Abstract
Purpose
Given the psychosocial burdens patients in advanced stages of cancer face, innovative care concepts are needed. At the same time, such vulnerable patient groups are difficult to reach for participation in intervention studies and randomized patient inclusion may not be feasible. This article aims to identify systematic biases respectively selection effects occurring during the recruitment phase and to discuss their potential causes based on a non-randomized, multicenter intervention study with patients in advanced stages of cancer.
Methods
Patients diagnosed with at least one of 16 predefined cancers were recruited at four hospitals in three German cities. The effect of social care nurses’ continuous involvement in acute oncology wards was measured by health-related quality of life (EORTC QLQ-C30), information and participation preferences, decisional conflicts, doctor-patient communication, health literacy and symptom perception. Absolute standardized mean difference was calculated as a standardized effect size to test baseline characteristics balance between the intervention and control groups.
Results
The study enrolled 362 patients, 150 in the intervention and 212 in the control group. Except for gender, both groups differed in relevant socio-demographic characteristics, e.g. regarding age and educational background. With respect to the distribution of diagnoses, the intervention group showed a higher symptom burden than the control group. Moreover, the control group reported better quality of life at baseline compared to the intervention group (52.6 points (SD 21.7); 47.8 points (SD 22.0), ASMD = 0.218, p = 0.044).
Conclusion
Overall, the intervention group showed more social and health vulnerability than the control group. Among other factors, the wide range of diagnoses included and structural variation between the recruiting clinics increased the risk for bias. We recommend a close, continuous monitoring of relevant social and health-related characteristics during the recruitment phase as well as the use of appropriate statistical analysis strategies for adjustment, such as propensity score methods.
Trial registration: German Clinical Trials Register (DRKS-ID: DRKS00013640); registered on 29th December 2017.
Similar content being viewed by others
Background
Cancer diagnoses burden patients with extensive medical procedures and have a far-reaching impact on all individual life contexts [1]. Oncological diseases are prevalently associated with psychological stress, and related mood disorders such as depression, but also anxiety disorders and insomnia are known to occur as complications in cancer patients [2, 3]. Depending on the type of cancer and the stage of the disease, there is a high incidence of disability, which contributes to a low quality of life [4]. Medical care of psychosocial burdens of disease in the context of a cancer diagnosis are heterogeneously dealt with and distinct services are provided. The spectrum ranges from state-funded health services and mental health case managers to specific offers aimed at more effective patient-provider communication to achieve improved care outcomes [5, 6].
Difficulties in accessing appropriate end-of-life care
Studies evaluating palliative care interventions in patients with advanced cancer could demonstrate positive effects of early palliative care. Thereby, compared to patients provided with standard care, patients receiving early palliative care services showed an increased overall quality of life (QoL), a better perception of the own care situation as well as a longer life expectancy, while they were less prevalently diagnosed with depression and anxiety disorders [7, 8]. Moreover, family members experienced the process of dying as less painful if their deceased patients had received more than 22 days of palliative or hospice care as compared to relatives of patients with shorter care intervals [9].
Nevertheless, not all patients are provided with such kind of end-of-life care, as a US American study emphasizes, in which only a small proportion of patients had received palliative or hospice care [10]. Hence, several barriers to access appear to exist. A systematic review identified patients’ sociodemographic and socioeconomic characteristics (e.g. gender, origin, and housing situation) as well as communication problems with the care institutions to be essential factors that minimize access to palliative and hospice care [11]. Furthermore, existing barriers to care access may be explained by the often prevailing curative-oriented treatment approaches patients are faced with until the very end of their lives [12]. At the same time, patients’ ability to assess the severity of their disease has shown to be limited. For instance, a study on patients with colorectal or lung cancer showed that about two-thirds of all patients were not aware that the likelihood of cure by means of chemotherapy is relatively low [13]. Moreover, limited care capacity further limits access to palliative care or inpatient hospices [14].
Additional types of care to overcome sector boundaries in the healthcare system
Given the psychosocial burdens, various deficiencies in health care systems, and the QoL benefits of empowering patients in advanced cancer stages, there is a need of additional care concepts [15]. For example, patients and their families appear to require specific information for medical care and for post-discharge care during inpatient oncology treatment [16]. Although such types of support services do exist within the German care context (e.g. social services), those are currently only available at the time of hospitalization. Standardized follow-up care in the outpatient setting is lacking [4]. Supportive care services have become increasingly important for patients to be able to engage in and manage their care more independently [17]. Characteristic to such programs is specialized trained staff, who are in regular contact with patients. Thereby, the personnel aims to decrease psychological distress and improve QoL by navigating their patients through the fragmented health care systems [18, 19]. The effectiveness of previous navigation interventions has been controversially discussed with respect to the stage of disease and the specific diagnoses [19,20,21,22].
Combination of psychosocial care and navigation
The intervention to be tested in the OSCAR study (acronym for Oncological Social CARe project) combines the use of well-trained nurse navigators with a strong focus on coordinating psychosocial counselling of patients. Previous supportive navigation approaches that start at earlier stages of the disease [19] or are only available in the context of inpatient treatment [17] or have a quite limited duration of care [17, 19] or are based on nonmedical personnel [22, 23] are further developed. Based on a curriculum of the Saxon Cancer Society a specific care concept for patients in advanced stages of cancer was designed. The concept includes a close-meshed care and nurse-based approach. For a period of 12 months patients and their relatives receive monthly regular counseling sessions during which they are supported with respect to psychosocial, medical as well as social security related issues to facilitate the path through the health system [24]. To this end, social care nurses (SCN) visit or call patients regularly and assess various dimensions of their QoL using a structured questionnaire. While OSCAR aimed to overcome existing gaps in the German cancer care and appears promising in improving patients’ QoL, research on the effectiveness of this care program is needed—as with other innovative approaches.
Challenges in intervention studies with vulnerable populations
Evidence of effectiveness is a prerequisite for a broad implementation of innovations in health care systems, but participation rates are low overall and studies show selection effects [25]. Studies that examined participation behavior in intervention trials based on responder-non-responder comparisons identified tumor stage, lymph node involvement and comorbidities as influencing participation [26, 27]. In addition, convenience, the expected success of treatment and side effects were identified as important factors for acceptance and participation in a recommended therapy and clinical trials [28, 29]. The treatment experiences of significant others were also influential in the participation decision [28]. This points to challenges inherent in trials in vulnerable patient populations as those with advanced cancer and poor prognosis. In addition to the described selection effects on the patient side, restrictive funding conditions, particular research questions, time restrictions or medical treatment reality can impede randomization in the recruitment and thus increase the risk of selection bias. Regardless of whether randomization is used or not, control of baseline distribution of covariates appears to be recommended [30, 31]. Further, when performing the analyses prespecified baseline covariates should be included to ensure that imbalances between intervention and control groups based on chance do not affect effect estimates [31, 32]. Non-randomized studies are inherently more susceptible to bias, due to a higher risk of systematically differences between intervention and control groups. To investigate and address recruitment based selection bias different approaches have been discussed before [33,34,35].
This article aims to identify systematic biases respectively selection effects occurring during the recruitment phase in a non-randomized intervention study with patients in advanced stages of cancer by performing comparative analyses of the baseline data. In addition, we will shed light on mechanisms and potential causes for bias and discuss suitable compensation strategies in order to improve future analyses and recruitment practices.
Methods
Study design
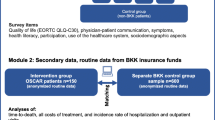
The OSCAR-study was designed as a non-randomized, controlled, multicenter trial to assess the effect of the social care nurse intervention. Data were collected from February 2018 to February 2020 [24]. Four clinics in three German cities served as recruitment channels, of which two belonged to university hospitals. The other two were maximum and standard care hospitals, that also provide a comprehensive and differentiated range of services as well as appropriate medical and technical facilities fulfilling supra-local priority tasks. The health insurers participating in the project show regionally very different shares of insured patients. By means of a Germany-wide patient potential analysis based on the defined cancer entities, the hospitals with the greatest patient potential were identified and recruited for the study.
Study population
Inclusion criteria for patients were defined as follows: ≥ 18 years of age, a combination of at least one of 16 types of cancer in combination with a burdensome therapy (e.g., surgical operation, radiotherapy, cytotoxic chemotherapy, etc.) (see Additional file 1: ICD-10 codes and OPS codes). Moreover, membership in one of 37 predefined German statutory health insurance companies (out of a total of 102 statutory health insurance funds in Germany) was a prerequisite for participation in the intervention group. All of the 37 health insurance funds have a common historical background, since as company health insurance funds they exclusively insured the employees of a particular industrial company or group. Patients who were not member with one of the predefined insurance companies were recruited into the control group. Exclusion criteria were advanced dementia and acute addiction. After providing written consent, patients were interviewed by the study team using a paper questionnaire for the scientific evaluation.
Study intervention
The intervention was provided as an additional service alongside the patients’ regular oncological care [24]. Following Kelly et la., the activities and roles of the social care nurse can be described as follows [36]. The nurses were employed by the four participating hospitals and worked in regular shifts on the oncological wards. In addition, they were given a fixed quota of hours to function as social care nurses.
At least once a month patients were actively contacted by their personal social care nurse. The contact was via the telephone, email in or a face-to-face meeting in the hospital. The meetings were to assess patient needs and identify gaps in care by using the cancer-specific quality of life questionnaire (EORTC QLQ-C30) [37]). Key functions of the social care nurses were to screen for support needs and to provide assistance in coordinating medical, psychosocial and palliative support services. Additional functions were to educate patients about the healthcare system (e.g., application for assistance) and navigate services (e.g., contact to therapists, support groups, early palliative care) to reduce barriers to receiving timely services. All social care nurses had at least five years of professional experience as trained nurse. The majority had an additional training in psycho-oncology. In preparation for their role, the SCNs received further three weeks of full-time training including: e.g., knowledge about tumor diseases, therapy options as well as special issues of oncological care and palliative medical services, knowledge of psychological aspects of the diseases (side-effect management, pain therapy, nutritional and wound therapy), psycho-oncology incl. dealing with grief and processing strategies as well as the inclusion of intercultural peculiarities, information on social security support and care services for affected persons and their relatives, theory and practice of participatory decision-making.
Patient-reported outcome measures
The primary outcome – quality of life (QoL) – was assessed by the EORTC QLQ-C30. The average of the global health status scale and the quality of life scale was used as the key scale. Scores ranges between 0 and 100. Higher scores indicate a better QoL [37, 38]. For secondary outcomes, patients’ health literacy was assessed by means of a validated assessment tool (HLS-EU-Q6 [39], score ranges between 1 and 4; score is grouped into insufficient (1–2 score), problematic (2–3 score), and sufficient (3-4 score). The relationship between physicians and patients was measured by the quality of the doctor-patient communication using an adapted version of the PRA-D (score ranges from 5 to 35; higher scores indicate better doctor-patient communication) [40]. Information and participation preferences (API-DM) were surveyed using the modified German version of the Autonomy Preference Index [41] (score ranges from 0 to 100; higher scores indicate a greater preference of information or participation). Moreover, the Decisional Conflict Scale (DCS, [42]) was utilized for evaluating patients’ perceptions on conflicts with decision-making and choosing treatment options (score ranges from 0 to 100; higher scores present a higher decision conflict). In order to assess illness coherence, the five item Illness Perception Scale was used (IPQ-R [43]). The total score ranges from 5 to 25 whereby higher scores present better illness coherence. Additionally the five single items are presented.
In addition, information on healthcare system utilization such as receiving therapies (e.g., operations, radiations therapy, hormone therapy, antibody therapy, targeted therapy, immune therapy, alternative therapies, psychotherapy, or other therapies) and health care services consultations (e.g., counseling regarding work-incapacity and pension, rehabilitation, aids, care counselling, counselling for improvement of living environment, financial advice, psychological support, addiction counseling, or other support) was retrieved [44].
The questionnaire further addressed sociodemographic characteristics (age, gender, family status, care level, and migration background), educational and professional background, social support (OSSS-3 [45], as well as the patients’ perceived social status (MacArthur Scale [46]. The questionnaire was surveyed by face-to-face interviews following patient recruitment in hospital. For follow-up interviews after three, six and twelve months, participants having been offered the choice between a face-to-face, a telephone or a handwritten postal interview. A detailed description of the study design and methods used was previously published [24].
Statistical analysis
The Intervention and control groups were comparatively characterized with respect to the predefined measures. Thereby, differences between baseline characteristics and outcomes were analyzed using the Chi-square test, Independent t-test, or Mann–Whitney U test, as appropriate. The absolute standardized mean difference (ASMD) was calculated as a standardized effect size to check the balance of the baseline characteristics, whereby ASMD < 0.1 was considered indicating an adequate balance between groups [47]. The level of significance was set to 0.05. All statistical tests were performed using Stata IC15 (StataCorp, 2017, College Station, TX, USA).
Results
A total of 362 patients were enrolled in the study, with 150 patients belonging to the intervention group and 212 to the control group. Screening data for the intervention group was not available due to data protection in relation of the small number of patients insured in the defined health insurances. For the control group 616 patients were requested. Four patients were excluded from the study; three due to non-compliance and a fourth one declined to participate in the study after enrolment. Differences in baseline characteristics between both groups are shown in Table 1. The participants in the intervention and control groups were unevenly distributed across the study sites. On average, patients in the intervention group were four years older compared to the control group members (66 vs. 62 years, ASMD = 0.0309, p = 0.004). Further differences were evident with respect to the diagnoses, such as diagnosis of acute leukemia (23.6% for the control group vs. 12.7% for the intervention group) and lung cancer (13.7% for the control group vs. 22.0% for the intervention group). The proportion of participants with a relative low level of education was 4.7% in the control group, while it was 15.2% in the intervention group (ASMD 0.323, p = 0.003). Patients in the control group rated their social status as generally higher than patients in the intervention group (6.4/10 vs. 6.1/10 points, ASMD = 0.223, p = 0.055).
Overall, patients belonging to the control group reported better global quality of life and physical functioning as in comparison to those in the intervention group (Table 2). The mean QoL was 52.6 (SD = 21.7) in the control group and 47.8 (SD = 22.0) in the intervention group (ASMD = 0.218, p = 0.044). Furthermore, patients in the intervention group reported worse outcomes in the symptom scale for pain and fatigue than the control group. Participants in the control group reported financial difficulties more often compared to patients in the intervention group, with a mean difference of 10 points between the two groups (ASMD = 0.343, p = 0.002). The proportions of the quality of life (EORTC QLQ-C30), functioning scales and symptom burden at baseline are summarized in Table 2 and illustrated in Fig. 1.
Results regarding the secondary outcomes at baseline are provided in Table 3 whereby a ASMD < 0.1 indicates balanced distribution of characteristics between intervention and control group. A significant difference was observed between both groups with respect to scores of doctor-patient communication (PRA-D). The mean of PRA-D was higher in the control group compared to the intervention group (30.6 vs. 28.4, ASMD = 0.339, p = 0.003). Moreover, the median of Decisional Conflict Scale (DCS) showed to be 20 points (IQR = 5 to 42) for those who were exposed to the intervention and 15 (IQR = 0 to 35) for participants of the control group, indicating more decisional conflicts in the intervention group. Inadequate health literacy was reported by 22.8% of patients in the intervention group and 11.0% in the control group. In line with these findings, 31.6% of patients in the intervention group and 40.2% of those belonging to the control group showed sufficient health literacy (ASMD = 0.256, p = 0.064). Furthermore, the control group scored higher on the Coherence of Disease Subscale (mean score 16.6 vs. 15.6, ASMD = 0.241, p = 0.038). No substantial group differences were found for the API-DM scale – neither regarding patients’ information preferences (mean score 96.5 compared to 95.4, ASMD = 0.172, p = 0.118) nor for participation preferences (mean score 53.7 compared to 51.5, ASMD = 0.151, p = 0.185).
Patients’ utilization of therapies and health care services during the last three months before study enrolment differed across patient groups (see Table 4). In the control group, higher usage rates of antibody therapy, targeted therapy, and chemotherapy were reported compared to the intervention group (each ASMD > 0.2). Similarly, patients in the control group were found to use health care support – such as disability and pension counseling, rehabilitation, therapeutic or medical aids, and psychological support – more frequently than participants belonging to the intervention group.
Discussion
The aim of the analysis of the OSCAR-study baseline characteristics was to identify and discuss possible systematic differences between the two study groups. Our comparative analysis revealed that the recruited patients were not evenly distributed to the intervention and control groups, respectively. More specifically, the study site location, age, diagnosis, QoL, doctor-patient communication, illness perception, and the educational background varied considerably between the two groups. Thereby, the results indicate a higher social and health-related vulnerability of the patients in the intervention group compared to those in the control group. Such a comparative analysis is valuable to assess the comparability of results between the intervention and control groups and to develop appropriate statistical analysis procedures for existing group differences.
Measurement of patient-reported outcomes in the OSCAR-study
At baseline, patients belonging to the control group reported a better global health status and quality of life, as well as lower symptom burdens as in comparison to the intervention group. As to be expected, QoL values measured in OSCAR were below the average found in a representative sample of the German general population (71.5 points, [48]) and European reference values (75.7 points) [49]. However, the values are lower for both groups compared to those of other studies: For instance, in a study on patients undergoing immunotherapy or chemotherapy treatments the average QoL score was 62.6 points [50]. Similarly, a German study of oncological patients, who were interviewed six months after a rehabilitation stay achieved an average score of 69.3 points [51]. QoL values measured in the OSCAR Study are comparable to the quality of life of palliative oncology patients in the last year of life [52]. The comparatively low scores in the intervention as well as control groups emphasize the relatively high burden of disease among the participating patients. Furthermore, social support – measured by means of the OSSS-3 scale – showed to be similar to mean values in the general German population [45]. The transformed values of our version of the PRA-D, which was adapted to assess doctor-patient communication, were higher than (regarding the control group) or comparable with (regarding the intervention group) reference values of the PRA-D [40]. In the light of the publication by Brenk-Franz et al., the baseline doctor-patient-communication in OSCAR can be interpreted as mostly satisfactory for both groups.
With respect to potential decision conflicts (DCS-score), results from our intervention and control groups indicate a low potential for decision conflicts about the hypothetical decision between treatment alternatives. The majority of patients felt relatively confident about their potential decision and thus seemed to be aware of the (dis)advantages of one therapy or another. In contrast, a validation of this instrument in a study on 149 patients with prostate cancer screening showed higher DCS-values at baseline [53]. These patients had more potential for internal conflicts relating to decisions about screening plans in the future as compared to the OSCAR-patients.
Results from the Participation and Information Preference Instrument (API-DM) indicated a strong need for information about the course of the disease and treatment as well as different therapy options for both the intervention and the control group. Additionally, both groups showed a similar preference for their involvement in medical decisions. Thereby, patients preferred a shared decision-making process together with their attending physician. For both categories, a French validation study of API for cancer patients found lower results (information preference = 85.3/100; participation preference = 45.6/100) [54]. The reference values for health literacy in the general German population (HLS-EU-6 [55]) largely correspond to those of the OSCAR control group. However, in the intervention group, the proportion of patients, who were classified in the “inadequate” category, was twice as high compared to the control group.
Understanding the differences in baseline characteristics
Benefits of participation
Having identified differences between the control and intervention groups the question arises, how those discrepancies can be explained. Following the observations of previous studies, we have to assume that participation in intervention studies is a challenge and selective in any case [26, 56]. Reasons for (non-)selection can be identified on the patient side as well as on the recruiter side: With respect to the recruitment, older patients with comorbid conditions, for example, were shown to be less likely to be offered participation [27]. On the patient side, Cottin et al. showed that severely affected patients are less likely to participate in trials [57]. Moreover, non-included patients show a significantly higher symptom-related limitation of activity, comorbidity, and lower self-determination, whereby older ones had a lower response rate to treatment and a shorter rate of survival [26, 57]. The OSCAR-study focused on patients with advanced cancers, whose disease burden and thereby affected life situations made a reduced study participation expectable. At the same time, participation in the study promised a concrete benefit for the patients in the intervention, which could lead to recruitment of sicker patients compared to the control group. As Puts et al. and Wright et al. showed, a higher willingness to participate in treatment and trials depend on the individually perceived benefit, probable success rate of treatment, side effects as well as the support offered in making a decision regarding trial entry—in our case, this support was provided by the personal social care nurses [28, 29].
The price for the required sample size
Recruiting participants for surveys among vulnerable and weakened patient populations is more resource-intensive, however is limited by the preset project durations and funding situation. In OSCAR, patients with multiple indications – each indicative of an advanced cancer – were eligible for study participation. The inclusion of such a broad range of diagnoses increases the number of potential study participants, while also raising the risk of bias between the intervention and control groups, especially if not all diagnoses are identical in their course of disease, symptom burden and prognosis [58]. Previous studies showed clear differences across tumor types with respect to symptoms and supportive care needs, specifically for respiratory in contrast to hematopoietic/lymphatic disease sites. These entities were not equally distributed between intervention and control group in OSCAR and might explain a bias towards a lower symptom burden in patients of the control group.
The role of funding conditions
Furthermore, the recruitment criterion of membership in certain health insurance companies limited access to the intervention group, which did not apply to the control group. Randomization into either of the two groups could not be realized and may thus have been a potential source of selection bias. The reason for this is the special funding framework within which the current study took place – the innovation fund of the German Federal Joint Committee. The existing economic competition between the types of health insurance funds in Germany favors the pursuit of individual projects and initiatives in order to distinguish themselves from one another and to be able to offer their own and potential new members particularly innovative services. The health insurance funds in Germany are characterized by different social and morbidity structures due to the historical background of their emergence [59, 60]. Basically, a distinction can be made between five types of health insurance funds: employees' health insurance fund, company health insurance funds, guild health insurance funds, miners' health insurance, local health insurance funds. Although the former allocation law has been replaced, according to which most statutory health insurance members were allocated to a specific health insurance fund depending on the characteristics of their workplace, the differences in the patient clientele persist [59].
Although patients in the intervention group were insured in 37 different health insurance funds, all of these are part of the same type of insurance (company health insurance funds). Hoffman & Icks pointed to differences in the distribution of person characteristics such as age, gender, weight, education level, as well as of the health status, smoking behavior, and specific diseases that varied by insurance type [59]. Those findings are meaningful in contextualizing our baseline findings. Their observations regarding higher proportion of women and lower educated in that type of insurance could be confirmed. Additionally, higher consumption rates in smokers were reported before, which we did not assess. The differences in the social composition and health behavior in the intervention group are well explored health predictors and may therefore be well related to higher symptom burdens in the intervention groups [61].
Study site effects
In addition, there were regional differences in the proportion of health insurance memberships among the recruited study participants. While the control group was enrolled equally at all three study sites, half of the patients in the intervention group were recruited at the third study site. This hospital had a palliative focus, which may be reflected in a higher percentage of patients being severely affected and particularly vulnerable. Although symptom burden and supportive care needs are often already high at the time of diagnosis, studies could demonstrate an increase of symptoms and decline of functioning with disease progression and increasing number of therapies of cancer patients [62].
Differences by chance
In relation to a cancer diagnosis, stratified randomization should lead to an evenly balanced distribution of known and unknown characteristics in the intervention and control groups. However, even in a randomized study design significant differences between study groups can occur, as in the study by Wagner et al., for instance, which examined the influence of nursing navigators on patients’ QoL [19]. This was the case for the distribution of educational qualifications within the control and intervention group in the context of cluster randomization. The authors also noted that cluster randomization prevented the random distribution of diagnoses within the groups [19]. More generally, Deaton and Cartwright pointed out that randomization procedures in medical intervention studies primarily determine whether patients are assigned to the intervention or control group but do not automatically lead to a random distribution of outcomes [63]. Irrespective of whether randomization is performed or not, it seems advisable to control for the baseline distribution of patients characteristics [30,31,32]. Despite non-randomized trials are inherently more prone to bias this does not necessarily result in different intervention effects due to adjustment for confounders or baseline imbalances [33, 34, 64]. A Cochrane review of methodological papers that comparatively analyzed healthcare outcomes from randomized studies and observational studies revealed only limited evidence for significant differences in the results of both study types [64].
Limitations and approaches to statistical address group differences
As shown above, our results show a higher general quality of life and lower symptom burden in the control group as in comparison to the intervention group. Such a phenomenon can bias study outcomes, and it seems likely that these differences will result in a substantial bias in the intervention effect [65]. However, the study has strengths in areas that are important for the generalizability of the results. The participating hospitals represent the diversity of the oncology care landscape, as university hospitals and (supra)regional hospitals are involved. Although rural areas appear to be underrepresented.
Adjustments for unbalanced sample distributions can be accomplished by several common statistical methods, including matched sampling, stratification, and multiple regression models. However, restrictions on the number of covariates to be considered often pose a challenge [65]. Propensity Score Methods are a set of methods for reducing such bias and has become popular for observational studies. The four common methods used in the propensity score are matching, stratification, covariate adjustment, and Inverse Probability of Treatment Weighting (IPTW). Regarding the limited sample size in the OSCAR study, the IPTW method using all available data will be considered in future analyses. The use of this method is advantageous because it allows the inclusion of all participants and reduces bias more effectively compared to other adjustment methods such as stratification and covariate adjustment [66]. A difficulty that can arise is the presence of large weights assigned to groups which could be assigned to those observations [67]. To address this problem, the use of stabilized, trimmed or truncated weights will be considered for further analyses of the OSCAR data [67, 68]. In addition, sensitivity analyses will stratify the results by diagnosis and study site, enabling the identification of selection effects during the recruitment phase. Finally, the sample will be repeatedly characterized at each follow-up point to identify potential changes in the composition of the study groups.
Conclusion
Intervention studies are commonly confronted with recruitment requirements and difficulties that favor selection effects, especially when severely ill patients with a poor prognosis are to be involved. Comparative analyses of central context and patient characteristics at baseline facilitate the identification of systematic differences between study groups and allow the reasons for such differences to be uncovered and discussed. Unobserved group differences may preclude a valid evaluation and correct judgement of the effectiveness of the intervention being tested. Against this background, a monitoring procedure accompanying the recruitment phase is recommended, by means of which the distribution of essential characteristics such as the study site and various participant characteristics (age, gender, or health status) are systematically verified to detect potential selection effects. Such a procedure may optimize the recruitment process in intervention studies, since appropriate measures can eventually be taken to adequately address identified selection effects as well as to reduce their occurrence in the first place. Moreover, in order to establish a valid comparability between different study groups, any differences at baseline need to be accounted for in the statistical analyses by means of appropriate methodological measures.
Availability of data and materials
The datasets generated and analyzed during the current study are not publicly available since the storage of the data in a public register is not covered by the patients’ declaration of consent. An anonymous data set are however available upon reasonable request. The corresponding author [DS] will provide data, analytical codes and material.
Change history
24 August 2022
Open access funding note has been updated.
Abbreviations
- API-DM:
-
German modified version of the Autonomy Preference Index
- ASMD:
-
Absolute standardized mean difference
- DCS:
-
Decision Conflict Scale
- DRKS:
-
German Clinical Trials Register
- EORTC:
-
European Organization for Research and Treatment of Cancer
- HLS-EU-Q6:
-
European Health Literacy Survey
- ICD:
-
International Statistical Classification of Diseases and Related Health Problems
- IPQ:
-
Illness Perception Questionnaire
- IPTW:
-
Inverse probability of treatment weighting
- IQR:
-
Interquartile range
- OECD:
-
Organization for Economic Co-operation and Development
- OPS:
-
Operation and Procedure Codes (German adaption of the International Classification of Procedures in Medicine (ICPM))
- OSCAR:
-
Oncological Social Care Project
- OSSS-3:
-
Oslo Social Support Scale
- PRA-D:
-
Patient Reaction Assessment
- PRO:
-
Patient-reported outcome
- QoL:
-
Quality of life
- SCN:
-
Social Care Nurse
- SD:
-
Standard deviation
References
Flatten G, Jünger S, Gunkel S, Singh J, Petzold E. Traumatic and psychosocial distress in patients with acute tumors. Psychother Psychosom Med Psychol. 2003;53(3–4):191–201.
Kim GM, Kim SJ, Song SK, Kim HR, Kang BD, Noh SH, et al. Prevalence and prognostic implications of psychological distress in patients with gastric cancer. BMC Cancer. 2017;17(1):283.
Mitchell AJ, Chan M, Bhatti H, Halton M, Grassi L, Johansen C, et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. Lancet Oncol. 2011;12(2):160–74.
Joshy G, Thandrayen J, Koczwara B, Butow P, Laidsaar-Powell R, Rankin N, et al. Disability, psychological distress and quality of life in relation to cancer diagnosis and cancer type: population-based Australian study of 22,505 cancer survivors and 244,000 people without cancer. BMC Med. 2020;18(1):372-.
Grassi L, Fujisawa D, Odyio P, Asuzu C, Ashley L, Bultz B, et al. Disparities in psychosocial cancer care: a report from the International Federation of Psycho-oncology Societies. Psychooncology. 2016;25(10):1127–36.
Fann JR, Ell K, Sharpe M. Integrating psychosocial care into cancer services. J Clin Oncol. 2012;30(11):1178–86.
Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733–42.
Zimmermann C, Swami N, Krzyzanowska M, Hannon B, Leighl N, Oza A, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet. 2014;383(9930):1721–30.
Choi JY, Kong KA, Chang YJ, Jho HJ, Ahn EM, Choi SK, et al. Effect of the duration of hospice and palliative care on the quality of dying and death in patients with terminal cancer: A nationwide multicentre study. Eur J Cancer Care. 2018;27(2): e12771.
Shin JA, Parkes A, El-Jawahri A, Traeger L, Knight H, Gallagher ER, et al. Retrospective evaluation of palliative care and hospice utilization in hospitalized patients with metastatic breast cancer. Palliat Med. 2016;30(9):854–61.
Parajuli J, Tark A, Jao YL, Hupcey J. Barriers to palliative and hospice care utilization in older adults with cancer: A systematic review. Journal of geriatric oncology. 2020;11(1):8–16.
Wedding U, Meran JG, Höffken K. Übertherapie in der Onkologie: Wann ist weniger mehr? Onkologe. 2008;14(7):691–700.
Weeks JC, Catalano PJ, Cronin A, Finkelman MD, Mack JW, Keating NL, et al. Patients’ expectations about effects of chemotherapy for advanced cancer. N Engl J Med. 2012;367(17):1616–25.
Radbruch L, Payne S. Standards und Richtlinien für Hospiz- und Palliativversorgung in Europa: Teil 2. Zeitschrift für Palliativmedizin. 2011;12:260–70.
Cuthbert CA, Boyne DJ, Yuan X, Hemmelgarn BR, Cheung WY. Patient-reported symptom burden and supportive care needs at cancer diagnosis: a retrospective cohort study. Support Care Cancer. 2020;28(12):5889–99.
Dougherty M. Assessment of patient and family needs during an inpatient oncology experience. Clin J Oncol Nurs. 2010;14(3):301–6.
Berezowska A, Passchier E, Bleiker E. Evaluating a professional patient navigation intervention in a supportive care setting. Support Care Cancer. 2019;27(9):3281–90.
Porzig R, Neugebauer S, Heckmann T, Adolf D, Kaskel P, Froster UG. Evaluation of a cancer patient navigation program (“Onkolotse”) in terms of hospitalization rates, resource use and healthcare costs: rationale and design of a randomized, controlled study. BMC Health Serv Res. 2018;18(1):413.
Wagner EH, Ludman EJ, Aiello Bowles EJ, Penfold R, Reid RJ, Rutter CM, et al. Nurse navigators in early cancer care: a randomized, controlled trial. J Clin Oncol. 2014;32(1):12–8.
Skrutkowski M, Saucier A, Eades M, Swidzinski M, Ritchie J, Marchionni C, et al. Impact of a pivot nurse in oncology on patients with lung or breast cancer: symptom distress, fatigue, quality of life, and use of healthcare resources. Oncol Nurs Forum. 2008;35(6):948–54.
Tho PC, Ang E. The effectiveness of patient navigation programs for adult cancer patients undergoing treatment: a systematic review. JBI Database System Rev Implement Rep. 2016;14(2):295–321.
Hendren S, Griggs JJ, Epstein R, Humiston S, Jean-Pierre P, Winters P, et al. Randomized controlled trial of patient navigation for newly diagnosed cancer patients: effects on quality of life. Cancer Epidemiol Biomarkers Prev. 2012;21(10):1682–90.
Fiscella K, Whitley E, Hendren S, Raich P, Humiston S, Winters P, et al. Patient Navigation for Breast and Colorectal Cancer Treatment: A Randomized Trial. Cancer Epidemiol Biomarkers Prev. 2012;21(10):1673–81.
Frick J, Schindel D, Gebert P, Grittner U, Schenk L. Improving quality of life in cancer patients through higher participation and health literacy: study protocol for evaluating the oncological social care project (OSCAR). BMC Health Serv Res. 2019;19(1):754.
Harrison JD, Solomon MJ, Young JM, Meagher A, Hruby G, Salkeld G, et al. Surgical and oncology trials for rectal cancer: Who will participate? Surgery. 2007;142(1):94-101.e20.
Gollhofer SM, Wiskemann J, Schmidt ME, Klassen O, Ulrich CM, Oelmann J, et al. Factors influencing participation in a randomized controlled resistance exercise intervention study in breast cancer patients during radiotherapy. BMC Cancer. 2015;15(1):186.
Kemeny MM, Peterson BL, Kornblith AB, Muss HB, Wheeler J, Levine E, et al. Barriers to Clinical Trial Participation by Older Women With Breast Cancer. J Clin Oncol. 2003;21(12):2268–75.
Puts MTE, Tapscott B, Fitch M, Howell D, Monette J, Wan-Chow-Wah D, et al. A systematic review of factors influencing older adults’ decision to accept or decline cancer treatment. Cancer Treat Rev. 2015;41(2):197–215.
Wright JR, Whelan TJ, Schiff S, Dubois S, Crooks D, Haines PT, et al. Why Cancer Patients Enter Randomized Clinical Trials: Exploring the Factors That Influence Their Decision. J Clin Oncol. 2004;22(21):4312–8.
Senn S. Testing for baseline balance in clinical trials. Stat Med. 1994;13(17):1715–26.
Kahan BC, Jairath V, Doré CJ, Morris TP. The risks and rewards of covariate adjustment in randomized trials: an assessment of 12 outcomes from 8 studies. Trials. 2014;15:139.
Karrison T, Kocherginsky M. Restricted mean survival time: Does covariate adjustment improve precision in randomized clinical trials? Clin Trials. 2018;15(2):178–88.
Nohr EA, Liew Z. How to investigate and adjust for selection bias in cohort studies. Acta Obstet Gynecol Scand. 2018;97(4):407–16.
Harvey RA, Hayden JD, Kamble PS, Bouchard JR, Huang JC. A comparison of entropy balance and probability weighting methods to generalize observational cohorts to a population: a simulation and empirical example. Pharmacoepidemiol Drug Saf. 2017;26(4):368–77.
Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–107.
Kelly KJ, Doucet S, Luke A. Exploring the roles, functions, and background of patient navigators and case managers: A sco** review. Int J Nurs Stud. 2019;98:27–47.
Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A, et al. The EORTC QLQ-C30 Scoring Manual (3rd Edition). Brussels; 2001.
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–76.
Lorini C, Santomauro F, Grazzini M, Mantwill S, Vettori V, Lastrucci V, et al. Health literacy in Italy: a cross-sectional study protocol to assess the health literacy level in a population-based sample, and to validate health literacy measures in the Italian language. BMJ Open. 2017;7(11): e017812.
Brenk-Franz K, Hunold G, Galassi JP, Tiesler F, Herrmann W, Freund T, et al. Quality of the Physician-Patient Relationship – Evaluation of the German Version of the Patient Reactions Assessment (PRA-D). ZFA Zeitschrift für Allgemeinmedizin. 2016;92(3):103–8.
Simon D, Kriston L, Loh A, Spies C, Scheibler F, Wills C, et al. Confirmatory factor analysis and recommendations for improvement of the Autonomy-Preference-Index (API). Health Expect. 2010;13(3):234–43.
O'Connor AM. User Manual - Decisional Conflict Scale [Update 2010]. Ottawa Hospital Research Institute; 1993.
Gaab J, Latanzia-Bunschoten S, Sprott H. Illness Perception Questionnaire. In: Begel, Wirtz, Zwingmann, editors. Kompendium: Diagnostische Verfahren in der Rehabilitation. Göttingen: Hogrefe Verlag; 2008.
Rattay P, Butschalowsky H, Rommel A, Prütz F, Jordan S, Nowossadeck E, et al. Utilization of outpatient and inpatient health services in Germany: results of the German Health Interview and Examination Survey for Adults (DEGS1). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56(5–6):832–44.
Kocalevent R-D, Berg L, Beutel ME, Hinz A, Zenger M, Härter M, et al. Social support in the general population: standardization of the Oslo social support scale (OSSS-3). BMC Psychology. 2018;6(1):31.
Hoebel J, Müters S, Kuntz B, Lange C, Lampert T. Measuring subjective social status in health research with a German version of the MacArthur Scale. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2015;58(7):749–57.
Stuart EA, Lee BK, Leacy FP. Prognostic score–based balance measures can be a useful diagnostic for propensity score methods in comparative effectiveness research. Journal of Clinical Epidemiology. 2013;66(8, Supplement):S84-S90.e1.
Schwarz R, Hinz A. Reference data for the quality of life questionnaire EORTC QLQ-C30 in the general German population. Eur J Cancer. 2001;37(11):1345–51.
Hinz A, Singer S, Brähler E. European reference values for the quality of life questionnaire EORTC QLQ-C30: Results of a German investigation and a summarizing analysis of six European general population normative studies. Acta Oncol. 2014;53(7):958–65.
Steffen McLouth LE, Lycan TW, Levine BJ, Gabbard J, Ruiz J, Farris M, et al. Patient-Reported Outcomes From Patients Receiving Immunotherapy or Chemoimmunotherapy for Metastatic Non–Small-Cell Lung Cancer in Clinical Practice. Clin Lung Cancer. 2020;21(3):255-63.e4.
Hinz A, Mehnert A, Dégi C, Reissmann DR, Schotte D, Schulte T. The relationship between global and specific components of quality of life, assessed with the EORTC QLQ-C30 in a sample of 2019 cancer patients. Eur J Cancer Care. 2017;26(2):e12416.
Verkissen MN, Hjermstad MJ, Van Belle S, Kaasa S, Deliens L, Pardon K. Quality of life and symptom intensity over time in people with cancer receiving palliative care: Results from the international European Palliative Care Cancer Symptom study. PLoS ONE. 2019;14(10): e0222988.
Linder SK, Swank PR, Vernon SW, Mullen PD, Morgan RO, Volk RJ. Validity of a low literacy version of the Decisional Conflict Scale. Patient Educ Couns. 2011;85(3):521–4.
Colombet I, Rigal L, Urtizberea M, Vinant P, Rouquette A. Validity of the French version of the Autonomy Preference Index and its adaptation for patients with advanced cancer. PLoS ONE. 2020;15(1): e0227802.
Pelikan J, Röthlin F, Ganahl K, Boltzmann L. Measuring comprehensive health literacy in general populations: validation of instrument, indices and scales of the HLS-EU study. Health Literacy Research Conference; 3.11.2014; Bethesda, Maryland (United Staates)2014.
Al-Refaie WB, Vickers SM, Zhong W, Parsons H, Rothenberger D, Habermann EB. Cancer Trials Versus the Real World in the United States. Annals of Surgery. 2011;254(3):438–43.
Cottin V, Arpin D, Lasset C, Cordier JF, Brune J, Chauvin F, et al. Small-cell lung cancer: Patients included in clinical trials are not representative of the patient population as a whole. Ann Oncol. 1999;10(7):809–16.
Bubis LD, Davis L, Mahar A, Barbera L, Li Q, Moody L, et al. Symptom Burden in the First Year After Cancer Diagnosis: An Analysis of Patient-Reported Outcomes. J Clin Oncol. 2018;36(11):1103–11.
Hoffmann F, Icks A. Structural differences between health insurance funds and their impact on health services research: results from the Bertelsmann Health-Care Monitor. Gesundheitswesen. 2012;74(5):291–7.
Werner A, Reitmeir P, John J. Switching sickness funds and risk compensation mechanisms in the statutory health insurance system in Germany–empirical results from the cooperative health research in the region of Augsburg (KORA). Gesundheitswesen. 2005;67(Suppl 1):S158–66.
Hartung TJ, Johansen C. Sozioökonomischer Status und Krebs. Forum. 2017;32(4):318–23.
Wilson MK, Karakasis K, Oza AM. Outcomes and endpoints in trials of cancer treatment: the past, present, and future. Lancet Oncol. 2015;16(1):e32–42.
Deaton A, Cartwright N. Understanding and misunderstanding randomized controlled trials. Soc Sci Med. 2018;210:2–21.
Anglemyer A, Horvath HT, Bero L. Healthcare outcomes assessed with observational study designs compared with those assessed in randomized trials. Cochrane Database Syst Rev. 2014(4):Mr000034.
D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265–81.
Deb S, Austin PC, Tu JV, Ko DT, Mazer CD, Kiss A, et al. A Review of Propensity-Score Methods and Their Use in Cardiovascular Research. Can J Cardiol. 2016;32(2):259–65.
Elze MC, Gregson J, Baber U, Williamson E, Sartori S, Mehran R, et al. Comparison of Propensity Score Methods and Covariate Adjustment: Evaluation in 4 Cardiovascular Studies. J Am Coll Cardiol. 2017;69(3):345–57.
Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661–79.
Acknowledgements
We would like to thank all participants, study nurses, and social care nurses who were involved in and facilitated the conduct of our study. Further, we would also like to thank Nadja-Raphaela Baer for her valuable comments on the manuscript. We acknowledge financial support from the Open Access Publication Fund of Charité – Universitätsmedizin Berlin and the German Research Foundation (DFG).
Funding
This research project received a grant from the German Innovation Fund of the Federal Joint Committee (G-BA). Reference number: 01NVF17016. The G-BA was not involved in the study design, data collection, data analysis, and publication. Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
All authors were involved in the implementation of the study. DS and LS developed the study design. PG was involved in the statistical analysis plan and analyzed data. PG, JF and DS drafted the manuscript. UG reviewed the statistical analysis. AL and LS provided critical reviews. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the ethics committees of the Charité – Universitätsmedizin Berlin (EA2/192/17) and the Medical Association of North Rhine (2017429). Patients were enrolled in the study after having provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Included ICD-10- and OPS-codes.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Frick, J., Gebert, P., Grittner, U. et al. Identifying and handling unbalanced baseline characteristics in a non-randomized, controlled, multicenter social care nurse intervention study for patients in advanced stages of cancer. BMC Cancer 22, 560 (2022). https://doi.org/10.1186/s12885-022-09646-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-09646-6