Abstract
Background
Birds are key indicator species in extant ecosystems, and thus we would expect extinct birds to provide insights into the nature of ancient ecosystems. However, many aspects of extinct bird ecology, particularly their diet, remain obscure. One group of particular interest is the bizarre toothed and long-snouted longipterygid birds. Longipterygidae is the most well-understood family of enantiornithine birds, the dominant birds of the Cretaceous period. However, as with most Mesozoic birds, their diet remains entirely speculative.
Results
To improve our understanding of longipterygids, we investigated four proxies in extant birds to determine diagnostic traits for birds with a given diet: body mass, claw morphometrics, jaw mechanical advantage, and jaw strength via finite element analysis. Body mass of birds tended to correspond to the size of their main food source, with both carnivores and herbivores splitting into two subsets by mass: invertivores or vertivores for carnivores, and granivores + nectarivores or folivores + frugivores for herbivores. Using claw morphometrics, we successfully distinguished ground birds, non-raptorial perching birds, and raptorial birds from one another. We were unable to replicate past results isolating subtypes of raptorial behaviour. Mechanical advantage was able to distinguish herbivorous diets with particularly high values of functional indices, and so is useful for identifying these specific diets in fossil taxa, but overall did a poor job of reflecting diet. Finite element analysis effectively separated birds with hard and/or tough diets from those eating foods which are neither, though could not distinguish hard and tough diets from one another. We reconstructed each of these proxies in longipterygids as well, and after synthesising the four lines of evidence, we find all members of the family but Sheng**gornis (whose diet remains inconclusive) most likely to be invertivores or generalist feeders, with raptorial behaviour likely in Longipteryx and Rapaxavis.
Conclusions
This study provides a 20% increase in quantitatively supported fossil bird diets, triples the number of diets reconstructed in enantiornithine species, and serves as an important first step in quantitatively investigating the origins of the trophic diversity of living birds. These findings are consistent with past hypotheses that Mesozoic birds occupied low trophic levels.
Similar content being viewed by others
Background
The diet of most non-avian avialans has been largely speculative so far [1,2,3]. We use Aves in this paper to refer to crown group birds, and Avialae to refer to crown group birds plus all coelurosaurian theropods closer to them than to either dromaeosaurids or troodontids [4]. Among Enantiornithes, the most diverse and widespread avialans in the Mesozoic, only Eoalulavis and Shenqiornis have good evidence (i.e. fossilised digestive tract contents or more than one line of quantitative proxy evidence) backing any particular diet [1, 5] out of nearly 100 known species [6]. A more robust understanding of non-avian avialan diet will allow us to test key hypotheses in bird evolution, such as beak evolution allowing for extant bird dietary diversity [7, 8], trophic level reduction driving powered flight development [9], and birds occupying a low-level consumer role in Mesozoic ecosystems [6) find hard invertivory and piscivory most likely for longipterygids followed by tetrapod hunting and generalist feeding. Both morphotypes of Longipteryx have lesser affinities for medium invertivory and scavenging and Longirostravis and Rapaxavis have lesser affinities for swallowing granivory (significant affinities with semi-specialists excluded). The large-toothed morphotype of Longipteryx shows an affinity for nectarivory, Longirostravis and Rapaxavis show an affinity for folivory, and Shanweiniao for scavenging only with semi-specialists included. DAPC (Additional file 1: Fig. S2D-E) and its predictions (Table 6) are consistent with LDA.
Phylogenetic HSD results comparing strain intervals of extant diet categories are given in Additional file 1: Table S5. Folivores are significantly different from medium invertivores, piscivores, and tetrapod hunters at the p < 0.05 level. Generalists are significantly different from hard invertivores at the p < 0.01 level and from medium invertivores and piscivores at the p < 0.05 level. These differences are noted above the violin plots in Fig. 7.
No statistically significant phylogenetic signal was detected in intervals data with or without semi-specialists included (Table 3). With semi-specialists excluded, Kmult is 0.41 for the PCA dataset and 0.43 for the LDA dataset (Table 3). With semi-specialists included, Kmult is 0.33 for the PCA dataset and 0.32 for the LDA dataset (Table 3).
Discussion
Body mass
Body masses of all longipterygid species were estimated in past publications [1, 21] using multivariate regression of avian skeletal measurements. For convenience, these are supplied in Table 7 alongside body mass estimations of two undescribed specimens of Longipteryx sp. (STM 7-156 and STM 8-117).
Within carnivores and herbivores, body mass appears to be generally conserved phylogenetically. In both groups, excluding and including semi-specialists, K statistics are above 1 (Table 3). This means that the masses of species are more similar to their close relatives than expected from random evolution. For mass of birds across all diets, however, K is just below 1 with semi-specialists excluded. There is no significant phylogenetic signal in body mass data with semi-specialists included.
In a previous review [1], we hypothesised that body mass could distinguish between vertivores and invertivores (Fig. 3C, D), with vertivores typically larger than invertivores, and our findings support this hypothesis. Phylogenetic HSD finds masses of vertivores and invertivores to be significantly different (p = 1.00E−3). We determine a mass cut-off point between vertivores and invertivores of either 324 g or 439 g depending on the dataset. To be conservative, this discussion will treat masses of 300 to 450 g as indeterminate for distinguishing between carnivores, with invertivores predicted below 300 g and vertivores above 450 g. Our more precise breakdown of diets (Fig. 3B) provides preliminary support for our previous hypothesis [1] that separation of predators by body mass occurs based on prey body mass. Tetrapod-hunting birds have a relatively narrow peak body mass (standard deviation = 2.0 g) while fish-hunting (standard deviation = 4.3 g) and invertebrate-hunting birds (standard deviation = 4.9 g) have a wider, flatter distribution. Accordingly, mass distributions of tetrapods tend to have narrower peaks than those of non-tetrapod fish [48]. While large-scale data for invertebrate mass distribution is not available, local distributions of invertebrate mass [49] and global distributions of insect length [50] seem to imply a broad peak of invertebrate masses as well. Together these imply that, at least on macroecological scales, predatory bird masses increase with prey body mass. However, future studies with more precise records of bird prey items and their masses are needed to fully validate this hypothesis. Soft invertivores, mostly filter feeders in this study (sensu [51], see “Diet assignment” in “Methods”), oppose this trend by being, on average, as massive as vertivores. Our sample size of filter feeding birds is small (n = 2), so further work is needed to determine if this persists in larger samples. We expect it will, as other aquatic filter feeders including baleen whales, whale sharks, and pachycormiform fish all convergently evolved gigantism [52], presumably to increase the volume of water/sediment sieved in each pum** cycle. Scavengers, the carnivores with the largest average body masses in this study, have no need to kill prey and no obligation to lift it. Thus, we would not expect their mass to scale to prey mass. Instead, they are believed to experience selective pressures towards larger body size to avoid kleptoparasitism [53], have larger fat stores, and search for carrion more efficiently [54].
When plotting body mass by diet (Fig. 3A, B), we noticed an apparent separation by diet in herbivores as well. While less significantly so than in carnivores (phylogenetic HSD p = 3.60E−02 for herbivores, p = 1.00E−3 for carnivores), folivores and frugivores tend to be more massive than granivores and nectarivores (Fig. 4A, B). Two cut-off points are calculated as optimal: 249 g and 408 g. As above, for this discussion, herbivores below 250 g will be assumed as granivores or nectarivores, those between 250 g and 400 g as indeterminate, and those above 400 g as folivores or frugivores. There is heavy phylogenetic influence on this split, however, signified by phylogenetic HSD and Tukey’s HSD returning very different p-values when comparing the two (Table 2). Food size can explain some of the mass trends once again: seeds are housed inside fruits, so fruits of a given plant species are necessarily larger than their seeds. This is exacerbated by semantic biases, where small fruits are often called seeds. For instance, grains, from whence “granivore” takes its name, are botanically fruits [55]. In other words, the mass distinction between frugivores and granivores seems to be based on the size of food taken, but differences between the botanical definition of seeds and fruits and their operational definitions in diet studies could artificially reinforce this distinction. Nectarivore and folivore masses have more established explanations. Nectarivore mass has long been known to be constrained by the small amounts of nutrient-poor nectar in flowers [56] and increasing specialisation for nectar consumption accompanies a reduction in body mass [57, 58]. Folivory is believed to necessarily increase gut mass and gut retention time, increasing body mass to the point that may impede flight [59]. Indeed, most folivorous taxa in our study are terrestrial (Anhimidae, Phasianidae) or aquatic (Anatidae, Rallidae). Of the two arboreal folivorous taxa studied, Opisthocomus (the hoatzin) is a poor flier [60] and Micropsitta (a pygmy parrot) is a lichen-eating specialist [61] whose assignment as a folivore is debatable.
Predicted body masses for longipterygids range from 32 g as a lower estimate for Longirostravis [21] and 406 g as an upper estimate for Sheng**gornis [1] (Table 7). Sheng**gornis is unusually large for the clade, with the next largest taxa, Longipteryx and Shanweiniao, having upper mass estimates of 246 and 68 g [21] (Table 7), respectively. Sheng**gornis falls within the indeterminate range (i.e. between the two cut-off points calculated) for both carnivore and herbivore masses, so there is no evidence of its diet from mass. Masses for all other longipterygids fall below cut-off point values for carnivores and herbivores, so no longipterygid other than Sheng**gornis is likely to be a vertivore, folivore, or frugivore.
Traditional morphometrics
We find pedal TM generally effective at distinguishing raptorial birds, ground birds, and perching birds. This is congruent with several previous studies [26, 62,63,64]. Ground birds tend to have very straight claws with relatively large DII, raptorial birds tend to have very curved claws with relatively large DI and DII, and perching birds tend to have very curved claws with relatively small DI and DII. However, the heavy overlap of some groups in PCA (e.g. scavenger and perch, strike, and restrain; see Fig. 5A) and distinct clusters within some groups (see “Results”) highlights the subjectivity of our ecological categories and indicates that some may need to be split or merged. If so, this can introduce artificial trends into LDA and DAPC, so we will primarily refer to PCA results when interpreting these data.
Unlike past studies [1, 24, 27], we find raptorial pedal morphologies do not separate cleanly in the morphospace. Striking and restraining raptors other than shrikes overlap almost completely in PCA (Fig. 5A), restraint is the category which striking overlaps with most heavily in LDA (Fig. 5B), and the two are not significantly different in phylogenetic HSD (Additional file 1: Table S1). Suffocation specialists partially overlap with these two groups in PCA, though three of the four taxa that overlap (Bubo virginianus, Pulsatrix perspicillata, and Ninox novaeseelandiae) are known to occasionally hunt large mammals [65, 66] or, in the case of N. novaeseelandiae, mammals much larger than its typical insect prey [67]. Thus, it is possible that this region of the morphospace, representing curved claws and enlarged DI and DII, more generally represents adaptations for taking large prey (sensu [24]) rather than any specific raptorial style. The region inhabited by the remaining owls, with claws roughly equal in size and less recurved than most perching birds, might therefore be specialised for hunting only small prey. Shrikes, however, have straight claws and reduced DI and DII, an even more extreme divergence from birds taking large prey despite shrikes taking prey nearly as large as they are [68, 69]. The “reduction” in DI and DII appears to be artificial from choosing DIII as the reference digit, as DIII is enlarged in shrikes (DIII/DIV ALo average 1.73 in shrikes, DII/DIV ALo average 1.61 in other restraint predators) and DIII likely performs the pinning role DII has previously been reported playing in raptors [24]. When size ratios are made to digit IV (Additional file 1: Fig. S6), shrikes cluster with other restraint raptors. Why shrike claws are so straight is less clear. One explanation would be a difference in handling time, as prey handling time for the loggerhead shrike [68] is on average 300 times shorter than in traditional raptors [70]. Another possibility is that a flatter foot may increase stability during the distinct vertebrate-prey-shaking behaviour true shrikes use on large prey like mice [71]. This behaviour is not reported in the similarly sized helmetshrike Prionops plumatus [72] whose claws plot alongside hawks and eagles.
Previous studies which find resolution between types of raptorial predation in pedal morphometrics [1, 24, 27] incorporate toe lengths as well as claw measurements. Thus, their exclusion here may be the reason for our lack of resolution. Einoder and Richardson [27] found talon measurements to describe raptorial specialisation better than any other hindlimb measurements including those of toes (compare their table 2 and fig. 2, 3, 5, and 8), undermining this possibility. Toe length does play a prominent role in the analyses of [1, 24], however. In particular, claw size ratios to toe lengths play a prominent role in separating restraining raptors from striking raptors along CA2/PC2 and suffocating raptors from all other birds along CA1/PC1 (compare fig. S1 in [1] to Additional file 1: Fig. S1; CA refers to the axes in Correspondence Analysis used by [1, 24, 73]). Future work may seek to focus on skeletal specimens in which toes are articulated due to incomplete maceration. All previous studies which successfully discriminate raptorial subtypes use data from fresh carcasses or skins rather than skeletal specimens, focus heavily on raptorial birds, and have sample sizes just over half that of the current study. It therefore remains unclear if the lack of resolution between raptors is due to the exclusion of toe measurements, use of skeletal material, or simply from more complete sampling capturing greater morphological diversity. It is noteworthy that the study of avian pedal morphometrics with the largest sample size [25] also found some of the greatest overlap between the pedal ecological groups studied.
Hedrick et al. ([36] pg. 11553) have recently proposed that morphospace grou** of raptorial bird claws reflects phylogeny rather than function. Significant phylogenetic signal is present in the TM data overall (Table 3) and each individual variable (Additional file 1: Table S2), and phylogenetic HSD finds most groups not significantly different (Additional file 1: Table S1). Taxonomic affiliation also explains some clusters in PCA (see “Results”). Most notably, the ground birds are split into megapodes and passerines (specifically larks) with very straight claws and cranes and tinamous with weakly recurved claws. We suspect this relates to habitat differences, as cranes and tinamous tend to inhabit wet floodplains or swamps while larks and megapodes are more known to inhabit dry scrub areas. The large separation of cranes along PC2 may reflect sexual dimorphism, with a female Grus canadensis having a much smaller and less recurved DII than a male Balearica pavonina. Alonso et al. [74] found male Grus grus to have a longer DIII than females, proposing the difference as related to elevated rates of combat in males. We suspect this extends to the highly recurved DII in cranes as well, but have been unable to confirm this in the literature. Our results also show most phylogenetic groups of perching birds being distinct from one another, but all are enveloped within the parrot morphospace. Despite this, however, we believe phylogeny does a worse job of explaining the data than pedal ecology (Additional file 1: Fig. S7). Passerine birds cover nearly the entire morphospace, and restraint predatory passerines have far more recurved claws and more interdigital size variation than ground bird passerines. Despite some being distinct, all subgroups of perching birds inhabit a region of the morphospace shared only with scavengers (who, in turn, mainly use their feet for perching). Most telling, though, is the clustering of macropredatory raptors (Accipitriformes, Strigiformes, Falconiformes), the seriema Cariama cristata (Cariamiformes), and the helmetshrike Prionops plumatus (Passeriformes) (Additional file 1: Fig. S7) representing five orders across Telluraves [75]. Also, while phylogenetic signal is present in all of the TM data, Kmult (= 0.66) and all K values (0.42–0.93, \(\overline{\mathrm{x}}\) = 0.69) are less than 1, meaning claw morphometrics are less similar than expected under a random evolution model. This in turn implies the pes is evolving under adaptive evolutionary pressures [45]. Given these polyphyletic clusters and homoplastic signal, we suggest that our pedal morphometric data reflects pedal ecology despite the effects of phylogeny.
Longipterygids generally have intermediate levels of claw curvature and little interdigital size variation. The major exception to this is Shanweiniao, whose very low claw curvature makes it by far the most similar to extant ground birds. Sheng**gornis seems at first glance to have similar proportions to extant pinning and striking raptors, though these seem driven more by a noticeably reduced DIII than an enlargement of DI and DII [13]. As Sheng**gornis plots in regions of heavy overlap in all graphs, we consider pedal morphometrics to be inconclusive in this taxon. Rapaxavis and both Longipteryx specimens plot in a central region of the morphospace due to their relatively equal claw sizes and intermediate curvatures. While this region is undiagnostic when DIII is used as a reference (Fig. 5), PCA with DIV used as the reference digit (Additional file 1: Fig. S6) and LDA predictions (Table 4) find these taxa most similar to suffocating raptors (in this study exclusively owls). Longipteryx has higher claw curvature than any other longipterygid genus (average 91° in Longipteryx, 78° in other longipterygids), driving its affinity towards non-raptorial perching birds (Table 4), with Rapaxavis, Shanweiniao, and Sheng**gornis having more affinity with ground birds. The results for Rapaxavis run counter to the findings of Morschhauser et al. [17] who found Rapaxavis to be arboreal based on non-ungual phalangeal proportions, though as owls are mainly arboreal this possibility cannot be ruled out. We thus find Shanweiniao most likely to be a ground-dwelling bird and Rapaxavis and Longipteryx likely to display raptorial behaviour with small animals (sensu [24]). Results for Sheng**gornis remain ambiguous due to its pedal proportions overlap** with most pedal ecological groups.
It is possible that the ratio of curvature between the ungual and keratin sheath is radically different in avians than non-avian avialans, meaning unguals may not accurately represent the whole claw used by longipterygids. Impressions resembling keratin sheaths are present alongside the right DI and DII unguals of Longipteryx DNHM D2889 [16], and the ratio of the sheath angle to the ungual angle (respectively 1.3 and 1.9) is akin to that previously reported for DI and DII in eagles (\(\overline{\mathrm{x}}\) = 1.4 (table S1 in 24)) and DIII across birds (\(\overline{\mathrm{x}}\) = 1.2 (table S1 in [26])). Rapaxavis, in contrast, preserves what appears to be a disarticulated keratin sheath [17, 76] less curved than the unguals. While it cannot be accurately measured due to loss of the base, a reasonable range of sheath to ungual curvature (~0.6–0.7) falls below that reported in any extant bird (0.9 in DIII of tinamou Rhynchotus rufescens (table S1 in [26])). Future work using techniques designed to identify soft tissue remains, e.g. laser-stimulated fluorescence [77], could help to evaluate the authenticity of these sheath impressions. While only small datasets for lepidosaurs have been examined, it does generally seem that birds and lepidosaurs have similar differences between ungual and claw sheath curvature (fig. 4 in [26]), meaning unguals should be reliable predictors of claw shape for a large extant phylogenetic bracket around Longipterygidae. For this reason, in lieu of thorough examinations of non-avian avialan claw sheath impressions, we consider our TM results ecologically informative of Longipterygidae.
Mechanical advantage and functional indices
We find mechanical advantage and the additional functional indices which seemed most promising in our previous review [1] to be very poor at predicting diet across birds. All three forms of measured mechanical advantage have low K values (Additional file 1: Table S4) which would predict that these have some adaptive significance [45, 78]. ACH, in contrast, has a high K value (Additional file 1: Table S4) which would imply some evolutionary constraint [78]. MA being undiagnostic is in line with the findings of Navalón et al. [18]. However, while some groups (generalists, invertivores, often frugivores and nectarivores) span the whole functional morphospace and thus can never be ruled out, several diet categories do display consistent mechanical traits that, if absent in fossil taxa, can be used to rule them out as possible diets.
Husking granivores, as noted previously [18, 29], have especially high jaw-closing mechanical advantage (AMA and PMA; Fig. 6, Additional file 1: Fig. S3A-D). This allows them to exert greater forces on seeds, which can allow for faster seed husking and more efficient feeding [42]. We do not, however, find AMA or PMA to be high in herbivores and low in invertivores, as previously reported [29, 79]. This is likely due to the large phylogenetic breadth of our sample. Miller and Pittman [1], reanalysing the work of Corbin et al. [29], found a moderate correlation between AMA and plant matter consumption in passerine birds, but nearly no correlation when a single columbiform data point was added. No bird diet groups have diagnostically low jaw-closing mechanical advantage.
Folivores are characterised by a high jaw-opening mechanical advantage (OMA; Fig. 6, Additional file 1: S3E-F). Why folivores require jaws that open slowly but powerfully, however, is unclear. Piscivores have relatively low OMA combined with a low relative articular offset (OA; Fig. 6, Additional file 1: S3E-H), to the point that they are the most commonly significantly different diet in phylogenetic HSD (Additional file 1: Table S3). We suspect both are adaptations to maintaining grip on slippery, muscular fish. A low relative articular offset causes the upper and lower jaws to meet in a scissor-like fashion, pushing fish in them forwards. Many piscivorous birds (e.g. Gavia stellate, Mergus serrator, Morus bassanus) have a rostral hook to the bill, which this scissor-like occlusion pushes the fish towards. This causes the bill to essentially wrap around the slippery prey, increasing contact area. Should the fish slip out of position, a low OMA allows the bird to quickly open its jaw and adjust position to maintain the grip. This may also aid in rapid swallowing, as kleptoparasitism is particularly common among seabirds [80] which are usually piscivorous.
Swallowing granivores, tetrapod hunters, scavengers, and soft frugivores separate in the functional morphospace to a lesser degree (scavengers only in LDA), and the driving forces of their separation are more complex than the diets above. Swallowing granivores and tetrapod hunters inhabit similar regions across multivariate analysis (Fig. 6, Additional files 4, 5, 6 and 7), driven mainly by a mixture of a below average AMA and PMA, relatively high OMA (but below that of folivores), and arguably a high MCH (neither group has particularly tall skulls, but they lack representatives with short skulls; Additional file 1: Fig. S3). One possible explanation is that the similarities stem from requirements of sensation rather than feeding (with all four indices able to be caused by an expansion of the cranial region), as locating both animal prey and ripened seeds require keen eyesight and advanced visual processing. Scavengers do not display strikingly high or low values of any functional index, and thus plot in a relatively tight cluster near the origin in PCA (Fig. 6A, B). They do, however, have less range in functional indices than other groups (Additional file 1: Fig. S3) which we suspect reflects tight constraints on what, mechanically, makes a successful scavenging bird. As Hertel [81] points out, scavenging birds often have little control over their food sources and abundant competition, so they need to feed particularly efficiently in order to survive.
Longipterygids’ MA and functional indices give no clear diagnosis of their diet, as one may expect from the poor predictive power in avians. Hard/medium invertivory and generalist feeding are recovered as the most likely diets of longipterygids (Table 5), but these are also the diets which spread farthest across the functional morphospace and so likely represent a “default” prediction for this analysis. Scavenging and piscivory are also recovered as somewhat likely but their relative likelihood is sensitive to the position of the quadrate. When the quadrate is placed to the extreme posterior, scavenging becomes the most likely longipterygid diet and piscivory enters the top three (Additional file 1: Table S6). Some of the particularly distinct diets mentioned above, however, can be ruled out. Longipterygids plot far from husking granivores in PCA (Fig. 6A, B) and GranivoreH is assigned the lowest probability for longipterygids by discriminant equations (Table 5). Most longipterygids also plot far from folivores and soft frugivores, though the unusually high OMA of Longirostravis (0.24, \(\overline{\mathrm{x}}\) = 0.17 for other longipterygids; Additional file 1: Fig. S3E-F) gives it an unusually high affinity with these groups. This involves some uncertainty, however, discussed in the following paragraph. In short, functional indices of the skull indicate that longipterygids were unlikely to be husking granivores, soft frugivores, or folivores (except possibly Longirostravis, see below).
The unusually high OMA of Longirostravis may be a by-product of the limits of our reconstruction (see “Methods”). The cranium of the only described specimen of Longirostravis (IVPP V11309) is crushed and indistinct with a prominent crack running through it ([82] pg. 86), meaning its caudal extent is obscure. Our initial reconstruction of Longirostravis which used the full extent of the skull had an unusually long cranium, and even after a roughly 30% reduction in its length to create the version in Fig. 1C, it is still more elongate than other longipterygids. It may be that the cranial bones of IVPP V11309 were sheared caudally or spread apart by cracks in the slab to a greater extent than we believe, and we thus overestimate its OMA. It is of note that in the results of sensitivity analysis of our reconstructions (Additional file 1: Table S6) folivory assignment is highly sensitive to the placement of the quadrate (with a rostral shift of the quadrate increasing OMA and folivore likelihood), and in fact folivory becomes the most likely assignment for Longirostravis at our anterior extreme for the error of the quadrate placement. If this is not an artefact of reconstruction, the increased OMA in Longirostravis may represent a unique feeding adaptation. Longirostravis has a uniquely narrow snout tip among longipterygids, which has led previous studies to propose it as a probing feeder [15, 17]. However, unlike modern probing feeders who insert and remove their beaks from substrate while remaining nearly closed [83], we propose the higher jaw-opening mechanical advantage in Longirostravis (if truly present) could have allowed it to open its jaws after inserting them into substrate, increasing its tactile range and allowing it to more efficiently remove larger prey items from the substrate. If true, one would also expect a large retroarticular process on the lower jaw for increased attachment area of jaw-opening muscles [84], though the holotype does not preserve this part of the jaw [15] (Fig. 1C).
Finite element analysis
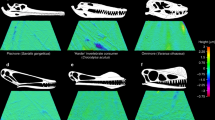
We find FEA to be overall ineffective at isolating specific diets, but very effective at separating swathes of diets. The large dietary overlap in MWAM strain (Fig. 7) and the first three principal components of intervals data (Fig. 8A, B, Additional files 8 and 9) suggests the overall structure of the data is not driven by diet. A reasonable null hypothesis would be that the data is driven by phylogeny, but Kmult tests of intervals data find no significant phylogenetic signal (Table 3). The partial separation of herbivores and carnivores in strain-space (Fig. 8A, B, Additional files 8 and 9), high separation when plotting LDA (Fig. 8C, D), and high confidence of LDA predictions (Table 6) all indicate that diet information can be reliably extracted from FEA results. LDA in particular is effective at splitting birds into two dietary groups. However these groups are not, as expected [1], those feeding on hard foods (in which initiating a crack via puncture is difficult) and soft foods (in which initiating a crack via puncture is easy). Nor does LDA discriminate between birds consuming hard foods or tough foods (in which propagating a crack is difficult, regardless of the mode or ease with which the initial crack forms). Instead, generalists and birds specialising in foods that are neither hard nor tough tend to have weak jaws (high strain when loaded). Specialists feeding on hard foods, tough foods, or foods both hard and tough tend to have stronger jaws (low strain when loaded).
Husking granivores and scavengers have the most consistently strong jaws. Both tend to have low MWAM strains (\(\overline{\mathrm{x}}\) = 110 and 160 με respectively; Fig. 7), partially separate out in PCA (Fig. 8; scavenger separation clearer in Additional files 8 and 9), and plot in low-strain regions in LDA (Fig. 8C, D). This is unsurprising for husking granivores, which need to break through seed coats when feeding and have been found to have high jaw strength in past studies [85, 86]. High jaw strength in scavenging birds has less clear reasoning. While mammals which heavily scavenge have been recorded as having high bite forces [87], this is associated with bone-crushing, which avian scavengers do not perform (aside from the bearded vulture Gypaetus barbatus [88] which had the weakest jaw of non-semi-specialist scavengers and typically uses tools to aid in crushing). There may be phylogenetic bias at play, as most scavenging birds are members of Accipitrimorphae (sensu [89]), but Phalcoboenus australis (Falconidae) and Leucophaeus scoresbii (Laridae) both plot alongside them. The vultures themselves are also ecologically diverse, with representatives of rippers, gulpers, and scrappers in the sample [90, 91]. We hypothesise, then, that scavenging birds need strong jaws to remove the tough, potentially desiccated, and hardened flesh of carcasses regardless of ecological specialty. This would explain why the semi-specialist scavenger Chionis minor has a weaker jaw than other avian scavengers, as it chiefly steals freshly caught food from other birds [92]. Hard frugivores arguably belong here as well, as they follow similar trends when Mitu tuberosum (which only a juvenile specimen was available to represent) is removed. This would not be surprising, as mechanically their feeding is similar to husking granivores.
Folivores, soft frugivores, swallowing granivores, and soft invertivores have the next most distinctly strong jaws. While they do not separate cleanly in PCA, they have generally low MWAM strains (\(\overline{\mathrm{x}}\) = 170, 110, 200, and 160 με respectively; Fig. 7) and plot in low-strain regions in LDA (Fig. 8C, D; note soft invertivores separate more when semi-specialists are included and plot very far from the origin along LD9 with them excluded [LD9 has weightings similar to the weightings which separate soft invertivores in the LDA plot with semi-specialists included]). As explained in the “Methods” section, two of the three soft invertivores included in this study are filter feeders. While trends are only tenuous given a small sample size, it would make sense for filter feeders to have jaws adapted for the unique demands of forcefully sieving water through their mouths [51, 93]. Alternatively, as discussed in the “Body mass” section, filter feeders face selection for larger body size and particularly larger head size to increase the volume of material filtered. It may be particularly easy to do this by increasing the depth of the jaw, which strengthens the jaw during biting as a side effect by increasing the second moment of area. Folivores and soft frugivores are both feeding on food that is tough but not hard [94]. So, while high jaw strength is not required to process their food directly, their food is tough enough that greater strength is needed to acquire and/or disassemble it. This aspect of disassembly likely explains the higher strength adaptations in swallowing granivores as well, as while they are not crushing hard food in their jaws, they are often still detaching them from plants housing the seeds. Thus, it appears that, in avian jaws, FEA has trouble distinguishing between toughness and hardness of foods in the diet.
Unexpectedly, the final diet group with “strong” jaws is nectarivorous birds. Nectarivorous birds rarely bite down on anything, feeding on nectar by rapidly inserting and removing their tongues from flowers [95]. Thus, one would expect their jaws to experience high strains when loaded as if biting, but the opposite is true. Nectarivores in this study have some of the lowest MWAM strains (\(\overline{\mathrm{x}}\) = 110 με; Fig. 7) and consistently plot in multivariate regions of low strain. Some of this can be explained by phylogenetic inertia. Parrots (Psittaciformes) are known to have strong jaws and strong evolutionary control on skull shape [96], and make up roughly half of the nectarivores studied. The other half, hummingbirds (Trochilidae), are more surprising to recover having jaws with low strain when loaded. We attribute this to the unique muscle attachment in the group. The m. adductor mandibulae externus (MAME) in hummingbirds is situated at the extreme posterior of the jaw [97], presumably to allow the jaw to quickly open and close as they feed. Thus, when we loaded the finite element model, the leverage on the jaw overall was much lower than in birds with a more typical MAME placement. Thus, we contend that hummingbirds do not have strong jaws, but rather have weak jaws with muscles positioned to put them under less strain. Nectarivores in the fossil record, then, may be particularly hard to identify with FEA given the uncertain placement of muscles onto fossil taxa.
Generalists, medium and hard invertivores, piscivores, and tetrapod hunters all have consistently weak jaws. They all plot in the upper right of PCA (Fig. 8A, B) and close to the origin in LDA (Fig. 8C, D), the areas of weakest jaws. Generalists and tetrapod hunters seem slightly stronger than the other three, never reaching the same values of MWAM strain (\(\overline{\mathrm{x}}\) = 180 and 190 με respectively) as medium (\(\overline{\mathrm{x}}\) = 230 με) and hard invertivores (\(\overline{\mathrm{x}}\) = 240 με) or piscivores (\(\overline{\mathrm{x}}\) = 220 με; Fig. 7) and plotting slightly farther from the origin in LDA (Fig. 8C, D). Generalists presumably have stronger jaws due to more frequent consumption of one of the foods listed above, while tetrapod hunters still need to tear apart prey as scavengers do but are working with softer and less tough meat. Of the remaining specialists, all of these foods taken are neither hard (compared to seeds and nuts) nor tough (compared to leaves or fruit rind), so relatively weak jaws are to be expected. Thus, it appears that while FEA is ineffective at discriminating between hard and tough diets for birds, it is effective at isolating diets that are neither hard nor tough. There is some nuance which bears discussing, however. Why piscivores would feel less selection towards strong jaws than tetrapod hunters is not immediately clear. We suspect this comes from a combination of piscivorous birds often taking smaller prey (relative to predator size) than tetrapod hunters, and the lessened ability of fish to struggle when removed from the water by a piscivorous bird, making disassembly easier. There is also the question of why some medium invertivores have unusually strong jaws (plotting at an origin distance similar to tetrapod hunters). We note that these are mainly swifts (Apodidae) and jacamars (Galbulidae), families known for flying fast and catching insects in mid-air (“hawking”) [98]. These taxa may require a stronger jaw to withstand the high-speed, high-force action of hawking capture.
While the FEA results for extant birds are rather complicated, the diagnosis for longipterygids is relatively simple. Longipterygids have consistently weak jaws, with MWAM strain greater than most groups (\(\overline{\mathrm{x}}\) = 290 με, Fig. 7) and plotting in the weakest region of PCA (Fig. 8A, B). They also remain in the weak region of LDA with semi-specialists excluded (Fig. 8C), but (presumably due to a quirk of the LD2 loadings) the small-toothed Longipteryx enters a quite strong region of the space with semi-specialists included. LDA and DAPC predictions (Table 6) find all longipterygids likely to be piscivores or hard invertivores with lower likelihoods of being generalists, tetrapod hunters, or medium invertivores (presumably due to the stronger-jawed hawking-adapted forms mentioned above). Longirostravis and Rapaxavis have a lower but consistent likelihood of being swallowing granivores as well. There are a few odd inconsistencies: Longirostravis and Rapaxavis are recovered as likely to be folivores with semi-specialists included, but unlikely to be folivores with them excluded. Large-tooth Longipteryx is considered extremely likely to be a nectarivore with semi-specialists included, but unlikely with semi-specialists excluded. We regard these inconsistencies as inconclusive. Overall, FEA finds the jaw strength of all longipterygids to be most consistent with hard invertivory and piscivory. The jaws of Longirostravis and Rapaxavis are also consistent with swallowing granivory in strain-space, though their MWAM strain (280 and 380 με, respectively) is higher than any studied swallowing granivore (max 270 με).
Longipterygid ecology and evolution
We reconstruct longipterygids other than Sheng**gornis (whose skull is too fragmentary for reconstruction and whose claws are indeterminate in morphology) as likely to be invertivorous or generalist feeders. This is because while FEA reveals they all have weak lower jaws (consistent with invertivory, generalist feeding, piscivory, and tetrapod hunting), low body mass estimates make vertivory (and thus piscivory and tetrapod hunting) unlikely in longipterygids. While MA and functional index results are less conclusive than the other lines of evidence, they are consistent with this dietary reconstruction as well, particularly in that they also find invertivory and generalist feeding the most likely diagnoses.
Using TM, we find that owl-like raptorial behaviour, where the talons are adapted to completely encircle the prey, was likely in Longipteryx and Rapaxavis. As explained above, this affinity seems to more represent the size of prey taken rather than the manner of killing per se. Owls usually hunt animals that their talons can fully encircle [24], and some of the large insects of the Jehol Biota [99] would be exactly this size for Longipteryx (Fig. 9). It is common for extant small owls [100], falconets [101], and shrikes [102] to capture insect prey with the hindlimb and use the pes for manipulation during disassembly of insect prey.
Reconstruction of the pes of large-toothed morphotype of Longipteryx grip** the mayfly Epicharmeropsis hexavenulosus from the same geological formation. Note that the mayfly is just large enough to be completely encircled by Longipteryx’s toes, as is typical for prey of extant owls [24]. The hindwing of E. hexavenulosus is excluded to better show the position of digit I. Longipteryx pes redrawn from specimen DNHM-D2889 [16], E. hexavenulosus redrawn from specimen CNU-E-YX-2007004 [99]
We find the hypothesis that Longipteryx is a specialist piscivore [15, 16] to be unlikely, mainly on grounds that all longipterygids are smaller than extant piscivores. It is noteworthy that the one kingfisher in our dataset, Alcedo atthis, does fall within the body mass range of longipterygids [103], and longipterygid piscivory has specifically been proposed as similar to that of kingfishers [13]. However, we still consider specialised piscivory unlikely for two reasons. First, the jaw of Alcedo atthis is stronger than that of any longipterygid, with MWAM strain comparable to hawking invertivores (160 με in A. atthis, minimum 260 με in longipterygids; Fig. 7). Second, Alcedo atthis is unusual among kingfishers in relying so much on fish. Only 15% of kingfishers have fish as the majority of their diet versus 60% of kingfishers with invertebrates as the majority of their diet [40]. So longipterygid jaw strength renders kingfisher-like behaviour unlikely, and even if this were not the case, simply being kingfisher-like would not be a strong argument for piscivory.
Swallowing granivory is recovered as likely for Longirostravis and Rapaxavis by FEA intervals (Table 6) and is consistent with their small body masses. However, we do not consider swallowing granivory likely in these taxa. The MWAM strain of their jaws (280 and 380 με, respectively) are higher than any swallowing granivores analysed (max 270 με; Fig. 7C, D). Additionally, two qualitative factors render swallowing granivory less likely in these taxa. Firstly, swallowing granivory in extant birds is aided by the gastric mill, which is believed to be absent in enantiornithines [3]. Secondly, elongated rostra would seem, if anything, to make it more difficult to channel whole seeds to the throat. This assumption is supported by past research finding granivorous birds to generally plot in morphospace regions without elongate beaks (fig. 4 in [18]).
The diet of Sheng**gornis remains obscure, largely due to its skull being so poorly preserved. Its body mass falls in the indeterminate range for carnivores and herbivores and its unguals are undiagnostic. Sheng**gornis is much larger than other longipterygids, appears to have a different shaped rostrum, and has an unusually reduced DIII ungual. This suggests that it inhabited a niche distinct from other longipterygids, though what this niche may have been remains unclear.
Diet appears rather conservative in Longipterygidae, with all taxa studied most adapted for invertivory or generalist feeding (except for Sheng**gornis, whose diet is indeterminate). This conservation is consistent with previous propositions that adaptations for taking easy-to-acquire foods are ancestral in Avialae [104]. Avialans are generally reconstructed as generalist mid-order consumers in Jehol ecosystems [1] is effective at delineating bird diet, though MA methods in particular would benefit from further refinement.
Conclusions
We reconstruct the diet of longipterygid enantiornithines using 4 lines of evidence: body mass estimation, traditional morphometrics of pedal unguals, mechanical advantage and functional index analysis of upper jaws, and finite element analysis of lower jaws. These provide detailed evidence that refines the dietary reconstruction of four of the six published longipterygid genera. This shows that the long-snouted and rostrally toothed longipterygids retained a conservative diet likely dependent on invertebrates and/or generalist feeding. The Jehol Biota had a speciose and ecologically diverse invertebrate fauna [107], so the unusual rostral morphology of longipterygids still may represent dietary specialisation, as previously proposed [11, 15,16,17], to take advantage of a particular subset of the invertebrate fauna. The greater disparity in longipterygid body mass and pedal morphology may also represent subdivision of the invertivore or generalist niche. This is consistent with past reconstructions of avialans as mid-order consumers in Jehol ecosystems [118]. The tree in [118] is time-scaled using Bayesian uncorrelated relaxed molecular clock data from 15 genes in 6663 extant bird species constrained by seven fossil taxa. Longipterygid branches were then grafted onto this tree following the topology of [4] (Sheng**gornis, not included in their phylogeny, was assumed to form a polytomy with the [Rapaxavis + Longirostravis] clade as it was recovered sister to the latter in the describing study [13]). We chose to place the Ornithothoraces node at 131 Ma given the age of the oldest known ornithothoracine taxa [119], though the taxa this old are diverse enough that the split likely occurred earlier. The oldest longipterygid, Shanweiniao, is known from the Dawangzhangzi Beds of the Yixian Formation [11], formed approximately 122 Ma [120]. The most recent possible age of the Longipterygidae node was estimated using this datum. All species were placed at the age of their oldest discovery with species divergences taking 10,000 years. All grafted lengths were scaled linearly so that the total length of the avian portion of the tree was equal to 94 Ma after the estimate of [121].
Specimen selection
Extant skulls
Extant skull specimens (used in MA and FEA) were primarily taken as images in lateral view from the repository Skullsite. Cameras and focal lengths used to take photos on Skullsite vary (Jan Jansen pers com. 2021), though from our sampling roughly half the images have exif data with camera type and settings embedded. Generally, birds with known diets from EltonTraits 1.0 were searched for on Skullsite, and those with good lateral images (judged as those with very little of the dorsal surface of the skull showing) were used in the study. Several additional radiographs were taken from the literature [122,123,124,125,126], primarily as tests of whether realistic modelling of rhamphotheca thickness affected the results. Coraciiform radiographs mentioned but not pictured in [126] were provided by Kathryn C. Gamble. Finally, a CT scan (Nikon Metrology XT H 225 ST, 130 kV, 400 mA) of the mandible of Anser fabalis was obtained from Bjarnason and Benson [127] to help cover for the underrepresentation of Anseriformes in the dataset (only a lower jaw was available so it is not included in MA analysis). All skull specimens are adults except that of Mitu tuberosum, for which only a juvenile specimen was available and which has no record of ontogenetic diet shifts [128]. In total, this study incorporates skulls from 121 extant taxa.
Extant claws
Extant claw ungual specimens used in TM were measured and photographed in person using a Google Pixel 2 cell phone at Carnegie Museum of Natural History and Florida Museum of Natural History from their skeletal bird collections. All claws measured lacked a keratin sheath to allow comparison to ungual bones of fossil taxa. As predation or lack thereof is the main focus of this approach, sampling focused primarily on traditional raptors (Accipitriformes, Strigiformes, Falconidae) and birds known to be macrocarnivorous but not traditionally considered raptorial (Cathartidae, Laniidae, Malaconotidae, Vangidae, Aegypiinae, Gypaetinae). Parrots (Psittaciformes) were also favoured in sampling due to their talons’ superficial resemblance to those of traditional raptors, to avoid false positives for raptorial behaviour. Several other non-carnivorous perching taxa (Cuculidae, Musophagidae, Opisthocomus; all of which primarily live and feed in trees) and ground birds (Alaudidae, Gruidae, Megapodiidae, Tinamidae; all of which primarily live and feed on the ground) were sampled as well. All claws were checked for unusually high porosity, a pathological state common in captive birds with improper flooring (David Steadman pers. com. 2020; see also [129]). In total, the study incorporates claws from 61 extant taxa. Nine of these are from the same species as a sample in the skull dataset, though none are from the same specimen.
Fossils
Published longipterygid specimens were incorporated as scale photos from the literature [11, 13, 15,16,17, 114, 130]. Skull reconstructions used in MA and FEA (Fig. 1) can only be to approximate scale because they are composites that combine several individuals/species of different sizes, so unscaled photographs from [82] were also used in their creation. All data taken from the skull are size-independent so this should not present analytical issues. Claw measurements for the large-toothed morphotype of Longipteryx (see Longipterygid skull reconstruction for MA and FEA), Rapaxavis, Shanweiniao, and Sheng**gornis were taken from scale photos from the above literature as well. Two previously undescribed specimens of Longipteryx sp. from the Shandong Tianyu Museum of Nature (STM 7-156, STM 8-117) were included in body mass measurements with one (STM 8-117) also providing claw measurements, though their skulls were too poorly preserved to aid in skull reconstruction.
Diet assignment
Bird diet was assigned based on the EltonTraits 1.0 database [40], a database recording the diet of over 9000 bird species broken down into different food categories in intervals of 10%. We then placed birds with over a certain threshold percentage of their diet from one category (Table 1) into qualitative diet categories that are similar to but more specific than those in EltonTraits 1.0. EltonTraits 1.0 tallies endotherm and ectotherm tetrapod contributions to diet separately, but due to previous studies finding no difference in hunting requirements for the two [131, 132], we merged them. The “Unknown Vertebrate” category from EltonTraits 1.0 was split evenly between tetrapods and fish (this only affected Chionis minor and Grus japonensis. Chionis minor was reassigned as a scavenger due to the Birds of the World database, on which EltonTraits 1.0 is based, noting that vertebrates were kleptoparasited rather than hunted). For convenience, we refer to the group which feeds primarily on non-reproductive plant tissue (“other plants” in EltonTraits 1.0) as “folivores”. While folivory refers specifically to the consumption of leaves, non-reproductive tissues of plants universally are low in nutrients and contain defensive chemicals or structures that should lead herbivores to adapt similarly to consuming them [133]. The arguable exception are the energy-rich tubers which are defended structurally and chemically [134], but are only consumed by ducks to our knowledge [135]. The only bird which consumes tubers in our dataset is Anser fabalis [136].
To ensure dietary signal would be as clear as possible, high thresholds were set for diet assignment. No single cut-off could be used, however, as some dietary categories tend to have a stronger domination of the primary food type than others (e.g. granivores often subsist entirely on seeds while piscivores typically supplement their diets with foods other than fish). Some species were marked as “semi-specialists” if a lower percentage of their diet consisted of the relevant food source than most members of the group, but their inclusion increased the phylogenetic breadth of the group dramatically. Generally, semi-specialists were allowed an additional 20% of their diet to be from other food sources, but expanding this to 30% in invertivores allowed inclusion of Strigiformes and expanding to 50% in scavengers was required to include more than one taxon that was not a vulture. Seeing if trends persist when including semi-specialists helps ensure dietary results are not simply recapitulating phylogeny. Cut-offs for diet assignment are given in Table 1.
The Diet-Fruit category of EltonTraits 1.0 [40] includes food items that are very mechanically different, from soft-fleshed papaya to rock-solid palm nuts. Thus, we split this diet category into Soft Frugivores (FrugivoreS) and Hard Frugivores (FrugivoreH). While “hard” fruits have traditionally been operationalised as having a puncture resistance above about 0.25 kgmm−2 [137, 138], this precision is not possible with our current data on bird diet and food material properties. Plants fed on by birds are typically accurate only to the family level [116], while puncture resistance can vary by more than 0.25 kgmm−2 even at the genus level [139] meaning many families contain both hard and soft fruits. As a compromise, we used the Birds of the World database to catalogue the families of fruits eaten by the frugivores in this study (Arecaceae, Burseraceae, Caricaceae, Celastraceae, Fabaceae, Lauraceae, Moraceae, Myristicaceae, Myrtaceae, Oleaceae, Podocarpaceae, Primulaceae, Putranjivaceae, Sapotaceae, and Urticaceae) and identified two that are known for having particularly hard fruits (Arecaceae [140] and Myristicaceae [141, 142]). Taxa that fed on fruits from these two families were assigned to FrugivoreH, others to FrugivoreS. Mitu tuberosum was also assigned to FrugivoreH due to specific note of it eating fruits of Lecointea amazonica [143] which are on record as hard fruits [144].
The Diet-Seed category of EltonTraits 1.0 includes two distinct foraging styles: those that remove the hard outer coating of seeds (de-husk) and those that swallow them whole. These styles appear mutually exclusive among most birds [145] and require different adaptations of the skull, so we see fit to separate granivores into Granivores that Husk (GranivoreH) and Granivores that Swallow seeds whole (GranivoreS). We determined which studied granivorous taxa de-husk seeds and which do not based on notes and videos in the Birds of the World database. De-husking in Sporophila crassirostris is inferred from Sporophila telasco and was observed directly by CVM in Nymphicus hollandicus. Swallowing seeds in Pterocles orientalis is inferred from Pterocles lichtensteinii.
The Diet-Inv category of EltonTraits 1.0 [40] also includes foods with a wide range of mechanical properties, from fragile butterflies to rugged crabs. Bestwick et al. [146] compiled literature on the properties of invertebrate exoskeletons and defined three mechanical groups: Soft Invertebrates (invertebrate larvae, lepidopterans, spiders, and myriapods), Medium Invertebrates (orthopterans, formicid hymenopterans, and odonatans), and Hard Invertebrates (coleopterans, crustaceans, and shelled gastropods). We also assign springtails (Collembola) to Soft and flies (Diptera) and termites (Isoptera) to Medium Invertebrates based on indentation hardness of their body parts compared to the above groups (compare [147,148,156]; Dorival Lima, Falcoaria e Voo Livre, pers. comm. 2021) akin to hawks and eagles, so we assigned this genus to the restraint category. Shrikes (Laniidae) and Helmetshrikes (Vangidae) are both placed in the restraint category due to their prolonged manipulation and transport of prey prior to impalement [68], though it should be noted that large prey may be paralysed or killed without the use of the feet prior to impalement [71, 102]. Secretarybirds (Sagittarius) and seriemas (Cariamidae) are both well-known for kicking with their hindlimbs, and so were added to the strike category. It should be noted, however, that while secretarybirds use kicks as their main hunting strategy [157], seriemas mainly forage with their beaks and kick during interspecific combat [158].
Among non-raptorial birds, acquisition of food with the pes is rare and manipulation of it is limited [159], so non-raptorial ecological categories characterise habitat rather than diet. Birds in the Ground and Perching categories are somewhat self-explanatory: birds that spend the vast majority of their time on the ground or perched on a branch, respectively. We strived for a broad phylogenetic breadth for each group: tinamous (Tinamidae), megapodes (Megapodiidae), cranes (Gruiformes), and larks (Alaudidae) represent ground birds; hoatzins (Opisthocomus), cuckoos (Cuculidae), turacos (Musophagidae), and parrots (Psittaciformes) represent perching birds. We separated out new-world vultures (Cathartidae) as Scavengers to see if this lifestyle had any diagnostic characteristics in the talons, despite their talons plotting with ground [24] or perching [43] birds in past analyses.
In total, this study includes nine ground birds, 14 non-raptorial perching birds, one piercing raptor, 15 restraint raptors, four scavengers, eight striking raptors, and ten suffocating raptors.
Measurements
Linear measures of extant claws and tibiotarsi were taken with a tape measure to avoid damaging the fragile specimens, with callipers (digital at Carnegie, dial at Florida due to technical difficulties) used on any claws less than 1 cm in proximodistal length. Angular measures were taken from photos taken in lateral view imported into CorelDraw X8 and measured as in [24] using the “Angular Dimension” tool. If digit identifications were ever in doubt, the claws were compared to taxidermy specimens and identified based on relative size and curvature.
Landmarks for TM follow [24] with two deviations. We figure these landmarks in a previous work (fig. 1 and 2 in [1]). First, the bone cores of claws were measured rather than keratinous sheaths. This allowed direct comparison to fossils which rarely preserve an outline of the keratin sheath. Even if an outline of the keratinous sheath was preserved in a fossil, it could be deformed with no indication of the original structure [160]. Measuring bone cores obscures inner-curvature landmarks (based largely on the transition from the flesh to the claw sheath) so we affirm the use of outer-curvature landmarks (i.e. the proximal landmark at the dorsal base of the extensor tubercle) as has become standard in avian pedal TM [24, 36, 64, 161]. Second, use of extant skeletal specimens usually prohibits inclusion of toe lengths, as toes are disarticulated with no reliable way to be reassembled in extant skeletal specimens. Reliance on toe measurements would also exclude entire groups of fossil avialans from future study (most notably Avisauridae [162]), so we see this limitation as a necessary eventuality when studying fossil bird diet. Thus, the final measures used in TM were outer arc curvature (Oo) and ratios of outer arc length (ALo) (sensu [24]) for each digit.
As angle measurements were taken from photos, we tested the effect of parallax by comparing a perfectly level photograph to those taken at 5° tilts in each orthogonal direction. 5° was considered the maximum reasonable deviation from level, as the camera had an accelerometer-based level whose crosshairs decouple near 5° of tilt. Parallax was found to have little effect, consistent with previous studies [163]. We also took photographs of a grid with the camera and affirmed the gird lines remained orthogonal.
Both digit III (DIII) and digit IV (DIV) were investigated as reference digits, i.e. as the denominator of size ratios. LDA models based on each were almost identically accurate when re-classifying extant taxa (Fleiss’ Kappa = 0.7177 DIII, 0.7182 DIV; see “Multivariate” analysis). Visual comparison of PCA plots found better separation when using DIII as a reference digit, so DIII is used in this paper. Graphs of results where DIV is used as a reference digit are available in Additional file 1: Fig. S6.
Longipterygid skull reconstruction for MA and FEA
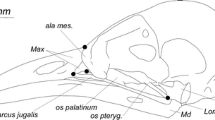
Final longipterygid skull reconstructions are pictured in Fig. 1. Because no longipterygid skull is complete, extrapolation of bones was necessary to create reconstructions. While not ideal due to intra- and interspecific morphological variation, this practice is common and necessary to create workable biomechanical models [115]. The ontogeny of enantiornithines remains largely uncertain so ontogenetic effects cannot be fully accounted for, though juvenile enantiornithines tend to have more gracile long bones and relatively larger orbits than any specimen used in this study [164]. Longipteryx specimen IVPP V12552 has been proposed as a juvenile due to several of its bones being unfused [76], though subsequent work has shown that the pattern of skeletal fusion in enantiornithines is highly variable [165]. Previous work reconstructed enantiornithine skulls based on the general morphology at the level of Enantiornithes [114]. We sought to improve on these reconstructions by leveraging newly published specimens, restricting extrapolated material to the family level (Longipterygidae), and making explicit what areas are reconstructed and where extrapolated material comes from.
Published images [2, 11, 13, 15, 16, 76, 82, 114, 130] were imported into CorelDraw X8. All skulls studied are preserved in lateral view. Skulls were then scaled to all have the same length (from tip of the rostrum to rear of the cranium). If a skull was disarticulated, its best-preserved bone was scaled to the same size at its closest phylogenetic relative per [4]. Once scaled, each distinct bone or set of bones (e.g. premaxilla + nasal with no clear suture preserved) in each skull was outlined and named according to its identification and source specimen. In every specimen, most individual bones of the cranium were indistinct, so a general “cranium” outline was made as well. The articular region between the upper and lower jaws was not clear in any studied specimen of Longipteryx, so descriptions from [84] were used to refine this area.
The most complete skull of a given genus was used as the base for reconstruction. Copies of its bone outlines were made and isolated, with upper and lower jaws moved into articulation. Missing or incomplete bones were then taken from the closest relative preserving the bone. These outlines were copied as well and placed between or over top of the existing bones, attempting to meet articulated bones cleanly and align with as many edges as possible of overlap** bones (akin to the process [115] recommends for 3D reconstruction). Once complete, new outlines were made by tracing over the composite of bones to make edges and articulations cleaner. If bone edges overlapped, those of the genus being reconstructed were favoured over its relatives, and among relatives those with the best preservation were favoured. Sutures were not intuited in bone sets so as to not overestimate the precision of the reconstruction. Finally, bones and bone sets were coloured based on the specimen they came from. Bones or bone sets that are amalgams of multiple specimens were given gradient fills approximating the regions with greatest contribution from a given specimen.
The sclerotic ring and lacrimal are only preserved in Longipteryx (BMNHC Ph-930B), the earliest-diverging member of the clade. Reconstruction of the sclerotic ring followed avian examples in [166], from which it appears shape in lateral view is conserved phylogenetically. We therefore believe the shape and arrangement of ossicles in our reconstructions are adequate, though their size and placement (based solely on BMNHC Ph-930B) are only tentative. The lacrimal is positioned in each skull as connecting the dorsal process of the maxilla to the frontal (except in Rapaxavis, where the maxillary process nearly contacts the dorsal premaxilla and the frontal begins more cranially than in other longipterygids), as in BMNHC Ph-930B. This often required removing one or both ends of the lacrimal as it appears in BMNHC Ph-930B and significant slimming of the bone for it to be similar in aspect ratio to the other bones in longipterygid skulls other than Longipteryx. As such, we note our reconstruction of the lacrimal as highly speculative. Additionally, the quadratojugal is not distinct in any enantiornithine except for Da**fangornis [167] in which it is a short cranially forked rod of bone contacting the jugal, quadrate, and squamosal, so we incorporate a similar morphology into these reconstructions using a dotted line. These bones do not affect any measurements taken so their uncertainties have no effect on any quantitative analyses in this work.
The position of the quadrate in enantiornithines is highly uncertain. An in situ quadrate has only been pictured in Pengornis houi [114], and while the quadrate of Longipteryx specimen IVPP V21702 is reported as articulated only an extreme close-up of the quadrate is figured [84]. We believe the cranial structure abutting the surangular in Longipteryx specimen BMNHC Ph-930B is also an in situ quadrate, and so use its positioning in this study. BMNHC Ph-930B preserves the orbital process of the quadrate as a broad anterior projection, common among non-avian avialans [84], so this feature is assumed to be present but unpreserved in other longipterygids. The quadrate is situated more cranially in BMNHC Ph-930B than in Pengornis and past skull reconstructions [114], which we attempt to replicate in other longipterygid skull reconstructions. This is attenuated by the depressions in the surangular of Rapaxavis and Shanweiniao which require the quadrate to be more rostrally positioned. As such, when placing the quadrate, we aligned the front of the upper and lower jaws and situated the quadrate as cranially as reasonable while still articulating with the surangular. As the articular condyle (located on the quadrate) is a landmark for every type of jaw mechanical advantage, this placement should, in theory, heavily influence the results of mechanical advantage calculation. However, a sensitivity analysis placing the articular condyle at biologically improbable cranial and rostral extremes (Additional file 1: Fig. S8) found dietary assignments to be overall robust to quadrate position (Additional file 1: Table S6).
In our reconstruction work, we noticed two distinct morphotypes of Longipteryx chaoyangensis: those with large teeth and more robust jaws (BMNHC Ph-930B, DNHM D2889, HGM-41HIII0319, SG2005-B1) and those with smaller teeth and more gracile jaws (BMNHC Ph-826, IVPP V12552, possibly BMNHC Ph-1071 and IVPP V12325). The difference may be interspecific or ontogenetic (IVPP V12552 has been identified as a subadult [2], but see above), but in this study, we err on the side of caution by classifying the two as “large-toothed Longipteryx” and “small-toothed Longipteryx”, since this difference requires further study. In phylogenetic trees, these morphotypes were given the smallest branch length possible [182,183,184,185,186,187,188,189,190,191,192,193,194,195] using the phylogenetic group closest to the modelled taxon. In fossil taxa, muscles were reconstructed based on the proposed non-avian dinosaur attachment sites and orientations [196] with preference for inferences based on birds over crocodilians. When in doubt, the MAME was assumed to insert ventral to the coronoid process of the mandible (or, when coronoid process was unclear, dorsal to the mandibular fenestra) and to be oriented roughly 45° from the coronal plane, as this appears to be the most common condition in archosaurs [194]. Load magnitude at the MAME attachment point was arbitrarily chosen to be 6N for the smallest model (Regulus regulus). Loads for all other models were scaled from this one using the Stress State Constant/Plane Strain equation in [177]. This effectively makes model strains size-independent, allowing for comparison of the effects of jaw shape and muscle attachment in isolation. Models were constrained from translation in all axes at the articular glenoid. Models also were constrained dorsoventrally at the rostral tip of the rhamphotheca in beaked taxa and at the apex of the first tooth in toothed taxa. All models were created and solved within HyperWorks 2019 Student Edition (HyperMesh and Optistruct, Altair Engineering, Inc., USA).
Intervals method
To compare the outputs of finite element models in a quantitative manner, we utilise the intervals method for comparing finite element outputs [34]. Conceptually, this technique is an extension of comparing contour plots, but allows them to be much more detailed than a subjective comparison. We split the full range of strain for all models into a number of equally sized intervals (analogous to colours on a contour plot), and the percent area of each model under each interval of strain is quantified. The outcome is akin to visually comparing the area of contour plots that are certain colours, but allows us to make these comparisons in a quantitative way. Convergence testing was used to determine what number of intervals was optimal. Deviating from [34], we transformed the raw intervals data matrix as it is compositional data, which cannot be used as-is in multivariate analyses [197]. We imputed zeroes using expected value multiplicative lognormal replacement [198] with the multLN function in R package zCompositions [199] version 1.3.4 before applying an isometric log ratio transformation [200] (ilr function in R package compositions [201] version 2.0-2) to the primary use FEA data and a centred log ratio transformation [197] (clr function in R package compositions [201] version 2.0-2) to the data used for character weight plotting. Imputation is necessary as the logarithm of zero is undefined. Isometric log ratio transformation more completely removes compositional effects from the data [200], while a centred log ratio transformation makes it much easier to interpret character weightings [197]. After transformation, FEA intervals data was subjected to multivariate analysis as described below.
Data analysis
All analyses of the data were performed in R version 4.1.2 [202], with scripts available from [203]. Additional files 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 and 15 also include interactive HTML-based three-dimensional graphs of all multivariate analysis results and two-dimensional multivariate FEA graphs annotated with contour plots of each jaw. Both were made using a package from Plotly (Plotly, Canada) for R [204], version 4.9.4.1. Univariate results in this study are compared in violin plots, a series of rotated and mirrored kernel density plots. When comparing subsets of carnivore and herbivore masses, we determined diagnostic cut-off values to compare fossil bird masses to using the R package OptimalCutpoints [205] version 1.1-5 (function optimal.cutpoints, optimised using Youden Index [44]). We performed two initial analyses on each multivariate dataset: principal component analysis (PCA; base R function prcomp) and linear discriminant analysis (LDA; caret package for R [206] version 6.0-90 function lda). Both seek to reduce dimensionality of data into a space easier to interpret, but do so by different means. PCA maximises the variance explained by each axis while LDA maximises the separation of predefined groups [197] (in this instance, diet or pedal ecological categories). In this sense, PCA can be seen as a more “objective” view of the data while LDA is more effective at identifying otherwise minor factors which distinguish groups. All PCAs in this study used the correlation matrix, which scales inputs to constant variance, removing effects of units and scale. All fossil data points were projected independently into multivariate space (i.e. they were not used in calculating the rotation of the data).
LDA has many more assumptions than PCA, the most troublesome of which is that all variables are uncorrelated. In biological systems, where mechanical traits are linked by a variety of developmental and evolutionary relationships that are often poorly understood, uncorrelated variables are difficult to isolate. As such, LDA performed in this study will inevitably defy this requirement for LDA. To account for this, we also incorporated discriminant analysis of principal components (DAPC) [46]. In essence, DAPC combines PCA and LDA into a single analysis. PCA de-correlates the input variables, and then LDA is performed on the principal components. Unfortunately, DAPC plots are very difficult to interpret. Each linear discriminant is made up of a combination of principal components, which are in turn made up of a combination of input variables. As such, we primarily use DAPC as a check on LDA. If LDA and DAPC plots of a given dataset look similar then we consider the LDA robust to the uncorrelated assumption, but if they were to differ then we would attempt to back-interpret the DAPC plot as best as possible. All LDA and DAPC outputs in this study are identical (compare relevant LDA results to Additional file 1: Fig. S2), so back-interpretation was not necessary.
When variable choice was in question (e.g. using digit III or IV as a reference digit in TM, how to measure skull area for MA, or the number of intervals to use in multivariate FEA), we compared sets in LDA using Fleiss’ Kappa [207] (obtained using caret package for R [206] version 6.0-90 function confusionMatrix). Fleiss’ Kappa is used to test the agreement of two observers in categorising data. We treated the a priori diet/ecology classification of birds used in this study as one observer and compared it to the LDA equations’ prediction of categories for said birds. Higher Fleiss’ Kappa means more agreement of the LDA predictions with reality, and thus a dataset producing more accurate results.
Extant groups with more than one member were compared in terms of TM variables (Additional file 1: Table S1), MA variables (Additional file 1: Table S3), and FEA intervals (Additional file 1: Table S5) using the pairwise() function in the RRPP package for R [39] (version 1.1.2) to test if they were distinct. In total, 1000 permutations were used by convention, with sensitivity analyses finding p-values to converge before this point. This test mimics the function of Tukey’s honestly significant differences (HSD) test [38] when comparing means, a nonparametric test for significant difference between each pairwise comparison within a large set of groups (in this case pedal ecological category or diet). It effectively differs from Tukey’s HSD in that it is able to incorporate and correct for phylogenetic signal in the data. As the authors of [39] did not provide a concise name for the output of the pairwise() function when comparing means, we refer to the results of this test herein as “phylogenetic HSD”.
In HSD, LDA, and DAPC of non-semi-specialist datasets for mass, MA, and FEA, Steatornis caripensis was removed from the analysis as it was the only representative of FrugivoreH. Single-member groups are not compatible with HSD and tend to interfere with LDA results, plotting at extreme values of a single discriminant and war** the remaining data. For the same reason, Pandion haliaetus was removed from phylogenetic HSD, LDA, and DAPC of TM datasets as the only representative of the Pierce category.
Phylogenetic signal was investigated in each dataset using the Kmult statistic [37] (function R code given in the paper), a summary statistic comparing the differences in high-dimensional traits across a given tree. A total of 1000 permutations were used by convention. A Kmult value of 1 indicates traits evolved under a Brownian motion model, i.e. random changes with no selection. Values less than 1 indicate taxa are more different from one another than in a Brownian motion model, values greater than 1 indicate taxa are more similar than expected [45]. The test also provides a p-value for the presence of phylogenetic signal (null hypothesis of no phylogenetic signal). Per the recommendation of Adams and Collyer [78] when Kmult was less than 1 but statistically significant phylogenetic signal was detected we also took K values for each individual input variable (Additional file 1: Tables S2 and S4). We used the same code to calculate Kmult and K values (the equivalent for univariate systems like body mass) as Adams [37] demonstrated Kmult = K for one-dimensional data.
Availability of data and materials
All data and computer code used in this study is stored at Mendeley Data, DOI 10.17632/w3c8p5w3hn.1 [203].
Abbreviations
- ACH:
-
Relative average cranial height
- AMA:
-
Anterior jaw-closing mechanical advantage
- ANOVA:
-
Analysis of variance
- bcL:
-
Length of bicipital crest
- BMNHC:
-
Bei**g Museum of Natural History, Bei**g, China
- CM:
-
Carnegie Museum of Natural History, Pittsburgh, USA
- CNU:
-
Capital Normal University, Bei**g, China
- dHW:
-
Dorsoventral width at midshaft of humerus
- dUW:
-
Craniocaudal width at midshaft of ulna
- DAPC:
-
Discriminant analysis of principal components
- DNHM:
-
Dalian Natural History Museum, Dalian, China
- FEA:
-
Finite element analysis
- FolFrug:
-
Folivores and frugivores
- FrugivoreH:
-
Hard frugivroe
- FrugivoreS:
-
Soft frugivore
- GranivoreH:
-
Husking granivore
- GranivoreS:
-
Swallowing granivore
- GranNect:
-
Granivores and nectarivores
- HL:
-
Length of humerus
- HSD:
-
Honest significant difference
- InvertivoreH:
-
Hard invertivore
- InvertivoreM:
-
Medium invertivore
- InvertivoreS:
-
Soft invertivore
- IVPP:
-
Institute of Vertebrate Paleontology and Paleoanthropology, Bei**g, China
- LD:
-
Linear discriminant
- LDA:
-
Linear discriminant analysis
- MA:
-
Mechanical advantage
- MCH:
-
Relative maximum cranial height
- MWAM:
-
Mesh-weighted arithmetic mean
- OA:
-
Relative articular offset
- OMA:
-
Jaw-opening mechanical advantage
- PC:
-
Principal component
- PCA:
-
Principal component analysis
- PMA:
-
Posterior jaw-closing mechanical advantage
- STM:
-
Shandong Tianyu Museum of Nature, **yi, China
- TL:
-
Length of tibiotarsus
- TM:
-
Traditional morphometrics
- UL:
-
Length of ulna
- \(\overline{\mathrm{x}}\) :
-
Mean value
- με:
-
Miscrostrain
References
Miller CV, Pittman M. The diet of early birds based on modern and fossil evidence and a new framework for its reconstruction. Biol Rev. 2021;96(5):2058–112.
O’Connor JK. The trophic habits of early birds. Palaeogeogr Palaeoclimatol Palaeoecol. 2019;513:178–95.
O'Connor JK, Zhou Z. The evolution of the modern avian digestive system: insights from paravian fossils from the Yanliao and Jehol biotas. Palaeontology. 2019;63:13–27.
Pittman M, O'Connor J, Field DJ, Turner AH, Ma W, Makovicky PJ, et al. Pennaraptoran systematics. In: Pittman M, Xu X, editors. Pennaraptoran theropod dinosaurs: past progress and new frontiers. New York: Bulletin of the American Museum of Natural History; 2020. p. 7–36.
Sanz JL, Chiappe LM, Pérez-Moreno BP, Buscalioni AD, Moratalla JJ, Ortega F, et al. An Early Cretaceous bird from Spain and its implications for the evolution of avian flight. Nature. 1996;382(6590):442.
Pittman M, O'Connor J, Tse E, Makovicky PJ, Field DJ, Ma W, et al. The fossil record of Mesozoic and Paleocene pennaraptorans. In: Pittman M, Xu X, editors. Pennaraptoran theropod dinosaurs: past progress and new frontiers. New York: Bulletin of the American Museum of Natural History; 2020. p. 37–96.
Bhullar BAS, Hanson M, Fabbri M, Pritchard A, Bever GS, Hoffman E. How to make a bird skull: major transitions in the evolution of the avian cranium, paedomorphosis, and the beak as a surrogate hand. Integr Comp Biol. 2016;56(3):389–403.
Zhou YC, Sullivan C, Zhou ZH, Zhang FC. Evolution of tooth crown shape in Mesozoic birds, and its adaptive significance with respect to diet. Palaeoworld. 2021;30(4):724–36.
Wu Y. Molecular phyloecology suggests a trophic shift concurrent with the evolution of the first birds. Commun Biol. 2021;4(1):1–13.
Matsukawa M, Shibata K, Sato K, **ng X, Lockley MG. The Early Cretaceous terrestrial ecosystems of the Jehol Biota based on food-web and energy-flow models. Biol J Linn Soc. 2014;113(3):836–53.
O'Connor JK, Wang XR, Chiappe LM, Gao CL, Meng QJ, Cheng XD, et al. Phylogenetic support for a specialized clade of Cretaceous enantiornithine birds with information from a new species. J Vertebr Paleontol. 2009;29(1):188–204.
O'Connor JK, Zhou ZH, Zhang FC. A reappraisal of Boluochia zhengi (Aves: Enantiornithes) and a discussion of intraclade diversity in the Jehol avifauna, China. J Syst Palaeontol. 2010;9(1):51–63.
Li L, Wang J, Zhang X, Hou S. A new enantiornithine bird from the Lower Cretaceous Jiufotang formation in **zhou area, Western Liaoning Province, China. 地质学报(英文版). 2012;86(5):1039–44.
Yun CG. Comments on the taxonomic validity of Camptodontornis yangi (Li, Gong, Zhang, Yang, and Hou, 2010) and its relationships to Longipteryx chaoyangensis Zhang, Zhou, Hou, and Gu, 2000 and Boluochia zhengi Zhou, 1995. Zootaxa. 2019;4652(2):391–2.
Hou LH, Chiappe LM, Zhang FC, Chuong CM. New Early Cretaceous fossil from China documents a novel trophic specialization for Mesozoic birds. Naturwissenschaften. 2004;91(1):22–5.
Wang XR, Zhao B, Shen CZ, Liu SZ, Gao CL, Cheng XD, et al. New material of Longipteryx (Aves: Enantiornithes) from the Lower Cretaceous Yixian Formation of China with the first recognized avian tooth crenulations. Zootaxa. 2015;3941(4):565–78.
Morschhauser EM, Varricchio DJ, Chunling G, Liu J, Wang X, Cheng X, et al. Anatomy of the Early Cretaceous bird Rapaxavis pani, a new species from Liaoning Province, China. J Vertebr Paleontol. 2009;29(2):545–54.
Navalón G, Bright JA, Marugán-Lobón J, Rayfield EJ. The evolutionary relationship among beak shape, mechanical advantage, and feeding ecology in modern birds. Evolution. 2018;73(3):422–35.
Pigot AL, Sheard C, Miller ET, Bregman TP, Freeman BG, Roll U, et al. Macroevolutionary convergence connects morphological form to ecological function in birds. Nat Ecol Evol. 2020;4:230–9.
Bright JA, Marugán-Lobón J, Cobbe SN, Rayfield EJ. The shapes of bird beaks are highly controlled by nondietary factors. Proc Natl Acad Sci U S A. 2016;113(19):5352–7.
Serrano FJ, Palmqvist P, Sanz JL. Multivariate analysis of neognath skeletal measurements: implications for body mass estimation in Mesozoic birds. Zool J Linn Soc. 2015;173(4):929–55.
Marcus LF. Traditional morphometrics. In: Rohlf FJ, Bookstein FL, editors. Proceedings of the Michigan morphometrics workshop. Ann Arbor: University of Michigan Museum of Zoology; 1990. p. 77–122.
Csermely D, Rossi O. Bird claws and bird of prey talons: where is the difference? Ital J Zool. 2006;73(01):43–53.
Fowler DW, Freedman EA, Scannella JB. Predatory functional morphology in raptors: interdigital variation in talon size is related to prey restraint and immobilisation technique. PLoS One. 2009;4(11):e7999.
Csermely D, Rossi O, Nasi F. Comparison of claw geometrical characteristics among birds of prey and non-raptorial birds. Ital J Zool. 2012;79(3):410–33.
Cobb SE, Sellers WI. Inferring lifestyle for Aves and Theropoda: a model based on curvatures of extant avian ungual bones. PLoS One. 2020;15(2):e0211173.
Einoder LD, Richardson AMM. Aspects of the hindlimb morphology of some Australian birds of prey: a comparative and quantitative study. Auk. 2007;124(3):773–88.
Stayton CT. Testing hypotheses of convergence with multivariate data: morphological and functional convergence among herbivorous lizards. Evolution. 2006;60(4):824–41.
Corbin CE, Lowenberger LK, Gray BL. Linkage and trade-off in trophic morphology and behavioural performance of birds. Funct Ecol. 2015;29(6):808–15.
Adams NF, Rayfield EJ, Cox PG, Cobb SN, Corfe IJ. Functional tests of the competitive exclusion hypothesis for multituberculate extinction. R Soc Open Sci. 2019;6(3):181536.
Ma W, Pittman M, Lautenschlager S, Meade LE, Xu X. Functional morphology of the oviraptorosaur and scansoriopterygid skull. In: Pittman M, Xu X, editors. Pennaraptoran theropod dinosaurs: past progress and new frontiers. New York: Bulletin of the American Museum of Natural History; 2020. p. 229–49.
Bathe K-J. Finite element procedures. 2nd ed. Watertown: Bathe, Klaus-Jürgen; 2014.
Marcé-Nogué J, de Esteban-Trivigno S, Escrig Pérez C, Gil Espert L. Accounting for differences in element size and homogeneity when comparing finite element models: armadillos as a case study. Palaeontol Electron. 2016;19(2):2T.
Marcé-Nogué J, De Esteban-Trivigno S, Püschel TA, Fortuny J. The intervals method: a new approach to analyse finite element outputs using multivariate statistics. PeerJ. 2017;5:e3793.
Felice RN, Tobias JA, Pigot AL, Goswami A. Dietary niche and the evolution of cranial morphology in birds. Proc R Soc London Ser B. 2019;286(1897):20182677.
Hedrick BP, Cordero SA, Zanno LE, Noto C, Dodson P. Quantifying shape and ecology in avian pedal claws: the relationship between the bony core and keratinous sheath. Ecol Evol. 2019;9:11545–56.
Adams DC. A generalized K statistic for estimating phylogenetic signal from shape and other high-dimensional multivariate data. Syst Biol. 2014;63(5):685–97.
Abdi H, Williams LJ. Tukey’s honestly significant difference (HSD) test. In: Salkind N, editor. Encyclopedia of research design. 3rd ed. Thousand Oaks: SAGE Publications, Inc.; 2010. p. 1–5.
Collyer ML, Adams DC. RRPP: an r package for fitting linear models to high-dimensional data using residual randomization. Methods Ecol Evol. 2018;9:1772–9.
Wilman H, Belmaker J, Simpson J, de la Rosa C, Rivadeneira MM, Jetz W. EltonTraits 1.0: species-level foraging attributes of the world’s birds and mammals. Ecology. 2014;95(7):2027.
Pineda-Munoz S, Alroy J. Dietary characterization of terrestrial mammals. Proc R Soc London Ser B. 2014;281(1789):20141173.
van der Meij MAA, Bout RG. Seed husking time and maximal bite force in finches. J Exp Biol. 2006;209(17):3329–35.
Tsang LR, Wilson LAB, Ledogar J, Wroe S, Attard M, Sansalone G. Raptor talon shape and biomechanical performance are controlled by relative prey size but not by allometry. Sci Rep. 2019;9:7076.
Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J. 2005;47(4):458–72.
Blomberg SP, Garland T, Ives AR. Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution. 2003;57(4):717–45.
Jombart T, Devillard S, Balloux F. Discriminant analysis of principal components: a new method for the analysis of genetically structured populations. BMC Genet. 2010;11(1):94.
Bright JA. A review of paleontological finite element models and their validity. J Paleontol. 2014;88(4):760–9.
O’Gorman EJ, Hone DW. Body size distribution of the dinosaurs. PLoS One. 2012;7(12):e51925.
Bourassa N, Morin A. Relationships between size structure of invertebrate assemblages and trophy and substrate composition in streams. J North Am Benthol Soc. 1995;14(3):393–403.
Finlay BJ, Thomas JA, McGavin GC, Fenchel T, Clarke RT. Self-similar patterns of nature: insect diversity at local to global scales. Proc R Soc London Ser B. 2006;273(1596):1935–41.
Zweers G, Dejong F, Berkhoudt H, Vandenberge JC. Filter-feeding in flamingos (Phoenicopterus ruber). Condor. 1995;97(2):297–324.
Friedman M. Parallel evolutionary trajectories underlie the origin of giant suspension-feeding whales and bony fishes. Proc R Soc London Ser B. 2012;279(1730):944–51.
Wallace MP, Temple SA. Competitive interactions within and between species in a guild of avian scavengers. Auk. 1987;104(2):290–5.
Ruxton GD, Houston DC. Obligate vertebrate scavengers must be large soaring fliers. J Theor Biol. 2004;228(3):431–6.
Bechtel DB, Abecassis J, Shewry PR, Evers AD. Development, structure, and mechanical properties of the wheat grain. In: Khan K, editor. Wheat: chemistry and technology. 4th ed. Cambridge: Woodhead Publishing and AACC International Press; 2009. p. 51–95.
Brown JH, Calder WA III, Kodric-Brown A. Correlates and consequences of body size in nectar-feeding birds. Am Zool. 1978;18(4):687–738.
Fleming TH, Muchhala N. Nectar-feeding bird and bat niches in two worlds: pantropical comparisons of vertebrate pollination systems. J Biogeogr. 2008;35(5):764–80.
Navalón G, Nebreda SM, Bright JA, Fabbri M, Benson RB, Bhullar B-A, et al. Craniofacial development illuminates the evolution of nightbirds (Strisores). Proc Royal Soc B. 2021;288(1948):20210181.
Dudley R, Vermeij GJ. Do the power requirements of flap** flight constrain folivory in flying animals? Funct Ecol. 1992;6:101–4.
Pagès F. Compared and functional morphology of the hoatzin (Opisthocomus hoazin). Paris: Museum national d’histoire naturelle - MNHN PARIS; 2019.
Collar N, Boesman PFD. Red-breasted Pygmy-Parrot (Micropsitta bruijnii), version 1.0. In: del Hoyo J, Elliott A, Sargatal J, Christie DA, de Juana E, editors. Birds of the world. Ithaca: Cornell Lab of Ornithology; 2020.
Feduccia A. Evidence from claw geometry indicating arboreal habits of Archaeopteryx. Science. 1993;259(5096):790–3.
Hopson JA. Ecomorphology of avian and nonavian theropod phalangeal proportions: implications for the arboreal versus terrestrial origin of bird flight. In: Ostrom JH, Gall LF, Gauthier J, editors. New perspectives on the origin and early evolution of birds: proceedings of the international symposium in honor of John H Ostrom. New Haven: Peabody Museum of Natural History; 2001. p. 211–35.
Pike AVL, Maitland DP. Scaling of bird claws. J Zool. 2004;262:73–81.
Artuso C, Houston CS, Smith DG, Rohner C. Great horned owl (Bubo virginianus), version 1.0. In: Poole AF, editor. Birds of the world. Ithaca: Cornell Lab of Ornithology; 2020.
Holt DW, Berkley R, Deppe C, Enríquez PL, Petersen JL, Rangel Salazar JL, et al. Spectacled owl (Pulsatrix perspicillata), version 1.0. In: del Hoyo J, Elliott A, Sargatal J, Christie DA, de Juana E, editors. Birds of the world. Ithaca: Cornell Lab of Ornithology; 2020.
del Hoyo J, Olsen PD, Marks JS, Collar N. Morepork (Ninox novaeseelandiae), version 1.0. In: Billerman SM, Keeney BK, Rodewald PG, Schulenberg TS, editors. Birds of the world. Ithaca: Cornell Lab of Ornithology; 2020.
Craig RB. An analysis of the predatory behavior of the loggerhead shrike. Auk. 1978;95(2):221–34.
Pedersen L, Geertsma M, Tottrup AP. Prey diversity is affected by climate and differs between age classes in the red-backed shrike (Lanius collurio). Ornis Fenn. 2012;89(2):99–108.
Slagsvold T, Sonerud GA. Prey size and ingestion rate in raptors: importance for sex roles and reversed sexual size dimorphism. J Avian Biol. 2007;38(6):650–61.
Sustaita D, Rubega MA, Farabaugh SM. Come on baby, let’s do the twist: the kinematics of killing in loggerhead shrikes. Biol Lett. 2018;14(9):20180321.
Allan D. White Helmetshrike (Prionops plumatus), version 1.0. In: del Hoyo J, Elliott A, Sargatal J, Christie DA, de Juana E, editors. Birds of the world. Ithaca: Cornell Lab of Ornithology; 2020.
Fowler DW, Freedman EA, Scannella JB, Kambic RE. The predatory ecology of Deinonychus and the origin of flap** in birds. PLoS One. 2011;6(12):e28964.
Alonso JC, Bautista LM, Alonso JA. Sexual size dimorphism in the Common Crane, a monogamous, plumage-monomorphic bird. Ornis Fenn. 2019;96(4):194–205.
Cooney CR, Bright JA, Capp EJR, Chira AM, Hughes EC, Moody CJA, et al. Mega-evolutionary dynamics of the adaptive radiation of birds. Nature. 2017;542(7641):344–7.
O'Connor JK, Chiappe LM, Gao C, Zhao B. Anatomy of the Early Cretaceous enantiornithine bird Rapaxavis pani. Acta Palaeontol Pol. 2011;56(3):463–75.
Kaye TG, Falk AR, Pittman M, Sereno PC, Martin LD, Burnham DA, et al. Laser-stimulated fluorescence in paleontology. PLoS One. 2015;10(5):e0125923.
Adams DC, Collyer ML. Phylogenetic comparative methods and the evolution of multivariate phenotypes. Annu Rev Ecol Evol Syst. 2019;50(1):405–25.
Olsen AM. Feeding ecology is the primary driver of beak shape diversification in waterfowl. Funct Ecol. 2017;31(10):1985–95.
Brockmann HJ, Barnard CJ. Kleptoparasitism in birds. Anim Behav. 1979;27:487–514.
Hertel F. Ecomorphological indicators of feeding behavior in recent and fossil raptors. Auk. 1995;112(4):890–903.
Chiappe LM, Meng Q. Birds of stone: Chinese avian fossils from the age of dinosaurs. Baltimore: John Hopkins University Press; 2016.
Barbosa A, Moreno E. Evolution of foraging strategies in shorebirds: an ecomorphological approach. Auk. 1999;116(3):712–25.
Stidham TA, O’Connor JK. The evolutionary and functional implications of the unusual quadrate of Longipteryx chaoyangensis (Avialae: Enantiornithes) from the Cretaceous Jehol Biota of China. J Anat. 2021;239(5):1066–74.
Soons J, Herrel A, Genbrugge A, Aerts P, Podos J, Adriaens D, et al. Mechanical stress, fracture risk and beak evolution in Darwin’s ground finches (Geospiza). Philos Trans R Soc Lond B Biol Sci. 2010;365(1543):1093–8.
Soons J, Genbrugge A, Podos J, Adriaens D, Aerts P, Dirckx J, et al. Is beak morphology in Darwin’s finches tuned to loading demands? PLoS One. 2015;10(6):e0129479.
Christiansen P, Wroe S. Bite forces and evolutionary adaptations to feeding ecology in carnivores. Ecology. 2007;88(2):347–58.
Margalida A, Bertran J. Function and temporal variation in use of ossuaries by Bearded Vultures (Gypaetus barbatus) during the nestling period. Auk. 2001;118(3):785–9.
Jarvis ED, Mirarab S, Aberer AJ, Li B, Houde P, Li C, et al. Whole-genome analyses resolve early branches in the tree of life of modern birds. Science. 2014;346(6215):1320–31.
Hertel F. Diversity in body size and feeding morphology within past and present vulture assemblages. Ecology. 1994;75(4):1074–84.
Linde-Medina M, Guerra C, Alcover JA. A revision of vulture feeding classification. Zoology. 2021;148:125946.
Burger AE. Time budgets, energy needs and kleptoparasitism in breeding Lesser Sheathbills (Chionis minor). Condor. 1981;83(2):106–12.
Pennycuick CJ, Bartholomew GA. Energy budget of the lesser flamingo (Phoeniconaias minor Geoifroy). Afr J Ecol. 1973;11(2):199–207.
Vogel SA. Matter of materials. Comparative biomechanics: life’s physical world. Princeton: Princeton University Press; 2013. p. 287–312.
Collins BG. Nectar intake and foraging efficiency: responses of honeyeaters and hummingbirds to variations in floral environments. Auk. 2008;125(3):574–87.
Bright JA, Marugán-Lobón J, Rayfield EJ, Cobb SN. The multifactorial nature of beak and skull shape evolution in parrots and cockatoos (Psittaciformes). BMC Evol Biol. 2019;19(1):104.
Zusi RL, Bentz GD. Myology of the purple-throated carib (Eulampis jugularis) and other hummingbirds (Aves: Trochilidae). Smithson Contrib Zool. 1984;385:1-70.
Rico-Guevara A, Sustaita D, Gussekloo S, Olsen A, Bright J, Corbin C, et al. Feeding in birds: thriving in terrestrial, aquatic, and aerial niches. In: Bels V, Whishaw IQ, editors. Feeding in vertebrates: evolution, morphology, behavior, biomechanics. Gewerbesrasse: Springer International Publishing; 2019. p. 643–93.
Huang J, Ren D, Sinitshenkova ND, Shih C. New genus and species of Hexagenitidae (Insecta: Ephemeroptera) from Yixian Formation, China. Zootaxa. 2007;1629(1):39–50.
Ligon JD. The biology of the elf owl, Micrathene whitneyi. Misc Publ Mus Zool Univ Mich. 1968;136:1–70.
Kemp AC, Crowe TM. Morphometrics of falconets and hunting behaviour of the black-thighed falconet Microhierax fringillarius. Ibis. 1994;136(1):44–9.
Yosef R. Prey transport by loggerhead shrikes. Condor. 1993:231–3.
Dunning JB Jr. CRC handbook of avian body masses. Boca Raton: CRC Press; 2008.
Li Z, Wang C-C, Wang M, Chiang C-C, Wang Y, Zheng X, et al. Ultramicrostructural reductions in teeth: implications for dietary transition from non-avian dinosaurs to birds. BMC Evol Biol. 2020;20:46.
Kettel EF, Gentle LK, Yarnell RW, Quinn JL. Breeding performance of an apex predator, the peregrine falcon, across urban and rural landscapes. Urban Ecosyst. 2019;22(1):117–25.
Robinson BW, Wilson DS. Optimal foraging, specialization, and a solution to Liem's paradox. Am Nat. 1998;151(3):223–35.
Zhang H, Wang B, Fang Y. Evolution of insect diversity in the Jehol Biota. Sci China Earth Sci. 2010;53(12):1908–17.
Zhang J. New genera and new species of Chironomidae (Diptera, Insecta) from Late Jurassic of China. Acta Palaeontol Sin. 1991;30:556–69.
Zhang W, Engel MS, Yao Y, Ren D, Shih C. The Mesozoic family Archegocimicidae and phylogeny of the infraorder Leptopodomorpha (Hemiptera). J Syst Palaeontol. 2014;12(1):93–111.
Peñalver E, Arillo A, Delclòs X, Peris D, Grimaldi DA, Anderson SR, et al. Parasitised feathered dinosaurs as revealed by Cretaceous amber assemblages. Nat Commun. 2017;8:1924.
Gao T, Yin X, Shih C, Rasnitsyn AP, Xu X, Chen S, et al. New insects feeding on dinosaur feathers in mid-Cretaceous amber. Nat Commun. 2019;10(1):5424.
Button DJ, Zanno LE. Repeated evolution of divergent modes of herbivory in non-avian dinosaurs. Curr Biol. 2020;30(1):158–68.
Ungar PS. Mammalian dental function and wear: a review. Biosurf Biotribol. 2015;1(1):25–41.
O'Connor JK, Chiappe LM. A revision of enantiornithine (Aves: Ornithothoraces) skull morphology. J Syst Palaeontol. 2011;9(1):135–57.
Lautenschlager S. Reconstructing the past: methods and techniques for the digital restoration of fossils. R Soc Open Sci. 2016;3(10):160342.
Billerman SM, Keeney BK, Rodewald PG, Schulenberg TS. Birds of the world. 2021 https://birdsoftheworld.org/bow/home.
Van Gestel W, Jansen J. Skullsite bird skull repository. 2020. http://skullsite.com/.
Jetz W, Thomas GH, Joy JB, Hartmann K, Mooers AO. The global diversity of birds in space and time. Nature. 2012;491(7424):444–8.
Wang M, Zheng X, O’Connor JK, Lloyd GT, Wang X, Wang Y, et al. The oldest record of Ornithuromorpha from the Early Cretaceous of China. Nat Commun. 2015;6(1):6987.
Zhou Z, Barrett PM, Hilton J. An exceptionally preserved Lower Cretaceous ecosystem. Nature. 2003;421(6925):807.
Kuhl H, Frankl-Vilches C, Bakker A, Mayr G, Nikolaus G, Boerno ST, et al. An unbiased molecular approach using 3′-UTRs resolves the avian family-level tree of life. Mol Biol Evol. 2020;38(1):108–27.
Smith BJ, Smith SA, Spaulding KA, Flammer K, Smallwood JE. The normal xeroradiographic and radiographic anatomy of the cockatiel (Nymphicus hollandis). Vet Radiol. 1990;31(5):226–34.
Smith SA, Smith BJ. Normal xeroradiographic and radiographic anatomy of the red-tailed hawk (Buteo jamaicencis), with reference to other diurnal raptors. Vet Radiol. 1990;31(6):301–12.
Smith BJ, Smith SA. Normal xeroradiographic and radiographic anatomy of the bobwhite quail (Colinus virginianus), with reference to other galliform species. Vet Radiol. 1991;32(3):127–34.
Smith SA, Smith BJ. Normal xeroradiographic and radiographic anatomy of the great horned owl (Bubo virginianus), with special reference to the barn owl (Tyto alba). Vet Radiol. 1991;32(1):6–16.
Gamble KC. Internal anatomy of the hornbill casque described by radiography, contrast radiography, and computed tomography. J Avian Med Surg. 2007;21(1):38–49.
Bjarnason A, Benson RBJ. A 3D geometric morphometric dataset quantifying skeletal variation in birds. MorphoMuseuM. 2021;7(1):125.
Udoye KC, Schulenberg TS. Razor-billed Curassow (Mitu tuberosum), version 1.0. In: Billerman SM, Keeney BK, Rodewald PG, Schulenberg TS, editors. Birds of the world. Ithaca: Cornell Lab of Ornithology; 2020.
Hawkins P. The welfare implications of housing captive wild and domesticated birds. In: Duncan IJH, Hawkins P, editors. The welfare of domestic fowl and other captive birds. Dordrecht: Springer Netherlands; 2010. p. 53–102.
Li L, Gong E, Zhang L, Yang Y, Hou L. A new enantiornithine bird (Aves) from the Early Cretaceous of Liaoning, China. Acta Palaeontol Sin. 2010;49(4):524–31.
Si G, Dong Y, Ma Y, Zhang Z. Shape similarities and differences in the skulls of scavenging raptors. Zoolog Sci. 2015;32(2):171–8.
Sun Y, Si G, Wang X, Wang K, Zhang Z. Geometric morphometric analysis of skull shape in the Accipitridae. Zoomorphology. 2018;137(3):445–56.
Karban R, Agrawal AA. Herbivore offense. Annu Rev Ecol Syst. 2002;33(1):641–64.
Wang D, Wang Q, Sun X, Gao Y, Ding J. Potato tuberworm Phthorimaea operculella (Zeller) (Lepidoptera: Gelechioidea) leaf infestation affects performance of conspecific larvae on harvested tubers by inducing chemical defenses. Insects. 2020;11(9):633.
Lovvorn JR. Biomechanics and foraging profitability: an approach to assessing trophic needs and impacts of diving ducks. Hydrobiologia. 1994;279(1):223–33.
Carboneras C, Kirwan GM. Taiga Bean-Goose (Anser fabalis), version 1.0. In: Billerman SM, Keeney BK, Rodewald PG, Schulenberg TS, editors. Birds of the world. Ithaca: Cornell Lab of Ornithology; 2020.
Dumont ER. The effect of food hardness on feeding behaviour in frugivorous bats (Phyllostomidae): an experimental study. J Zool. 1999;248(2):219–29.
Dumont ER, O’neal R. Food hardness and feeding behavior in Old World fruit bats (Pteropodidae). J Mammal. 2004;85(1):8–14.
Lambert JE, Chapman CA, Wrangham RW, Conklin-Brittain NL. Hardness of cercopithecine foods: implications for the critical function of enamel thickness in exploiting fallback foods. Am J Phys Anthropol. 2004;125(4):363–8.
Reis SB, Mercadante-Simões MO, Ribeiro LM. Pericarp development in the macaw palm Acrocomia aculeata (Arecaceae). Rodriguésia. 2012;63(3):541–9.
Burubai W, Amula E, Davies RM, Etekpe GWW, Daworiye SP. Determination of Poisson’s ratio and elastic modulus of African nutmeg [Monodora myristica]. Int Agrophys. 2008;22(2):99–102.
Bowler M, Bodmer RE. Diet and food choice in Peruvian red uakaris (Cacajao calvus ucayalii): selective or opportunistic seed predation? Int J Primatol. 2011;32(5):1109.
Torres B. La dieta del paujil (Mitu mitu): o las vicisitudes de ser frugívoro. Bol Lima. 1989;66:87–90.
Norconk MA, Veres M. Physical properties of fruit and seeds ingested by primate seed predators with emphasis on sakis and bearded sakis. Anat Rec. 2011;294(12):2092–111.
Prosser P, Hart ADM. Assessing potential exposure of birds to pesticide-treated seeds. Ecotoxicology. 2005;14(7):679–91.
Bestwick J, Unwin DM, Purnell MA. Dietary differences in archosaur and lepidosaur reptiles revealed by dental microwear textural analysis. Sci Rep. 2019;9(1):11691.
Cribb BW, Lin C-L, Rintoul L, Rasch R, Hasenpusch J, Huang H. Hardness in arthropod exoskeletons in the absence of transition metals. Acta Biomater. 2010;6(8):3152–6.
Olerud H. Lessons from nature-surface characterisation by means of nanotechnology. Trondheim: Institutt for Produktutvikling og Materialer; 2013.
Rong J, Lin Y, Sui Z, Wang S, Wei X, **ao J, et al. Amorphous calcium phosphate in the pupal cuticle of Bactrocera dorsalis Hendel (Diptera: Tephritidae): a new discovery for reconsidering the mineralization of the insect cuticle. J Insect Physiol. 2019;119:103964.
Seear PJ, Tarling GA, Burns G, Goodall-Copestake WP, Gaten E, Özkaya Ö, et al. Differential gene expression during the moult cycle of Antarctic krill (Euphausia superba). BMC Genomics. 2010;11(1):582.
Pütz K, Buchholz F. Comparative ultrastructure of the cuticle of some pelagic, nektobenthic and benthic malacostracan crustaceans. Mar Biol. 1991;110(1):49–58.
Sykes PW Jr. The feeding habits of the snail kite in Florida, USA. Colon Waterbirds. 1987;10(1):84–92.
Kruuk H. Competition for food between vultures in East Africa. Ardea. 1967;55:171–93.
Lopes LE, Fernandes AM, Medeiros MCI, Marini MA. A classification scheme for avian diet types. J Field Ornithol. 2016;87(3):309–22.
Kerkhoff AJ, Enquist BJ. Multiplicative by nature: why logarithmic transformation is necessary in allometry. J Theor Biol. 2009;257(3):519–21.
Bierregaard RO, Kirwan GM, Boesman PFD. Collared Forest-Falcon (Micrastur semitorquatus), version 1.0. In: del Hoyo J, Elliott A, Sargatal J, Christie DA, de Juana E, editors. Birds of the world. Ithaca: Cornell Lab of Ornithology; 2020.
Portugal SJ, Murn CP, Sparkes EL, Daley MA. The fast and forceful kicking strike of the secretary bird. Curr Biol. 2016;26(2):R58–R9.
Silva AN, Nunes R, Estrela DC, Malafaia G, Castro ALS. Behavioral repertoire of the poorly known Red-legged Seriema, Cariama cristata (Cariamiformes: Cariamidae). Ornithol Res. 2016;24(2):73–9.
Sustaita D, Pouydebat E, Manzano A, Abdala V, Hertel F, Herrel A. Getting a grip on tetrapod gras**: form, function, and evolution. Biol Rev. 2013;88(2):380–405.
Saitta ET, Rogers CS, Brooker RA, Vinther J. Experimental taphonomy of keratin: a structural analysis of early taphonomic changes. Palaios. 2017;32(10):647–57.
Mosto MC, Tambussi CP. Qualitative and quantitative analysis of talons of diurnal bird of prey. Anat Histol Embryol. 2014;43(1):6–15.
Atterholt J, Hutchison JH, O'Connor JK. The most complete enantiornithine from North America and a phylogenetic analysis of the Avisauridae. PeerJ. 2018;6:e5910.
Macdonald DA, Royal K, Buchanan B. Evaluating the effects of parallax in archaeological geometric morphometric analyses. Archaeol Anthropol Sci. 2020;12(7):149.
Chiappe LM, Shu’An J, Qiang J. Juvenile birds from the early cretaceous of China: Implications for enantiornithine ontogeny. Am Mus Novit. 2007;3594:1–46.
Knoll F, Chiappe LM, Sanchez S, Garwood RJ, Edwards NP, Wogelius RA, et al. A diminutive perinate European Enantiornithes reveals an asynchronous ossification pattern in early birds. Nat Commun. 2018;9:937.
Lima FC, Vieira LG, Santos ALQ, De Simone SBS, Hirano LQL, Silva JMM, et al. Anatomy of the scleral ossicles in brazilian birds. J Morphol Sci. 2017;26(3-4):165–9.
Wang M, Hu H. A comparative morphological study of the jugal and quadratojugal in early birds and their dinosaurian relatives. Anat Rec. 2017;300(1):62–75.
Brusatte SL, Sakamoto M, Montanari S, Harcourt Smith WEH. The evolution of cranial form and function in theropod dinosaurs: insights from geometric morphometrics. J Evol Biol. 2012;25(2):365–77.
Sakamoto M. Jaw biomechanics and the evolution of biting performance in theropod dinosaurs. Proc R Soc London Ser B. 2010;277(1698):3327–33.
Dumont ER, Grosse IR, Slater GJ. Requirements for comparing the performance of finite element models of biological structures. J Theor Biol. 2009;256(1):96–103.
Schileo E, Taddei F, Cristofolini L, Viceconti M. Subject-specific finite element models implementing a maximum principal strain criterion are able to estimate failure risk and fracture location on human femurs tested in vitro. J Biomech. 2008;41(2):356–67.
Yosibash Z, Tal D, Trabelsi N. Predicting the yield of the proximal femur using high-order finite-element analysis with inhomogeneous orthotropic material properties. Philos Trans R Soc Lond A. 1920;2010(368):2707–23.
Sternheim A, Traub F, Trabelsi N, Dadia S, Gortzak Y, Snir N, et al. When and where do patients with bone metastases actually break their femurs? A CT-based finite element analysis. Bone Joint J. 2020;102-B(5):638–45.
Cuff AR, Bright JA, Rayfield EJ. Validation experiments on finite element models of an ostrich (Struthio camelus) cranium. PeerJ. 2015;3:e1294.
Cost IN, Middleton KM, Sellers KC, Echols MS, Witmer LM, Davis JL, et al. Palatal biomechanics and its significance for cranial kinesis in Tyrannosaurus rex. Anat Rec. 2019;303:999–1017.
Rayfield EJ. Strain in the ostrich mandible during simulated pecking and validation of specimen-specific finite element models. J Anat. 2011;218(1):47–58.
Marcé-Nogué J, DeMiguel D, Fortuny J, de Esteban-Trivigno S, Gil EL. Quasi-homothetic transformation for comparing the mechanical performance of planar models in biological research. Palaeontol Electron. 2013;16(3):6T.
Miller CV, Pittman M, Kaye TG, Wang X, Bright JA, Zheng X. Disassociated rhamphotheca of fossil bird Confuciusornis informs early beak reconstruction, stress regime, and developmental patterns. Commun Biol. 2020;3:519.
Morales-García NM, Burgess TD, Hill JJ, Gill PG, Rayfield EJ. The use of extruded finite-element models as a novel alternative to tomography-based models: a case study using early mammal jaws. J R Soc Interface. 2019;16(161):20190674.
Shufeldt RW. The myology of the raven (Corvus corax sinuatus.): a guide to the study of the muscular system in birds. New York: Macmillan and Company; 1890.
Beecher WJ. Convergence in the Coerebidae. Wilson Bull. 1951;63(4):274–87.
Fisher HI, Goodman DC. The myology of the whoo** crane, Grus americana. Ill Biol Monogr. 1955;24(2):1–127.
Zusi RL. Structural adaptations of the head and neck in the black skimmer; 1962.
George JC, Berger AJ. Avian myology. New York: Academic; 1966.
Zusi RL, Storer RW. Osteology and myology of the head and neck of the pied-billed grebes (Podilymbus). Misc Publ Mus Zool Univ Mich. 1969;139:1–49.
Wild JM, Zeigler HP. Central representation and somatotopic organization of the jaw muscles within the facial and trigeminal nuclei of the pigeon (Columba livia). J Comp Neurol. 1980;192(1):175–201.
Holliday CM, Witmer LM. Archosaur adductor chamber evolution: integration of musculoskeletal and topological criteria in jaw muscle homology. J Morphol. 2007;268(6):457–84.
Tokita M, Schneider RA. Developmental origins of species-specific muscle pattern. Dev Biol. 2009;331(2):311–25.
Donatelli RJ. Jaw musculature of the Picini (Aves: Piciformes: Picidae). Int J Zool. 2012;2012:941678.
Bhattacharyya BN. Avian jaw function: adaptation of the seven–muscle system and a review. Proc Zool Soc. 2013;66(2):75–85.
Lautenschlager S, Bright JA, Rayfield EJ. Digital dissection–using contrast-enhanced computed tomography scanning to elucidate hard-and soft-tissue anatomy in the Common Buzzard Buteo buteo. J Anat. 2014;224(4):412–31.
Quayle MR, Barnes DG, Kaluza OL, McHenry CR. An interactive three dimensional approach to anatomical description-the jaw musculature of the Australian laughing kookaburra (Dacelo novaeguineae). PeerJ. 2014;2:e355.
Carril J, Degrange FJ, Tambussi CP. Jaw myology and bite force of the monk parakeet (Aves, Psittaciformes). J Anat. 2015;227(1):34–44.
Smith-Paredes D, Bhullar B-AS. The skull and head muscles of Archosauria. In: Ziermann JM, Diaz Jr RE, Diogo R, editors. Heads, jaws, and muscles: anatomical, functional, and developmental diversity in chordate evolution. Gewerbesrasse: Springer International Publishing; 2019. p. 229–51.
To KH, O’Brien HD, Stocker MR, Gignac PM. Cranial musculoskeletal description of black-throated finch (Aves: Passeriformes: Estrildidae) with DiceCT. Integr Org Biol. 2021;3(1):obab007.
Holliday CM. New insights into dinosaur jaw muscle anatomy. Anat Rec. 2009;292(9):1246–65.
Brückner A, Heethoff M. A chemo-ecologists’ practical guide to compositional data analysis. Chemoecology. 2017;27(1):33–46.
Palarea-Albaladejo J, Martin-Fernandez JA. Values below detection limit in compositional chemical data. Anal Chim Acta. 2013;764:32–43.
Palarea-Albaladejo J, Martín-Fernández JA. zCompositions—R package for multivariate imputation of left-censored data under a compositional approach. Chemometrics Intellig Lab Syst. 2015;143:85–96.
Filzmoser P, Hron K, Reimann C. Principal component analysis for compositional data with outliers. Environmetrics. 2009;20(6):621–32.
Van den Boogaart KG, Tolosana-Delgado R. “compositions”: a unified R package to analyze compositional data. Comput Geosci. 2008;34(4):320–38.
R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2020.
Miller CV, Pittman M, Wang X, Zheng X, Bright JA. Diet of Mesozoic toothed birds (Longipterygidae) inferred from quantitative analysis of extant avian diet proxies. Mendeley Data; 2022. https://doi.org/10.17632/w3c8p5w3hn.1.
Plotly. Plotly R Open Source Graphing Library. 2021. Available from: https://plotly.com/r/.
López-Ratón M, Rodríguez-Álvarez MX, Cadarso-Suárez CM, Gude-Sampedro F. OptimalCutpoints: an R package for selecting optimal cutpoints in diagnostic tests. J Stat Softw. 2014;61(8):1–36.
Kuhn M. Building predictive models in R using the caret package. J Stat Softw. 2008;28(1):1–26.
Sim J, Wright CC. The kappa statistic in reliability studies: use, interpretation, and sample size requirements. Phys Ther. 2005;85(3):257–68.
Acknowledgements
The authors would like to thank Stephen Rogers, Chase Mendenhall (Carnegie Museum of Natural History), Andrew Kratter, and David Steadman (Florida Museum of Natural History) for their assistance and expertise in selecting physical specimens for this study. We would also like to thank Kathryn C. Gamble DVM, MS, Dipl ACZM, Dip ECZM (ZHM), Veterinary Advisor Coraciiformes/Bucerotiformes TAG for providing coraciiform radiographs used in this study, and Michael Collyer and David Bapst for advice on statistical programming.
Funding
CVM is supported by a Postgraduate Scholarship from The University of Hong Kong (HKU PGS). MP is supported by the Research Grant Council of Hong Kong’s General Research Fund (17120920; 17103315; 17105221) and the School of Life Sciences at the Chinese University of Hong Kong.
Author information
Authors and Affiliations
Contributions
CVM, MP, and JAB conceived and designed the experiment. All authors acquired the data. CVM analysed the data. All authors contributed to interpreting the data. CVM, MP, and JAB drafted the manuscript. All authors read and approved the final manuscript.
Authors’ information
Twitter handle: @PaleoCase (Case Vincent Miller); @PalaeoPittman (Michael Pittman); @BeekGeak (Jen A. Bright).
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: FigS1.
Plot of TM character weights. FigS2. All DAPC plots. FigS3. Violin plots of MA/functional indices. FigS4. Plot of MA/functional indices character weights. FigS5. Plot of FEA character weights. FigS6. TM phylomorphospace with digit IV as reference. FigS7. TM phylomorphospace with phylogeny-based hulls. FigS8. Longipterygid skull reconstructions for MA/functional indices sensitivity analysis. FigS9. Dot plot of MWAM strain for FEA sensitivity analysis. FigS10. Intervals method PCA for FEA sensitivity analysis. Table S1. TM phylogenetic HSD results. Table S2. K values for individual TM variables. Table S3. MA/functional indices phylogenetic HSD results. Table S4. K values for individual MA/functional indices variables. Table S5. FEA phylogenetic HSD results. Table S6. LDA predictions from MA/functional indices sensitivity analysis. Table S7. Comparison of MWAM strain from FEA sensitivity analysis.
Additional file 2.
Interactive 3D PCA graph of TM data. Based on same data as Fig. 5A.
Additional file 3.
Interactive 3D LDA graph of TM data. Based on same data as Fig. 5B.
Additional file 4.
Interactive 3D PCA graph of MA/functional indices data, excluding semi-specialists. Based on same data as Fig. 6A.
Additional file 5.
Interactive 3D PCA graph of MA/functional indices data, including semi-specialists. Based on same data as Fig. 6B.
Additional file 6.
Interactive 3D LDA graph of MA/functional indices data, excluding semi-specialists. Based on same data as Fig. 6C.
Additional file 7.
Interactive 3D LDA graph of MA/functional indices data, including semi-specialists. Based on same data as Fig. 6D.
Additional file 8.
Interactive 3D PCA graph of FEA intervals data, excluding semi-specialists. Based on same data as Fig. 8A.
Additional file 9.
Interactive 3D PCA graph of FEA intervals data, including semi-specialists. Based on same data as Fig. 8B.
Additional file 10.
Interactive FEA PCA graph showing contour plots on hover, excluding semi-specialists. Based on same data as Fig. 8A.
Additional file 11.
Interactive FEA PCA graph showing contour plots on hover, including semi-specialists. Based on same data as Fig. 8B.
Additional file 12.
Interactive 3D LDA graph of FEA intervals data, excluding semi-specialists. Based on same data as Fig. 8C.
Additional file 13.
Interactive 3D LDA graph of FEA intervals data, including semi-specialists. Based on same data as Fig. 8D.
Additional file 14.
Interactive FEA LDA graph showing contour plots on hover, excluding semi-specialists. Based on same data as Fig. 8C.
Additional file 15.
Interactive FEA LDA graph showing contour plots on hover, including semi-specialists. Based on same data as Fig. 8D.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Miller, C.V., Pittman, M., Wang, X. et al. Diet of Mesozoic toothed birds (Longipterygidae) inferred from quantitative analysis of extant avian diet proxies. BMC Biol 20, 101 (2022). https://doi.org/10.1186/s12915-022-01294-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12915-022-01294-3