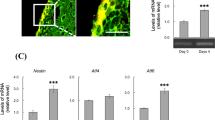
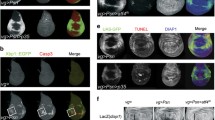
Abstract
Bardet-Biedl syndrome (BBS) is an autosomal recessive syndrome presenting with retinal dystrophy, cognitive impairment, and obesity. BBS is characterized by elevated endoplasmic reticulum (ER) stress in the early stages of adipocyte and retinal development. BBS expression in the CNS and indications of hippocampal dysgenesis suggest neural development abnormalities. However, the role of BBS in ER stress in neuronal cells has not yet been studied. Therefore, we aimed at studying the role of BBS4 in neuronal development under normal and ER stress conditions. ER stress and unfolded protein response (UPR) were studied in BBS4-silenced (SiBBS4) SH-SY5Y cells during differentiation under normal and stress states, using molecular and biochemical markers. ER stress was demonstrated at early neural differentiation, with significantly augmented expression of UPR markers corresponding to BBS4 expression. In the undifferentiated state, BBS4 silencing resulted in significantly reduced ER-stress markers’ expression under normal and ER-stress states. Independent of ER stress, SiBBS4 cells demonstrated significant reduction in activated phospho-IRE1α. Under BBS4 silencing, both sXBP-1 and activated ATF6α p50 failed to translocate to the nucleus. Transcript levels of apoptosis markers were upregulated under BBS4 depletion and ER-stress induction, corresponding to decreased viability. BBS4 depletion in neuronal cells results in reduced sensitivity to ER stress during differentiation and under ER-stress induction, partly due to failure in translocation of ER-transcription factors (TF) sXBP-1 and ATF6α p50 to the nucleus. Hence, BBS4 is essential for nuclear transport under ER-stress response in neuronal cells during early differentiation. Our studies shed light on molecular mechanisms through which BBS4 malfunction alters neuronal ER stress response.






Similar content being viewed by others

Abbreviations
- BBS:
-
Bardet-Biedl syndrome
- UPR:
-
Unfolded protein response
- ER:
-
Endoplasmic reticulum
- TM:
-
Tunicamycin
- BiP:
-
Binding protein
- XBP-1:
-
X-box binding protein 1
- IRE1α:
-
Inositol-requiring 1α
- ATF6α:
-
Activating transcription factor 6α
- CHOP:
-
C/EBP homologous protein
- Bax:
-
Pro-apoptotic Bcl-2 associated X-protein
- Bcl-2:
-
Anti-apoptotic marker B cell lymphoma 2
- PD:
-
Parkinson’s disease
- AD:
-
Alzheimer’s disease
- ALS:
-
Amyotrophic lateral sclerosis
- HD:
-
Huntington disease
References
Forsythe E, Beales PL (2013) Bardet–Biedl syndrome. Eur J Hum Genet 21:8–13. https://doi.org/10.1038/ejhg.2012.115
Álvarez-Satta M, Castro-Sánchez S, Valverde D (2017) Bardet-Biedl syndrome as a chaperonopathy: dissecting the major role of chaperonin-like BBS proteins (BBS6-BBS10-BBS12). Front Mol Biosci 4:55. https://doi.org/10.3389/fmolb.2017.00055
Anosov M, Birk R (2019) Bardet-Biedl syndrome obesity: BBS4 regulates cellular ER stress in early adipogenesis. Mol Genet Metab 126:495–503. https://doi.org/10.1016/j.ymgme.2019.03.006
Loktev AV, Zhang Q, Beck JS, Searby CC, Scheetz TE, Bazan JF, Slusarski DC, Sheffield VC et al (2008) A BBSome subunit links ciliogenesis, microtubule stability, and acetylation. Dev Cell 15:854–865. https://doi.org/10.1016/j.devcel.2008.11.001
Liu P, Lechtreck KF (2018) The Bardet–Biedl syndrome protein complex is an adapter expanding the cargo range of intraflagellar transport trains for ciliary export. Proc Natl Acad Sci 115:E934–E943. https://doi.org/10.1073/pnas.1713226115
Khan SA, Muhammad N, Khan MA, Kamal A, Rehman ZU, Khan S (2016) Genetics of human Bardet-Biedl syndrome, an updates: genetics of human Bardet-Biedl syndrome. Clin Genet 90:3–15. https://doi.org/10.1111/cge.12737
Chiang AP, Beck JS, Yen H-J, Tayeh MK, Scheetz TE, Swiderski RE, Nishimura DY, Braun TA et al (2006) Homozygosity map** with SNP arrays identifies TRIM32, an E3 ubiquitin ligase, as a Bardet-Biedl syndrome gene (BBS11). Proc Natl Acad Sci 103:6287–6292. https://doi.org/10.1073/pnas.0600158103
Leitch CC, Zaghloul NA, Davis EE, Stoetzel C, Diaz-Font A, Rix S, Alfadhel M, Lewis RA et al (2008) Hypomorphic mutations in syndromic encephalocele genes are associated with Bardet-Biedl syndrome. Nat Genet 40:443–448. https://doi.org/10.1038/ng.97
Otto EA, Hurd TW, Airik R, Chaki M, Zhou W, Stoetzel C, Patil SB, Levy S et al (2010) Candidate exome capture identifies mutation of SDCCAG8 as the cause of a retinal-renal ciliopathy. Nat Genet 42:840–850. https://doi.org/10.1038/ng.662
Heon E, Kim G, Qin S, Garrison JE, Tavares E, Vincent A, Nuangchamnong N, Scott CA et al (2016) Mutations in C8ORF37 cause Bardet Biedl syndrome (BBS21). Hum Mol Genet 25:2283–2294. https://doi.org/10.1093/hmg/ddw096
Weihbrecht K, Goar WA, Pak T et al (2017) Kee** an eye on Bardet-Biedl syndrome: a comprehensive review of the role of Bardet-Biedl syndrome genes in the eye. Med Res Arch 5. https://doi.org/10.18103/mra.v5i9.1526
Fattahi Z, Rostami P, Najmabadi A, Mohseni M, Kahrizi K, Akbari MR, Kariminejad A, Najmabadi H (2014) Mutation profile of BBS genes in Iranian patients with Bardet-Biedl syndrome: genetic characterization and report of nine novel mutations in five BBS genes. J Hum Genet 59:368–375. https://doi.org/10.1038/jhg.2014.28
Muller J, Stoetzel C, Vincent MC, Leitch CC, Laurier V, Danse JM, Hellé S, Marion V et al (2010) Identification of 28 novel mutations in the Bardet-Biedl syndrome genes: the burden of private mutations in an extensively heterogeneous disease. Hum Genet 127:583–593. https://doi.org/10.1007/s00439-010-0804-9
Xu Y, Cao J, Huang S, Feng D, Zhang W, Zhu X, Yan X (2015) Characterization of tetratricopeptide repeat-containing proteins critical for cilia formation and function. PloS One 10:e0124378. https://doi.org/10.1371/journal.pone.0124378
Domire JS, Green JA, Lee KG, Johnson AD, Askwith CC, Mykytyn K (2011) Dopamine receptor 1 localizes to neuronal cilia in a dynamic process that requires the Bardet-Biedl syndrome proteins. Cell Mol Life Sci 68:2951–2960. https://doi.org/10.1007/s00018-010-0603-4
Leitch CC, Zaghloul NA (2014) BBS4 is necessary for ciliary localization of TrkB receptor and activation by BDNF. PLoS ONE 9:e98687. https://doi.org/10.1371/journal.pone.0098687
Prieto-Echagüe V, Lodh S, Colman L, Bobba N, Santos L, Katsanis N, Escande C, Zaghloul NA et al (2017) BBS4 regulates the expression and secretion of FSTL1, a protein that participates in ciliogenesis and the differentiation of 3T3-L1. Sci Rep 7:9765. https://doi.org/10.1038/s41598-017-10330-0
Han Y-G, Alvarez-Buylla A (2010) Role of primary cilia in brain development and cancer. Curr Opin Neurobiol 20:58–67. https://doi.org/10.1016/j.conb.2009.12.002
Eichers ER, Abd-El-Barr MM, Paylor R et al (2006) Phenotypic characterization of Bbs4 null mice reveals age-dependent penetrance and variable expressivity. Hum Genet 120:211–226. https://doi.org/10.1007/s00439-006-0197-y
Carter CS, Vogel TW, Zhang Q, Seo S, Swiderski RE, Moninger TO, Cassell MD, Thedens DR et al (2012) Abnormal development of NG2+PDGFR-α+ neural progenitor cells leads to neonatal hydrocephalus in a ciliopathy mouse model. Nat Med 18:1797–1804. https://doi.org/10.1038/nm.2996
Lechtreck K-F, Delmotte P, Robinson ML, Sanderson MJ, Witman GB (2008) Mutations in Hydin impair ciliary motility in mice. J Cell Biol 180:633–643. https://doi.org/10.1083/jcb.200710162
Chamling X, Seo S, Bugge K, Searby C, Guo DF, Drack AV, Rahmouni K, Sheffield VC (2013) Ectopic expression of human BBS4 can rescue Bardet-Biedl syndrome phenotypes in Bbs4 null mice. PLoS ONE 8:e59101. https://doi.org/10.1371/journal.pone.0059101
Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, Leitch CC, Chapple JP et al (2005) Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet 37:1135–1140. https://doi.org/10.1038/ng1644
Aksanov O, Green P, Birk RZ (2014) BBS4 directly affects proliferation and differentiation of adipocytes. Cell Mol Life Sci 71:3381–3392. https://doi.org/10.1007/s00018-014-1571-x
Ariyasu D, Yoshida H, Hasegawa Y (2017) Endoplasmic reticulum (ER) stress and endocrine disorders. Int J Mol Sci 18. https://doi.org/10.3390/ijms18020382
Mercado G, Castillo V, Soto P, Sidhu A (2016) ER stress and Parkinson’s disease: pathological inputs that converge into the secretory pathway. Brain Res 1648:626–632. https://doi.org/10.1016/j.brainres.2016.04.042
Gerakis Y, Hetz C (2018) Emerging roles of ER stress in the etiology and pathogenesis of Alzheimer’s disease. FEBS J 285:995–1011. https://doi.org/10.1111/febs.14332
Montibeller L, de Belleroche J (2018) Amyotrophic lateral sclerosis (ALS) and Alzheimer’s disease (AD) are characterised by differential activation of ER stress pathways: focus on UPR target genes. Cell Stress Chaperones 23:897–912. https://doi.org/10.1007/s12192-018-0897-y
Liu Y, Connor J (2015) From adaption to death: endoplasmic reticulum stress as a novel target of selective neurodegeneration? Neural Regen Res 10:1397. https://doi.org/10.4103/1673-5374.165227
Fischer JW, Leung AKL (2017) CircRNAs: a regulator of cellular stress. Crit Rev Biochem Mol Biol 52:220–233. https://doi.org/10.1080/10409238.2016.1276882
Mockel A, Obringer C, Hakvoort TBM, Seeliger M, Lamers WH, Stoetzel C, Dollfus H, Marion V (2012) Pharmacological modulation of the retinal unfolded protein response in Bardet-Biedl syndrome reduces apoptosis and preserves light detection ability. J Biol Chem 287:37483–37494. https://doi.org/10.1074/jbc.M112.386821
Schönthal AH (2012) Endoplasmic reticulum stress: its role in disease and novel prospects for therapy. Scientifica 2012:1–26. https://doi.org/10.6064/2012/857516
Pagliassotti MJ, Kim PY, Estrada AL, Stewart CM, Gentile CL (2016) Endoplasmic reticulum stress in obesity and obesity-related disorders: an expanded view. Metabolism 65:1238–1246. https://doi.org/10.1016/j.metabol.2016.05.002
Stagg SM, LaPointe P, Balch WE (2007) Structural design of cage and coat scaffolds that direct membrane traffic. Curr Opin Struct Biol 17:221–228. https://doi.org/10.1016/j.sbi.2007.03.010
** H, White SR, Shida T, Schulz S, Aguiar M, Gygi SP, Bazan JF, Nachury MV (2010) The conserved Bardet-Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell 141:1208–1219. https://doi.org/10.1016/j.cell.2010.05.015
Walter F, O’Brien A, Concannon CG et al (2018) ER stress signaling has an activating transcription factor 6α (ATF6)-dependent “off-switch”. J Biol Chem 293:18270–18284. https://doi.org/10.1074/jbc.RA118.002121
Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K (2001) XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107:881–891. https://doi.org/10.1016/S0092-8674(01)00611-0
Puthalakath H, O’Reilly LA, Gunn P et al (2007) ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 129:1337–1349. https://doi.org/10.1016/j.cell.2007.04.027
Sun X, Li W, Deng Y, Dong B, Sun Y, Xue Y, Wang Y (2018) Hepatic conditional knockout of ATF6 exacerbates liver metabolic damage by repressing autophage through MTOR pathway. Biochem Biophys Res Commun 505:45–50. https://doi.org/10.1016/j.bbrc.2018.09.047
Vadde R, Radhakrishnan S, Reddivari L, Vanamala JKP (2015) Triphala extract suppresses proliferation and induces apoptosis in human colon cancer stem cells via suppressing c-Myc/cyclin D1 and elevation of Bax/Bcl-2 ratio. BioMed Res Int 2015:1–12. https://doi.org/10.1155/2015/649263
Yi J, Yang X, Zheng L, Yang G, Sun L, Bao Y, Wu Y, Huang Y et al (2015) Photoactivation of hypericin decreases the viability of RINm5F insulinoma cells through reduction in JNK/ERK phosphorylation and elevation of caspase-9/caspase-3 cleavage and Bax-to-Bcl-2 ratio. Biosci Rep 35:e00195. https://doi.org/10.1042/BSR20150028
Bu L-J, Yu H-Q, Fan L-L, Li XQ, Wang F, Liu JT, Zhong F, Zhang CJ et al (2017) Melatonin, a novel selective ATF-6 inhibitor, induces human hepatoma cell apoptosis through COX-2 downregulation. World J Gastroenterol 23:986. https://doi.org/10.3748/wjg.v23.i6.986
Dadey DYA, Kapoor V, Khudanyan A et al (2016) The ATF6 pathway of the ER stress response contributes to enhanced viability in glioblastoma. Oncotarget 7. https://doi.org/10.18632/oncotarget.6712
Hetz C, Saxena S (2017) ER stress and the unfolded protein response in neurodegeneration. Nat Rev Neurol 13:477–491. https://doi.org/10.1038/nrneurol.2017.99
**ang C, Wang Y, Zhang H, Han F (2017) The role of endoplasmic reticulum stress in neurodegenerative disease. Apoptosis 22:1–26. https://doi.org/10.1007/s10495-016-1296-4
Sarvani C, Sireesh D, Ramkumar KM (2017) Unraveling the role of ER stress inhibitors in the context of metabolic diseases. Pharmacol Res 119:412–421. https://doi.org/10.1016/j.phrs.2017.02.018
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that the results of this work are part of a filed patent.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Horwitz, A., Birk, R. BBS4 Is Essential for Nuclear Transport of Transcription Factors Mediating Neuronal ER Stress Response. Mol Neurobiol 58, 78–91 (2021). https://doi.org/10.1007/s12035-020-02104-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-02104-z