiful in the brain, particularly in the hippocampus, hypothalamus, cerebellum, and brainstem, indicating the prominent importance of WFS1 in the central nervous system (CNS) [15, 16]. Previous studies showed that the wfs1-deficient rats exhibited retinal gliosis, optic nerve atrophy, and medullary volume decrease, and retinal abnormalities were also found in the wfs1-deficient mice [17, 18]. At that time, a remarkable loss of retinal ganglion cells was detected in the wfs1b mutant zebrafish retinas [19]. In addition, knockdown of wfs1 in neurons in the fly brain led to age-dependent behavioral deficits and neurodegeneration [20]. More interestingly, WFS1 might participate in the pathogenesis of Alzheimer’s disease (AD) [21,22,23,24]. However, relatively little is known about the link between WFS1 and axon regeneration.
WFS1 is also a component of the unfolded protein response (UPR), which is a series of signal transduction pathways caused by ER stress response [25]. The mRNA expression level of wfs1 was upregulated in response to ER stress [26]. Concurrently, the ER stress response was stimulated by the WFS1 deficiency, which led to dysfunction of the pancreatic islets and the nervous system in the wfs1-deficient rats and WS patients, indicating that WFS1 is related to the ER stress response [6, 11,12,13,14, 17, 27].
The M-cell is a single pair of motor neurons in the brain stem of zebrafish which regulates the escape response triggered by abrupt stimuli [28, 29]. According to recent studies, M-cells show a strong ability to regenerate in zebrafish in contrast to the inability to regenerate in the adult mammalian CNS [30,31,32,33,34], allowing zebrafish to become an emerging model to investigate spinal-cord injury (SCI), which remains a major cause of morbidity and societal expense on account of its global prevalence [35, 36]. SCI and degenerative CNS disorders may cause axon degeneration, which is regarded as a therapeutic target for treating neurodegenerative diseases [37, 38]. Therefore, we intend to investigate M-cell axon regeneration using the SCI model in zebrafish, allowing us to better understand axonal degeneration in the degenerative CNS disease of WS and provide potential therapy for WS patients. Additionally, zebrafish is an ideal model for the study of human diseases because zebrafish have at least one obvious orthologue for over 70% of human genes, which means zebrafish are genetically similar to humans [39].
Here, we established a mutant zebrafish line that has a 23-bp deletion in wfs1b and showed that loss of wfs1b inhibited M-cell axon regeneration, which is partly caused by wfs1b deficiency-mediated ER stress response. Together, our study showed that wfs1b is essential for ER stress response that regulates M-cell axon regeneration, providing a new therapeutic target for WS.
Materials and methods
Zebrafish strains and maintenance
Adult zebrafish were maintained in an aquatic habitat system at 28.5 °C with a light/dark cycle of 14/10-hour (14-h light and 10-h dark cycle). Embryos were collected after natural spawning and raised at 28.5 °C in an incubator (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4, and 0.1% methylene blue, pH 7.0). From 24 hpf, embryos were supplemented with 0.003% N-phenylthiourea (PTU, Sigma-Aldrich, USA) to avoid pigmentation. The transgenic line employed in this study is Tg (Tol 056: EGFP), in which M-cells express Enhanced Green Fluorescence Protein (from RIKEN, Japan). The University of Science and Technology of China (USTC) Animal Resources Center and the University Animal Care and Use Committee provided the rules and regulations that all experiments implemented. All protocols were subject to approval by the Committee on the Ethics of Animal Experiments of the USTC (permit no. USTCACUC1103013).
Genome editing
CRISPR-mediated genome editing for the generation of wfs1b−/− mutants was performed. Cas9 mRNA was synthesized accordingly using appropriate plasmids (108301; Addgene) with the mMessage mMachine T7 Ultra Kit (Thermo Fisher). The single-guide RNA (sgRNA) targeting the wfs1b sequence was synthesized using the plasmids mentioned above with the Megashortscript T7 kit (Thermo Fisher). We blended the Cas9 mRNA (300 ng/μL) with the sgRNA (40 ng/ μL) gently and then microinjected this mixture into one-cell stage embryos.
Single-cell electroporation
Before electroporation, 4 dpf larvae were anesthetized with ethyl 3-aminobenzoic methanesulfonate (MS222, Sigma-Aldrich) and embedded in 1% low-melting agarose (Sangon, China) in an electroporation chamber. A micropipette tip pulled by a micropipette puller (Sutter Instrument, USA) was filled with plasmids and placed near the M-cell soma. Electric stimulation was applied to the zebrafish larvae, delivering the plasmids, whose concentration is 120 ng/μL, into the unilateral M-cell.
Two-photo axotomy
Anesthetic zebrafish larvae at 6 dpf were fixed in 1% low-melting agarose in a chamber prior to axotomy. A Zeiss microscope (LSM710; Carl Zeiss, Oberkochen, Germany) equipped with a two-photon was used at a wavelength of 800 nm and an intensity of 15%–35% to ablate the M-cell axons under a 25 × oil dip** lens.
In vivo imaging
Larvae were sedated with MS222 and then embedded in 1% low-melting agarose in a chamber. Larvae were photographed at 2 days after axotomy with a confocal system (FV1000; Olympus, Tokyo, Japan) and a water dip** lens (40 × , 0.85 numerical-aperture objective). Z-stack images were acquired at 3-μm intervals.
Quantitative real-time PCR
Total RNA was extracted from the whole larvae using RNAsio (TAKARA), and approximately 1 μg of RNA was reverse-transcribed into cDNA using HiScript II qRT SuperMix II (Vazyme). qPCR application was performed in a total volume of 10 μL containing 5 μL ChamQ Universal SYBR Green qPCR Master Mix and 1 μL cDNA template on a real-time quantification system (LightCycler 96, Roche). The mRNA expression levels were analyzed using the comparative Ct relative quantification method formula 2−△△CT, with housekee** gene β-actin mRNA used as invariant control to normalize the mRNA of target genes, which was repeated three times for each sample. All primers used are listed in Additional file 1: Supplementary Table S1.
Protein extraction and western blotting
Wildtype and wfs1b−/− mutant larvae at 5 dpf were collected and lysed with RIPA buffer supplemented with protease inhibitor and phosphatase inhibitor (Sangon). The lysates were centrifuged and the collected supernatant was kept on ice. Using BCA Protein Assay Kit (Beyotime) in accordance with the manufacturers’ instructions, the concentration of each protein sample was assessed on a microplate reader.
Samples were boiled for 4 min and run on a 10% SDS-PAGE gel with loading buffer (5 ×) and transferred to PVDF membrane. After incubation in 5% nonfat milk and TBST for 60 min at room temperature, the membranes were washed once with TBST and incubated with antibodies against WFS1 (1:1000; Proteintech) or β-Actin (1:2000; HuaAn) at 4 °C for 12 h. Subsequently, the membranes were incubated with secondary goat anti-rabbit antibodies (1:5000; Proteintech) for 1 h at room temperature. Blots were washed with TBST three times and visualized by enhanced chemiluminescence (ECL) system (Thermo Fisher). The densities of bands were quantified by ImageJ software and normalized to protein β-Actin.
Whole-mount in situ hybridization
Whole-mount in situ hybridizations were performed as described [40]. A partial fragment of wfs1b was amplified from cDNA generated from 5 dpf RNA with primers listed in Table S1 for the sake of preparing for the synthesis of digoxygenin (DIG)—labeled RNA probes. DIG-labeled RNA probes were synthesized using DIG RNA labeling mix (Roche) and T7 RNA polymerase (Thermo Fisher). A series of in situ hybridization was replicated three times with the use of independently collected embryos.
Drug treatment
The 4-Phenylbutyric acid (4-PBA) (P21005, Sigma-Aldrich) is a well-characterized ER stress antagonist while Tunicamycin (TM) (654,380, Sigma-Aldrich) is a well-known ER stress activator [41, 42]. We pretreated larvae with 4-PBA at a final concentration of 50 μM from 24 hpf to 8 dpf [2 days post axotomy(dpa)] and pretreated larvae with TM at a final concentration of 0.5 μg/ml from 4 to 8 dpf during all of the regeneration experiments. The treatment of EM containing DMSO and PTU was applied in the control larvae.
Transmission electron microscopy
Brain tissues were taken from 6 dpf zebrafish and immediately fixed in 2.5% glutaraldehyde solution overnight at 4 °C. After washing with PBS, specimens were then incubated in the post-fixation solution containing 1% osmic acid for 2 h. Subsequently, specimens were dehydrated with serial dilutions of ethanol in water (50%, 70%, 80%, 95%) for 10 min and 100% ethanol twice for 50 min each. The samples were then embedded in Epon resin with surrounding support tissue and polymerized at 45 °C for 12 h and at 72 °C for 24 h. Ultrathin (70 nm) transverse sections of the brain were segmented by Leica UC-7 and stained with uranyl acetate and lead citrate. Sections were viewed and photographed with a JEM-1400 transmission electron microscope (TEM). Statistical methods were utilized by reference to the previous study [43].
Optokinetic response assay
The optokinetic response (OKR) behavior test was accomplished according to the previous study in order to examine the visual function [44, 45]. A sine-wave grating, which is projected by an LCD projector (NEC 280+; NEC Corporation, Japan), was generated by the software LabVIEW. 5 dpf Zebrafish larvae were placed dorsal side up in 6% methylcellulose solution to hinder from body movement. An infrared-sensitive CCD camera (TCA-1.3BW; Nan**g, China) monitored the elicited eye movement in real time while the rotating grating was placed around the larvae. Wildtype and wfs1b mutant larvae were stimulated with a constant angular velocity of 7.5 degree/s and a fixed spatial frequency (SF) of 0.06 cycles/degree. The gain which equals the ratio of eye velocity and stimulus velocity was used to gauge contrast sensitivity.
Escape behavior assay
The device system is composed of a high-speed camera (1000 fps), a computer, a loudspeaker. The 8 dpf zebrafish larvae were placed in a petri dish with EM and moved to a platform with appropriate light around. The computer was connected to a loudspeaker near the petri dish and a high-speed camera were adjusted properly. Before each test, the larvae were left for 5 min without being disturbed. Movement trajectory was induced by sound stimulation of sinusoidal waves (500 Hz, 20 ms) and video acquisition was controlled by specialized software. For injured group, unilateral M-cells were ablated with two-photon axotomy in 6 dpf before escape behavior assay.
Statistical analysis
Graphs and statistical significance were analyzed with GraphPad Prism 8.0 software (San Diego, USA), Adobe Photoshop CC2020, and Adobe Illustrator CC 2020. Data are presented as the mean ± standard error of the mean (SEM). Experiments were analyzed using unpaired two-tailed Student’s t-tests. Experiments with more than two groups were analyzed using one-way analyses of variance (ANOVAs), and experiments involved two independent variables using two-way ANOVAs. Experiments were repeated at least three times. Differences were considered significant when *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001, ****P ≤ 0.0001. The figure legends provided all other pertinent information, such as sample size and precise statistical tests used.
Results
Developmental Expression and Characteristics of wfs1b in zebrafish larvae.
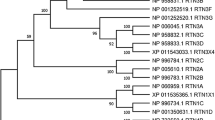
The wfs1 gene, which is only present as a single copy in humans, is present in two copies in zebrafish. Using whole-mount in situ hybridization (WISH), we assessed the pattern of mRNA expression of wfs1a and wfs1b at various development stages. Two wfs1 gene transcripts were examined at 48, 72, and 96 hpf, and during the developmental stage, they were all expressed throughout the CNS (Fig. 1a-l and a′-l′). Similar mRNA expression patterns were reported in their zebrafish embryos [46]. The Wfs1b protein consisted of 895 amino acids while the Wfs1a protein consisted of 1061 amino acids. A computational analysis by the Pfam database (pfam.xfam.org) predicted the domains of protein among Wfs1b, Wfs1a, and WFS1. Wfs1b has similar domains to WFS1 (Fig. 1m). The alignment of correlative amino acid sequences resulted in the creation of a phylogenetic tree with neighbor-joining and maximum-likelihood algorithms through MEGA-11 software. There was higher evolutionary conservation between the WFS1 in humans and the Wfs1b in zebrafish (Fig. 1n).
wfs1a and wfs1b genes in zebrafish larvae. a–f Expression pattern of wfs1b as shown by whole-mount in situ hybridization. a′–f′ were the images of wfs1b expression in the brain of zebrafish larvae. g–l Expression pattern of wfs1a as shown by whole-mount in situ hybridization. g′–l′ were the images of wfs1a expression in the brain of zebrafish larvae. The dorsal view (scale bar, 5 μm) and lateral view (scale bar, 10 μm) of the zebrafish larvae at different stages showed that wfs1b is expressed in the CNS (red arrows) as well as wfs1a. m The predicted protein structure of WFS1 between human and zebrafish. n The phylogenetic tree with the maximum-likelihood algorithm. The numbers represent the length of the evolutionary branch and it determines the degree of homology, Wfs1b has a shorter distance compared to Wfs1a, which means Wfs1b has higher evolutional conservation
Identification of wfs1b mutant zebrafish
Since zebrafish wfs1b has higher conservation, we then focused on wfs1b and designed a CRISPR-Cas9-targeted site in its second exon (Fig. 2a). The wfs1b sgRNA and Cas9 mRNA were transcribed and then co-microinjected into one-cell embryos (Fig. 2b). After two generations of gene identification and larvae cultivation, we obtained a homozygous wfs1b mutant zebrafish line. To precisely evaluate the type of mutation, we amplified about 678-base pair (bp) DNA fragment from genomic DNA by polymerase chain reaction (PCR) and then digested the DNA fragment with EciI endonuclease. Gel electrophoresis findings revealed that F2 zebrafish larvae were heritably homozygous mutants (Additional file 2: Supplementary Fig. S1). According to the sequencing results, a 23-bp loss was detected, which led to a premature termination codon in its third exon (Fig. 2c, d). In addition, we amplified genomic DNA and complementary DNA (cDNA) fragments with primers spanning the target site, and the results showed that no PCR band was displayed in wfs1b−/− groups (Additional file 2: Supplementary Fig. S2). The functional sequence domains of the F2 zebrafish Wfs1b protein were also found to be frame-shifted, according to bioinformatics analysis (Fig. 2e). Lastly, Western blot data revealed that the expression of WFS1 protein was decreased in wfs1b−/− mutant zebrafish (Fig. 2f, g). Meanwhile, the OKR behavior test was utilized to assess the visual function, and the test indicated that the visual function was obviously damaged in 5 dpf wfs1b−/− zebrafish (Additional file 2: Supplementary Fig. S3). Thus, the results above verified that we successfully generated a wfs1b−/− mutant zebrafish line.
Generation and identification of wfs1b mutant zebrafish. a Schematic of the Cas9-sgRNA targeted site located at the second exon of wfs1b. b schematic of the complex injected into one-cell embryos. c, d Representative sequencing results of wide-type and mutated zebrafish lines. The mutant sequencing result showed a 23-bp base deletion. e Bioinformatics analysis indicated that the mutated region is located in the front of the functional domain of Wfs1b. The wildtype translated to 895 amino acids, whereas the mutant translated to 59 normal amino acids. f, g Western-blotting analysis showed that WFS1 protein expression is inhibited in the mutant group compared with that of the wildtype. The protein expressions were quantified by Image J software. The experiment was repeated three times with three independent samples. P = 0.0394. Assessed by unpaired t test
Deficiency in Wfs1b suppresses Mauthner-cell axon regeneration in vivo
Previous studies have shown the M-cells’ ability for regeneration [32,33,34]. The transgenic line Tg (Tol 056: EGFP) was mated with the wfs1b mutant line and the Tg (Tol 056: EGFP)/ wfs1b−/− zebrafish line was obtained so as to explore what role wfs1b plays in M-cell axon regeneration (Fig. 3a). One of the M-cell axons was transected over the cloacal pores at 6 dpf utilizing two-photon laser axotomy (Fig. 3b). When compared to wildtype (wfs1b+/+) larvae, in vivo live imaging at 8 dpf revealed that wfs1b−/− mutant larvae exhibited a reduction in the length of M-cell axon regeneration, indicating that the regenerative capacity of the wfs1b−/− mutant is inferior to the wildtype (control: 461.7 ± 20.82 μm; wfs1b−/−: 268.8 ± 12.84 μm; Fig. 3c, d). We calculated the whole-body length from 4 to 6 dpf and the length of M-cell axons from the cloaca to the end and observed no discernible differences among wildtype, wfs1b+/−, and wfs1b−/− mutant larvae. This demonstrated that the wfs1b mutant had no impact on the development of the M-cell axon itself (Fig. 3e–h). Previous research has proved that heterozygous carriers of the gene for the WS are vulnerable to psychiatric illness and wfs1+/− mice exhibited higher sensitivity to the high-fat diet [47, 48], we assumed that the ability of M-cell axon regeneration in the wfs1b+/− zebrafish might be affected. Then we found that wfs1b+/− mutant larvae displayed a reduction in the length of M-cell axon regeneration as well (control: 472.5 ± 20.65 μm; wfs1+/-: 356.2 ± 21.59 μm; Additional file 2: Supplementary Fig. S4).
Wfs1b regulates M-cell axon regeneration in vivo. a Hybridization of the transgenic line: Tg (Tol 056: EGFP) and wfs1b mutants were crossed for two consecutive generations to obtain Tg (Tol 056: EGFP)/ wfs1b +/- and Tg (T056: EGFP); wfs1b −/− lines. b Representative images of the M-cell axon before and after ablations by a two-photon laser. Asterisk, injury site; arrowhead, cloacal pore; scale bar, 50 μm. c, d Confocal imaging of M-cell axons between wfs1b+/+ and wfs1b−/− groups at 8 dpf and the regeneration length at 2 dpa. Violin plot shows all data points, including minimum, maximum, median, and quartiles. Scale bar, 20 μm, P < 0.0001, control, n = 24; wfs1b−/−, n = 26. Assessed by unpaired t test. e, f Representative images of embryos from the wildtype and the mutant at 6 dpf (scale bar, 500 μm), and measured total body length from 4 to 6 dpf before axotomy (4 dpf, wildtype: 3.603 ± 0.01402 cm, wfs1−/−: 3.549 ± 0.02201 cm, P = 0.1708; 5 dpf, wildtype: 3.745 ± 0.01504 cm, wfs1−/−: 3.727 ± 0.01679 cm, P = 0.8255; 6 dpf, wildtype: 3.790 ± 0.01691 cm, wfs1−/−: 3.806 ± 0.01569 cm, P = 0.8842; n = 30). Assessed by two-way ANOVA. ns, not significant. g, h Defined lengths of M-cell axons from the cloaca to the end were not notably different among WT, homozygous, and heterozygous larvae (wfs1b+/+: 1042 ± 19.51 μm; wfs1b+/-: 985.2 ± 21.47 μm, P = 0.0689; wfs1b−/−: 995.6 ± 21.98 μm, P = 0.1363; n = 9). Assessed by ordinary one-way ANOVA/Tukey’s multiple-comparisons test (wfs1b+/+ versus wfs1b+/-: P = 0.1594; wfs1b+/+ versus wfs1b−/−: P = 0.2856; wfs1b+/- versus wfs1b−/−: P = 0.9342) White asterisk: ablation point. Scale bar, 20 μm. ns, not significant
M-cells are relevant to escape behavior, which is considered to have two obvious stages: the C-start and escape swimming [28]. In order to investigate the function of M-cells in the wfs1b−/− zebrafish larvae, an experimental device system was set up to monitor the movement trajectory (Fig. 4a). Two indicators were measured on the basis of the referenced methods: maximal turn angle and time to the maximal turn angle [49, 50]. Our results showed that the maximal turn angle was shorter when a unilateral M-cell was transected after 48 h and the time to the maximal turn angle was longer in the wfs1b−/− zebrafish larvae, whereas there was no obvious difference when M-cells were not transected between the control and wfs1b−/− groups (Fig. 4b–g).
Escape Behavior Test Exhibited the Impaired Function of M-cell. a Device for testing escape behavior. b Representative images of the original orientation and maximal turn angle position from the wfs1b+/+ and wfs1b−/−zebrafish larvae in the uninjured and injured groups. Red lines indicate the heading direction. c, d Statistical diagram of maximal turn angle, θ. uninjured: P = 0.0921 (wfs1b+/+:121.5° ± 5.879; wfs1b−/−:104.9° ± 7.200); injured: P = 0.0230(wfs1b+/+:94.44° ± 7.681; wfs1b−/−:69.31° ± 5.465); n = 10; ns, not significant. Scale bar, 1 mm. Assessed by unpaired t test. e A series of images of movement trajectory from the wfs1b+/+ and wfs1b−/−zebrafish larvae in the uninjured and injured groups. asterisk: maximal turn angle position. f, g Statistical diagram of time to maximal turn angle. uninjured, P = 0.6410 (wfs1b+/+:12.2 ± 1.879 ms; wfs1b−/−:13.6 ± 2.276 ms); injured, P = 0.0133 (wfs1b+/+:17.00 ± 1.983 ms; wfs1b−/−:25.33 ± 1.944 ms); n = 6; ns, not significant. Scale bar, 1 mm. Assessed by unpaired t test
Complementation of wfs1b promotes axon regeneration at a single cell level
To explore more about the function of wfs1b in M-cell axon regeneration further, we carried out cell-type-specific retro-complementation. Plasmids containing UAS-wfs1b and UAS-mCherry were constructed (Fig. 5a). Thereafter, plasmids CMV-GAL4-VP16/UAS-mCherry (served as control group) and CMV-GAL4-VP16/UAS-mCherry/ UAS-wfs1b were co-transfected into unilateral M-cell through single-cell electroporation at 4 dpf in wfs1b−/− mutant zebrafish respectively (Fig. 5b, c). Zebrafish larvae, whose M-cell exhibited red-fluorescent that mCherry fluorescent protein expressed, were selected 12 h after electroporation (Fig. 5d). Subsequently, we ablated red-fluorescent M-cell axons at 6 dpf with a two-photon laser scanning microscope and proceeded to image the regenerated length of M-cell axons at 8 dpf (2 dpa). After complementation of the wfs1b gene in a single M-cell axon, the capacity for regenerate was activated (Fig. 5e, f).
Complementation of wfs1b at single-cell electroporation promotes M-cell axon regeneration. a Construction of the wfs1b expression system. Plasmids express only mCherry served as the control vector. b Timeline of time points of electroporation, axotomy, and regeneration imaging. c Schematic of M-cell soma electroporation. d Confocal imaging of zebrafish larvae 12 h after electroporation (far left) and magnified images of the brain stem in zebrafish larvae, denoting the position of M-cell soma under different fluorescence in the white box. mCherry represents the axons that were labeled by mCherry. Scale bar, 50 μm. e, f wfs1b gene retro-complementation rescued the length of Mauthner cell axon regeneration (control: 233.1 ± 22.65 μm, n = 10; wfs1b overexpression: 341.4 ± 37.92 μm, n = 9). White asterisk: ablation point. scale bar, 50 μm. P = 0.0225. Assessed by unpaired t test
ER stress response was evoked due to loss of Wfs1b
Considering Wfs1b is mainly localized in the ER, we hypothesized that wfs1b knockout might affect the ER structure and stimulate the ER stress-associated signaling pathway. The UPR is comprised of three pathways: IRE1 (inositol requiring enzyme 1), PERK [double-stranded RNA-activated protein kinase (PKR)–like ER kinase], and ATF6 (activating transcription factor 6) [51, 52]. The expression levels of the UPR pathway were detected by qPCR and we found that the transcriptional level of several UPR-relevant genes, including hspa5, hsp90b1, atf6, and atf4b, were upregulated in wfs1b mutant zebrafish compared to the control group (Fig. 6a). Meanwhile, ER morphology in the zebrafish brain was observed employing Electron Microscopy (Fig. 6b). TEM imaging results showed that the ER structure was fractured both in homozygous and heterozygous wfs1b zebrafish (Fig. 6c–i). Abnormality of ER morphology was also observed in wfs1−/− rodent models and patients [14, 53, 54]. Above all, our findings indicated that wfs1b and ER stress are related.
Loss of Wfs1b provokes the ER stress response. a qPCR analyses of up-regulated downstream genes of the UPR pathway. hspa5, P < 0.0001; hsp90b1, P = 0.0046; atf6, P < 0.0001; atf4b, P < 0.0001. Assessed by unpaired t test. b Diagram of the electron microscope’s operation. c–h Representative TEM imaging showed the ER morphology in wildtype (upper), wfs1b−/− mutant (middle) and wfs1b± mutant (bottom) larvae brain, indicating that the ER morphology was ruptured. (C′-H′) were the ER ultrastructure under magnification from the white boxes in c–f. Scale bar, 500 nm. L, rough endoplasmic reticulum; N, cell nuclei; M, mitochondria. i Statistical diagram of perimeter and Feret’ diameter among the wildtype, the wfs1b−/− and wfs1b+/- mutant zebrafish larvae. Assessed by ordinary one-way ANOVA
wfs1b deficiency-induced ER stress affects Mauthener-cell axon regeneration
To better understand whether ER stress is involved in M-cell regeneration, we tested the involvement of ER stress in this model via a pharmacological approach. ER stress inhibitor 4-PBA was applied at 1 dpf (Fig. 7a), and wfs1b mutant zebrafish larvae pretreated with 4-PBA had boosted M-Cell axon regeneration while there was no obvious difference in wfs1b+/+ zebrafish (Fig. 7b, c). In addition, decreased mRNA expression of relevant ER stress genes at 6 dpf was observed, while the expression of atf4b did not decrease after treatment with 4-PBA (Fig. 7d–f and Additional file 2: Supplementary Fig. S5A). It should be noted that the application of 4-PBA didn’t relieve the abnormal morphology of ER (Additional file 2: Supplementary Fig. S6). The wfs1b+/+ zebrafish group served as a blank control. Then, the wfs1b+/+ zebrafish larvae pretreated with ER stress inducer TM had diminished M-Cell regeneration (Fig. 6g–i) while there was no obvious difference between wfs1b mutant zebrafish groups. qPCR analyses confirmed that the application of TM evoked ER stress response both in the wfs1b+/+ and wfs1b−/− zebrafish larvae (Fig. 7j–l and Additional file 2: Supplementary Fig. S5B). Together, these data demonstrated that ER stress is associated with M-Cell axon regeneration.
ER Stress Response Affects M-Cell Axon Regeneration. a Timeline of time points of 4-PBA treatment, axotomy, and regeneration imaging. b, c Representative confocal imaging of Mauthner-cell axon in wfs1b+/+ treated with DMSO, wfs1b+/+ treated with 4-PBA, wfs1b−/− treated with DMSO, and wfs1b−/− treated with 4-PBA at 8 dpf and the regeneration length at 2 dpa (wfs1b+/+ + DMSO: 431.7 ± 14.12 μm, n = 28; wfs1b+/+ + 4-PBA:432.7 ± 21.63 μm, n = 15; wfs1b−/− + DMSO: 274.2 ± 25.80 μm, n = 20; wfs1b−/− + 4-PBA: 432.0 ± 29.26 μm, n = 15). Assessed by two-way ANOVA/Tukey’s multiple-comparisons test (wfs1b+/+ + DMSO versus wfs1b+/+ + 4-PBA: P > 0.9999; wfs1b+/+ + DMSO versus wfs1b−/− + DMSO: P < 0.0001; wfs1b−/− + DMSO versus wfs1b−/− + 4-PBA: P < 0.0001; wfs1b+/+ + 4-PBA versus wfs1b−/− + DMSO: P < 0.0001). Scale bar, 20 μm. ns, not significant. d–f qPCR analyses of downstream genes of the UPR pathway. Assessed by ordinary one-way ANOVA. g Timeline of time points of TM treatment, axotomy, and regeneration imaging. h, i Representative confocal imaging of Mauthner-cell axon in wfs1b+/+ treated with DMSO, wfs1b+/+ treated with TM, wfs1b−/− treated with DMSO, and wfs1b−/− treated with TM at 8 dpf and the regeneration length at 2 dpa (wfs1b+/+ + DMSO: 456.9 ± 19.27 μm, n = 21; wfs1b+/+ + TM: 323.4 ± 15.80 μm, n = 23; wfs1b−/− + DMSO: 266.1 ± 21.94 μm, n = 13; wfs1b−/− + TM: 263.2 ± 17.29 μm, n = 16). Assessed by two-way ANOVA/Tukey’s multiple-comparisons test (wfs1b+/+ + DMSO versus wfs1b+/+ + TM: P < 0.0001; wfs1b+/+ + DMSO versus wfs1b−/− + DMSO: P < 0.0001; wfs1b+/+ + DMSO versus wfs1b−/− + TM: P < 0.0001; wfs1b−/− + DMSO versus wfs1b−/− + TM: P = 0.9997). Scale bar, 20 μm. ns, not significant. j–l qPCR analyses of downstream genes of the UPR pathway. Assessed by unpaired t test. White asterisk: ablation point
Discussion
WS is considered as a rare neurodegenerative disorder associated with ER dysfunction and its main causative gene wfs1 has been widely reported on its function in pancreatic beta cells but barely in the CNS [1, 12,13,14]. Here, we focused on the impact of wfs1b on axonal regeneration. As far as we know, at least, we first found that zebrafish gene wfs1b deficiency robustly suppressed M-cell axon regeneration through regulation in the ER stress pathway, which might shed light on the understanding of new therapeutic strategies not only for WS but also for neurodegeneration (Fig. 8).
Overview of wfs1b Deficiency-Mediated ER Stress Inhibits M-cell Axon Regeneration. The wfs1b knockout impeded the M-cell axon regeneration due to increased ER stress, resulting in up-regulated mRNA expression of the UPR pathway. The ER stress activator TM inhibited M-cell axon regeneration in wfs1b+/+ zebrafish larvae, whereas both the application of ER stress inhibitor 4-PBA and complementation of wfs1b at the single-cell level ameliorated the degenerated M-cell axon in wfs1b−/− zebrafish larvae
In this study, we established a mutant zebrafish line that has a 23-bp deletion in wfs1b, facilitating us to investigate how Wfs1b affects M-cell axon regeneration. The whole protein expression level of WFS1, including the protein expression level of Wfs1a and Wfs1b, was decreased. As shown in supplementary, we conducted PCR experiments on the basis of primers spanning the target sequence both at the genome and cDNA levels, and no PCR band was verified in the wfs1b−/− mutant zebrafish. The wfs1b mutant zebrafish exhibited OKR deficiency, by implication, optic dysfunction, which was consistent with previous studies in that the WS patients displayed malfunctions in the visual function [5].
M-cells, the biggest motor neurons that regulate the escape response triggered by threatening events in zebrafish, have a powerful capacity for regeneration after two-photon axotomy [28, 29]. Due to their limited numbers, large size, and ease to view, M-cells became a powerful model for investigating axon degeneration in vivo [32, 37, 38, 55, 56]. Our results revealed that M-cell axon regeneration was intensely inhibited in the wfs1b−/− mutant zebrafish as well as the function of M-cells, including shorter maximal turn angle and longer duration to maximal turn angle. The length and function of the M-cell axon did not vary from the mutant larvae compared to the wildtype before ablation, that is, inhibition of M-cell was likely due to wfs1b deficiency rather than development. Furthermore, the regeneration of the M-cell axon was promoted after complementation of the wfs1b gene at a single cell level in the wfs1b−/− zebrafish larvae, suggesting that wfs1b might be an intrinsic factor in regulating axonal regeneration.
ER is an organelle that participates in a wide variety of cellular functions, such as calcium regulation, post-translational modification, and folding of membrane and secretory proteins [26]. WFS1 plays a protective role in regulating ER functions and WS is considered the best prototype for ER diseases since WFS1 is localized in ER [57]. It has been reported that knockdown of WFS1 induced upregulation of ATF6α and its target genes in the rat insulinoma cells [27]. Lack of wfs1 in mice β-cells induced increased markers of ER stress and abnormal ER morphology [13, 14]. The wfs1 knockout mice exhibited increased BiP mRNA expression level in arginine vasopressin (AVP) neurons, as BiP has been used as a marker of ER stress [58]. A mass of evidence indicated that a proportion of neurodegenerative diseases are closely related to ER dysfunction, which resulted from exacerbated ER stress [59,60,61]. Our results showed that the interruption of wfs1b gave rise to the upregulation of several UPR-associated genes and impaired the ER structure in zebrafish larvae. Thus, M-cell axon degeneration might be in connection with the wfs1b deficiency-mediated ER stress response.
Research has proved that inhibition of ER stress through injecting 4-PBA subconjunctivally could accelerate corneal epithelial wound healing and nerve regeneration [41]. In our study, treating wfs1b−/− mutant zebrafish larvae with 4-PBA could ameliorate M-cell axon regeneration partly due to alleviated ER stress and 4-PBA itself has no significant effect on M-cell axon regeneration. However, it’s irreversible when wfs1b deficiency-induced abnormal ER morphology appeared. Induction of ER stress via administering TM, which causes UPR activation in all species studied [42], could hamper axonal regeneration in wfs1b+/+ zebrafish larvae. It was noteworthy that wfs1b−/− mutant zebrafish larvae that were treated with TM exhibited an increased ER stress response compared with wfs1b−/− zebrafish larvae treated with DMSO, suggesting the vulnerability to the disease in the wfs1b−/− mutant zebrafish, whereas the regenerative length showed no remarkable difference, indicating that a certain degree of ER stress response was enough to induce nerve degeneration.
There are, however, a few limitations. First of all, our study is to emphasize the relationship between the knock-out of wfs1b, strictly speaking, knockdown of wfs1 in the zebrafish, and M-cell axon regeneration. Double knock-out of wfs1a and wfs1b in the zebrafish are encouraged to investigate. Besides, Wfs1b is involved in not only the ER but also the mitochondria [54, 62]. Whether mitochondria are associated with M-cell axon regeneration in the wfs1b−/− zebrafish model or not is unknown. Finally, we merely utilized pharmacological approaches to investigate the connection of M-cell axon regeneration with ER stress. It remains to be clarified the underlying molecular pathways of how wfs1b deficiency-mediated ER stress renders M-cell axon degeneration.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Inoue H, Tanizawa Y, Wasson J, Behn P, Kalidas K, Bernal-Mizrachi E et al (1998) A gene encoding a transmembrane protein is mutated in patients with diabetes mellitus and optic atrophy (Wolfram syndrome). Nat Genet 20:143–148
Strom TM, Hortnagel K, Hofmann S, Gekeler F, Scharfe C, Rabl W et al (1998) Diabetes insipidus, diabetes mellitus, optic atrophy and deafness (DIDMOAD) caused by mutations in a novel gene (wolframin) coding for a predicted transmembrane protein. Hum Mol Genet 7:2021–2028
Takeda K, Inoue H, Tanizawa Y, Matsuzaki Y, Oba J, Watanabe Y et al (2001) WFS1 (Wolfram syndrome 1) gene product: predominant subcellular localization to endoplasmic reticulum in cultured cells and neuronal expression in rat brain. Hum Mol Genet 10:477–484
Seppa K, Toots M, Reimets R, Jagomae T, Koppel T, Pallase M et al (2019) GLP-1 receptor agonist liraglutide has a neuroprotective effect on an aged rat model of Wolfram syndrome. Sci Rep 9:15742
Hoekel J, Chisholm SA, Al-Lozi A, Hershey T, Tychsen L, Washington University Wolfram Study Group (2014) Ophthalmologic correlates of disease severity in children and adolescents with Wolfram syndrome. J AAPOS 18:461–465
Wang L, Liu H, Zhang X, Song E, Wang Y, Xu T et al (2021) WFS1 functions in ER export of vesicular cargo proteins in pancreatic beta-cells. Nat Commun 12:6996
Hershey T, Lugar HM, Shimony JS, Rutlin J, Koller JM, Perantie DC et al (2012) Early brain vulnerability in Wolfram syndrome. PLoS ONE 7:e40604
Hilson JB, Merchant SN, Adams JC, Joseph JT (2009) Wolfram syndrome: a clinicopathologic correlation. Acta Neuropathol 118:415–428
Chaussenot A, Bannwarth S, Rouzier C, Vialettes B, Mkadem SA, Chabrol B et al (2011) Neurologic features and genotype-phenotype correlation in Wolfram syndrome. Ann Neurol 69:501–508
Hardy C, Khanim F, Torres R, Scott-Brown M, Seller A, Poulton J et al (1999) Clinical and molecular genetic analysis of 19 Wolfram syndrome kindreds demonstrating a wide spectrum of mutations in WFS1. Am J Hum Genet 65:1279–1290
Hu K, Zatyka M, Astuti D, Beer N, Dias RP, Kulkarni A et al (2022) WFS1 protein expression correlates with clinical progression of optic atrophy in patients with Wolfram syndrome. J Med Genet 59:65–74
Ishihara H, Takeda S, Tamura A, Takahashi R, Yamaguchi S, Takei D et al (2004) Disruption of the WFS1 gene in mice causes progressive beta-cell loss and impaired stimulus-secretion coupling in insulin secretion. Hum Mol Genet 13:1159–1170
Yamada T, Ishihara H, Tamura A, Takahashi R, Yamaguchi S, Takei D et al (2006) WFS1-deficiency increases endoplasmic reticulum stress, impairs cell cycle progression and triggers the apoptotic pathway specifically in pancreatic beta-cells. Hum Mol Genet 15:1600–1609
Riggs AC, Bernal-Mizrachi E, Ohsugi M, Wasson J, Fatrai S, Welling C et al (2005) Mice conditionally lacking the Wolfram gene in pancreatic islet beta cells exhibit diabetes as a result of enhanced endoplasmic reticulum stress and apoptosis. Diabetologia 48:2313–2321
Rigoli L, Lombardo F, Di Bella C (2011) Wolfram syndrome and WFS1 gene. Clin Genet 79:103–117
Ivask M, Pajusalu S, Reimann E, Koks S (2018) Hippocampus and Hypothalamus RNA-sequencing of WFS1-deficient Mice. Neuroscience 374:91–103
Plaas M, Seppa K, Reimets R, Jagomae T, Toots M, Koppel T et al (2017) Wfs1- deficient rats develop primary symptoms of Wolfram syndrome: insulin-dependent diabetes, optic nerve atrophy and medullary degeneration. Sci Rep 7:10220
Waszczykowska A, Zmyslowska A, Braun M, Ivask M, Koks S, Jurowski P et al (2020) Multiple retinal anomalies in Wfs1-deficient mice. Diagnostics (Basel) 10:1
Cairns G, Burte F, Price R, O’Connor E, Toms M, Mishra R et al (2021) A mutant wfs1 zebrafish model of Wolfram syndrome manifesting visual dysfunction and developmental delay. Sci Rep 11:20491
Sakakibara Y, Sekiya M, Fujisaki N, Quan X, Iijima KM (2018) Knockdown of wfs1, a fly homolog of Wolfram syndrome 1, in the nervous system increases susceptibility to age- and stress-induced neuronal dysfunction and degeneration in Drosophila. PLoS Genet 14:e1007196
Yoshida H (2007) ER stress and diseases. FEBS J 274:630–658
Li L, Venkataraman L, Chen S, Fu H (2020) Function of WFS1 and WFS2 in the central nervous system: implications for wolfram syndrome and Alzheimer’s disease. Neurosci Biobehav Rev 118:775–783
Delpech JC, Pathak D, Varghese M, Kalavai SV, Hays EC, Hof PR et al (2021) Wolframin-1-expressing neurons in the entorhinal cortex propagate tau to CA1 neurons and impair hippocampal memory in mice. Sci Transl Med 13:eabe8455
Chen S, Acosta D, Li L, Liang J, Chang Y, Wang C et al (2022) Wolframin is a novel regulator of tau pathology and neurodegeneration. Acta Neuropathol 143:547–569
Fonseca SG, Fukuma M, Lipson KL, Nguyen LX, Allen JR, Oka Y et al (2005) WFS1 is a novel component of the unfolded protein response and maintains homeostasis of the endoplasmic reticulum in pancreatic beta-cells. J Biol Chem 280:39609–39615
Ueda K, Kawano J, Takeda K, Yujiri T, Tanabe K, Anno T et al (2005) Endoplasmic reticulum stress induces Wfs1 gene expression in pancreatic beta-cells via transcriptional activation. Eur J Endocrinol 153:167–176
Fonseca SG, Ishigaki S, Oslowski CM, Lu S, Lipson KL, Ghosh R et al (2010) Wolfram syndrome 1 gene negatively regulates ER stress signaling in rodent and human cells. J Clin Invest 120:744–755
Sillar KT (2009) Mauthner cells. Curr Biol 19:R353-355
Korn H, Faber DS (2005) The Mauthner cell half a century later: a neurobiological model for decision-making? Neuron 47:13–28
Curcio M, Bradke F (2018) Axon regeneration in the central nervous system: facing the challenges from the inside. Annu Rev Cell Dev Biol 34:495–521
Becker T, Becker CG (2014) Axonal regeneration in zebrafish. Curr Opin Neurobiol 27:186–191
Hu BB, Chen M, Huang RC, Huang YB, Xu Y, Yin W et al (2018) In vivo imaging of Mauthner axon regeneration, remyelination and synapses re-establishment after laser axotomy in zebrafish larvae. Exp Neurol 300:67–73
Huang R, Chen M, Yang L, Wagle M, Guo S, Hu B (2017) MicroRNA-133b negatively regulates zebrafish single Mauthner-cell axon regeneration through targeting tppp3 in vivo. Front Mol Neurosci 10:375
Yang LQ, Chen M, Ren DL, Hu B (2020) Dual oxidase mutant retards Mauthner-cell axon regeneration at an early stage via modulating mitochondrial dynamics in zebrafish. Neurosci Bull 36:1500–1512
McDonald JW, Sadowsky C (2002) Spinal-cord injury. Lancet 359:417–425
Karsy M, Hawryluk G (2019) Modern medical management of spinal cord injury. Curr Neurol Neurosci Rep 19:65
Lingor P, Koch JC, Tonges L, Bahr M (2012) Axonal degeneration as a therapeutic target in the CNS. Cell Tissue Res 349:289–311
Feng Y, Yan T, Zheng J, Ge X, Mu Y, Zhang Y et al (2010) Overexpression of WldS or Nmnat2 in mauthner cells by single-cell electroporation delays axon degeneration in live zebrafish. J Neurosci Res 88:3319–3327
Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M et al (2013) The zebrafish reference genome sequence and its relationship to the human genome. Nature 496:498–503
Thisse C, Thisse B (2008) High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc 3:59–69
Wang X, Li W, Zhou Q, Li J, Wang X, Zhang J et al (2020) MANF promotes diabetic corneal epithelial wound healing and nerve regeneration by attenuating hyperglycemia-induced endoplasmic reticulum stress. Diabetes 69:1264–1278
Vacaru AM, Di Narzo AF, Howarth DL, Tsedensodnom O, Imrie D, Cinaroglu A et al (2014) Molecularly defined unfolded protein response subclasses have distinct correlations with fatty liver disease in zebrafish. Dis Model Mech 7:823–835
Lam J, Katti P, Biete M, Mungai M, AshShareef S, Neikirk K et al (2021) A universal approach to analyzing transmission electron microscopy with ImageJ. Cells 10:2177
Mueller KP, Neuhauss SC (2010) Quantitative measurements of the optokinetic response in adult fish. J Neurosci Methods 186:29–34
Huang DF, Wang MY, Yin W, Ma YQ, Wang H, Xue T et al (2018) Zebrafish lacking circadian gene per2 exhibit visual function deficiency. Front Behav Neurosci 12:53
Crouzier L, Richard EM, Diez C, Alzaeem H, Denus M, Cubedo N et al (2022) Morphological, behavioral and cellular analyses revealed different phenotypes in Wolfram syndrome wfs1a and wfs1b zebrafish mutant lines. Hum Mol Genet 31:2711–2727
Swift RG, Polymeropoulos MH, Torres R, Swift M (1998) Predisposition of Wolfram syndrome heterozygotes to psychiatric illness. Mol Psychiatry 3:86–91
Ivask M, Volke V, Raasmaja A, Koks S (2021) High-fat diet associated sensitization to metabolic stress in Wfs1 heterozygous mice. Mol Genet Metab 134:203–211
Danos N, Lauder GV (2012) Challenging zebrafish escape responses by increasing water viscosity. J Exp Biol 215:1854–1862
Dunn TW, Gebhardt C, Naumann EA, Riegler C, Ahrens MB, Engert F et al (2016) Neural circuits underlying visually evoked escapes in Larval Zebrafish. Neuron 89:613–628
Walter P, Ron D (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334:1081–1086
Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8:519–529
Akiyama M, Hatanaka M, Ohta Y, Ueda K, Yanai A, Uehara Y et al (2009) Increased insulin demand promotes while pioglitazone prevents pancreatic beta cell apoptosis in Wfs1 knockout mice. Diabetologia 52:653–663
Angebault C, Fauconnier J, Patergnani S, Rieusset J, Danese A, Affortit CA et al (2018) ER-mitochondria cross-talk is regulated by the Ca2+ sensor NCS1 and is impaired in Wolfram syndrome. Sci Signal 11:eaaq1380
Hecker A, Anger P, Braaker PN, Schulze W, Schuster S (2020) High-resolution map** of injury-site dependent functional recovery in a single axon in zebrafish. Commun Biol 3:307
Bremer J, Marsden KC, Miller A, Granato M (2019) The ubiquitin ligase PHR promotes directional regrowth of spinal zebrafish axons. Commun Biol 2:195
Pallotta MT, Tascini G, Crispoldi R, Orabona C, Mondanelli G, Grohmann U et al (2019) Wolfram syndrome, a rare neurodegenerative disease: from pathogenesis to future treatment perspectives. J Transl Med 17:238
Kurimoto J, Takagi H, Miyata T, Hodai Y, Kawaguchi Y, Hagiwara D et al (2021) Deficiency of WFS1 leads to the impairment of AVP secretion under dehydration in male mice. Pituitary 24:582–588
Wang M, Kaufman RJ (2016) Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature 529:326–335
Hetz C, Mollereau B (2014) Disturbance of endoplasmic reticulum proteostasis in neurodegenerative diseases. Nat Rev Neurosci 15:233–249
Lindholm D, Wootz H, Korhonen L (2006) ER stress and neurodegenerative diseases. Cell Death Differ 13:385–392
Cagalinec M, Liiv M, Hodurova Z, Hickey MA, Vaarmann A, Mandel M et al (2016) Role of Mitochondrial Dynamics in Neuronal Development: Mechanism for Wolfram Syndrome. PLoS Biol 14:e1002511
Acknowledgements
This work was supported by the core facility center for life sciences, University of Science and Technology of China.
Funding
The National Natural Science Foundation of China, No. 82071357. The Ministry of Science and Technology of China, No. 2019YFA0405600 (both to BH). Hefei Independent Innovative Project of “Loan to Subsidy”, No. J2020Y08.
Author information
Authors and Affiliations
Contributions
Conception and design: ZYW, YC, BH; Acquisition of data: ZYW, XLW, LYS; Data analysis: ZYW; Manuscript draft and revision: ZYW, YC, BH. All authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Table S1. Plasmid constructs primer sequences.
Additional file 2
: Figure S1. Agarose gel electrophoresis of the wildtype, heterozygosis, and homozygosis. (a) Manipulation of Ecil endonuclease among the wildtype, wfs1b+/- and wfs1b-/-. (b) The targeted fragments were amplified by PCR from genomic DNA and then digested with Ecil. Red arrows represented the shorter cleaved PCR bands, blue arrows represent the longer cleaved PCR bands, and green arrows represented the uncleaved PCR bands. Figure S2. Examination of mutations in genomic DNA and cDNA levels. (a) Schematic of primers design in genomic DNA. (b) PCR bands from genomic DNA. About 1000 bp fragment was amplified. (c) Schematic of primers design in cDNA. (d) PCR bands from cDNA. About 500 bp fragment was amplified. No PCR band was amplified from genomic DNA and cDNA in wfs1b mutant zebrafish. Figure S3. wfs1b mutant zebrafish showed optokinetic response (OKR) deficiency. (a) Schematic of the apparatus used to measure the OKR of zebrafish larvae. (b) OKR behavior tests of wildtype and wfs1b mutant zebrafish larvae at 5 dpf under 0.04 cycle/degree and 0.6 contrast conditions. wildtype, n=12; wfs1b-/-, n=7. P = 0.0033. Assessed by unpaired t test. Figure S4. Regulation of heterozygote on M-cell axon regeneration. (a) M-cell axon regeneration was hindered in wfs1b+/- mutant zebrafish in vivo. (b) the regenerative length of the M-cell axons at 2 dpa. White asterisk: ablation point. Violin plot shows all data points, including minimum, maximum, median, and quartiles. Scale bar, 20 μm. wfs1b+/+, n=21; wfs1b+/-, n=25. P = 0.0004. Assessed by unpaired t test. Figure S5. qPCR analyses of atf4b genes after treatment with TM and 4-PBA. (a) Treatment of 4-PBA did not relieve the mRNA expression of atf4b. ns, not significant. Assessed by ordinary one-way ANOVA. (b) Treatment of TM accelerated the mRNA expression of atf4b. Assessed by two-way ANOVA/Tukey’s multiple-comparisons test. Figure S6. Electron microscope of zebrafish brain ultrastructure during application of 4-PBA. (a–f) Representative TEM imaging showed the normal ER morphology in WT (above), swelling and ruptured ER morphology in the wfs1b mutant (middle), and 4-PBA – applied (below) zebrafish larvae brain. (a´-f´) were the magnification of the white boxes in (a-f). Scale bar, 500 nm. L, rough endoplasmic reticulum; N, cell nuclei; M, mitochondria. (g) Statistical diagram of perimeter and Feret’ diameter among the wildtype +DMSO, wfs1b-/- +DMSO and wfs1b-/- +4-PBA groups. Assessed by ordinary one-way ANOVA.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, Z., Wang, X., Shi, L. et al. Wolfram syndrome 1b mutation suppresses Mauthner-cell axon regeneration via ER stress signal pathway. acta neuropathol commun 10, 184 (2022). https://doi.org/10.1186/s40478-022-01484-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40478-022-01484-8