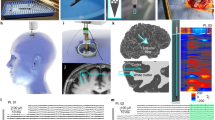
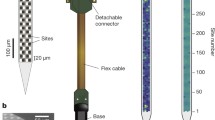
Abstract
Large-scale neural population recordings with single-cell resolution across the primate brain remain challenging. Here we introduce the Neuroscroll probe that isolates single neuronal activities simultaneously from 1,024 densely spaced channels that are flexibly distributed across the shank of the probe. The Neuroscroll probe length is easily tunable for individual probes from 10 mm to 90 mm, covering the brain size of non-human primates and humans, and the probes remain intact and functional after repeated bending deformations. The Neuroscroll probes provided reliable recordings from large neural populations with high chronic stability up to 105 weeks in rats. Recording with each Neuroscroll probe yielded hundreds of well-isolated single units simultaneously from multiple brain regions distributed across the entire depth of the rhesus macaque brain. With the thousand simultaneously recorded channels, unprecedented probe length, excellent mechanical stability and flexible recording site distribution, the Neuroscroll probes enable a wide range of new experimental paradigms in system neuroscience studies with great versatility.





Similar content being viewed by others
Data availability
The data analyzed during the current study are available at https://github.com/DuanLab-PKU/Liu_et_al_2024_Neuroscrolls. Raw data generated during the current study are available from the corresponding author upon reasonable request. Source data are provided with this paper.
Code availability
Thermo Fisher Scientific Quattro Environmental Scanning Electron Microscope software was used for SEM image collection. USB Interface Board software 1.5.4 was used for rat electrophysiology data acquisition, available at https://intantech.com/. Custom 1,024-channel recording software was used for rhesus macaque electrophysiology data acquisitions, available at https://github.com/DuanLab-PKU/Liu_et_al_2024_Neuroscrolls. MaxLab Live software (MaxWell Biosystems) was used for simulated neural signal acquisitions with CMOS-MEAs. ParaVision 6.0.1 was used for MRI acquisitions on rats. Insight Vision 1.0 was used for CT acquisitions on rhesus macaques. Syngo VE11C was used for MRI acquisitions on rhesus macaques. Olympus VS200 ASW 4.1 software was used for fluorescence imaging. All data analysis and visualization are based on Python 3.11.3, MATLAB R2021b, Origin 2022 and ImageJ 1.54f. MIPAV software (https://www.mipav.cit.nih.gov) was used for MRI and CT image co-registration to CIVM template, and itk-SNAP software (http://www.itksnap.org/pmwiki/pmwiki.php) was used for defining regions of interest of different brain areas. MATLAB R2021b was used for three-dimensional reconstruction of electrode trajectories. Kilosort 3.0 (https://github.com/MouseLand/Kilosort) was used for spike sorting, and ecephys_spike_sorting (https://github.com/AllenInstitute/ecephys_spike_sorting) was used for automatic post-processing, mainly double-counted spike removal. Phy (https://github.com/cortex-lab/phy) was used for manual curation. Custom code (https://github.com/DuanLab-PKU/Liu_et_al_2024_Neuroscrolls) was used for data format conversion, batch processing, analysis and visualization.
References
Marshall, N. J. et al. Flexible neural control of motor units. Nat. Neurosci. 25, 1492–1504 (2022).
Trepka, E. B., Zhu, S., **a, R., Chen, X. & Moore, T. Functional interactions among neurons within single columns of macaque V1. eLife 11, e79322 (2022).
Sun, X. et al. Cortical preparatory activity indexes learned motor memories. Nature 602, 274–279 (2022).
Bao, P., She, L., McGill, M. & Tsao, D. Y. A map of object space in primate inferotemporal cortex. Nature 583, 103–108 (2020).
Zylberberg, A., Fetsch, C. R. & Shadlen, M. N. The influence of evidence volatility on choice, reaction time and confidence in a perceptual decision. eLife 5, e17688 (2016).
Maynard, E. M., Nordhausen, C. T. & Normann, R. A. The Utah intracortical electrode array: a recording structure for potential brain–computer interfaces. Electroencephalogr. Clin. Neurophysiol. 102, 228–239 (1997).
Musallam, S., Bak, M. J., Troyk, P. R. & Andersen, R. A. A floating metal microelectrode array for chronic implantation. J. Neurosci. Methods 160, 122–127 (2007).
Knudsen, E. B. & Wallis, J. D. Hippocampal neurons construct a map of an abstract value space. Cell 184, 4640–4650 (2021).
Hesse, J. K. & Tsao, D. Y. A new no-report paradigm reveals that face cells encode both consciously perceived and suppressed stimuli. eLife 9, e58360 (2020).
Hong, G. & Lieber, C. M. Novel electrode technologies for neural recordings. Nat. Rev. Neurosci. 20, 330–345 (2019).
Steinmetz, N. A., Zatka-Haas, P., Carandini, M. & Harris, K. D. Distributed coding of choice, action and engagement across the mouse brain. Nature 576, 266–273 (2019).
Jun, J. J. et al. Fully integrated silicon probes for high-density recording of neural activity. Nature 551, 232–236 (2017).
Steinmetz, N. A. et al. Neuropixels 2.0: a miniaturized high-density probe for stable, long-term brain recordings. Science 372, eabf4588 (2021).
Peters, A. J., Fabre, J. M. J., Steinmetz, N. A., Harris, K. D. & Carandini, M. Striatal activity topographically reflects cortical activity. Nature 591, 420–425 (2021).
Eric, M. T. et al. Large-scale high-density brain-wide neural recording in nonhuman primates. Preprint at bioRxiv https://doi.org/10.1101/2023.02.01.526664 (2023).
Yang, C. & Naya, Y. Hippocampal cells integrate past memory and present perception for the future. PLoS Biol. 18, e3000876 (2020).
Naya, Y., Chen, H., Yang, C. & Suzuki, W. A. Contributions of primate prefrontal cortex and medial temporal lobe to temporal-order memory. Proc. Natl Acad. Sci. USA 114, 13555–13560 (2017).
Zhou, Y. & Freedman, D. J. Posterior parietal cortex plays a causal role in perceptual and categorical decisions. Science 365, 180–185 (2019).
Schwarz, D. A. et al. Chronic, wireless recordings of large-scale brain activity in freely moving rhesus monkeys. Nat. Methods 11, 670–676 (2014).
Urai, A. E., Doiron, B., Leifer, A. M. & Churchland, A. K. Large-scale neural recordings call for new insights to link brain and behavior. Nat. Neurosci. 25, 11–19 (2022).
Dotson, N. M., Hoffman, S. J., Goodell, B. & Gray, C. M. A large-scale semi-chronic microdrive recording system for non-human primates. Neuron 96, 769–782 (2017).
Harris, K. D., Henze, D. A., Csicsvari, J., Hirase, H. & Buzsáki, G. Accuracy of tetrode spike separation as determined by simultaneous intracellular and extracellular measurements. J. Neurophysiol. 84, 401–414 (2000).
Sakane, T. et al. Brain and nasal cavity anatomy of the cynomolgus monkey: species differences from the viewpoint of direct delivery from the nose to the brain. Pharmaceutics 12, 1227 (2020).
Rengachary, S. S. & Ellenbogen, R. G. Principles of Neurosurgery (Elsevier, 2005).
Chung, J. E. et al. High-density, long-lasting, and multi-region electrophysiological recordings using polymer electrode arrays. Neuron 101, 21–31 (2019).
Obaid, A. et al. Massively parallel microwire arrays integrated with CMOS chips for neural recording. Sci. Adv. 6, eaay2789 (2020).
Yoo, J. & Meng, E. Bonding methods for chip integration with Parylene devices. J. Micromech. Microeng. 31, 045011 (2021).
Zhao, Z. et al. Ultraflexible electrode arrays for months-long high-density electrophysiological map** of thousands of neurons in rodents. Nat. Biomed. Eng. 7, 520–532 (2023).
Lee, J. M. et al. Nanoenabled direct contact interfacing of syringe-injectable mesh electronics. Nano Lett. 19, 5818–5826 (2019).
Namima, T. et al. Inserting a Neuropixels probe into awake monkey cortex: two probes, two methods. J. Neurosci. Methods 402, 110016 (2024).
Müller, J. et al. High-resolution CMOS MEA platform to study neurons at subcellular, cellular, and network levels. Lab Chip 15, 2767–2780 (2015).
Musk, E. An integrated brain–machine interface platform with thousands of channels. J. Med. Internet Res. 21, e16194 (2019).
Pachitariu, M., Steinmetz, N., Kadir, S., Carandini, M. & Harris, K. Fast and accurate spike sorting of high-channel count probes with KiloSort. Advances in Neural Information Processing Systems 29 (NIPS 2016) 4455–4463 (NeurIPS, 2016).
Darji, M. et al. Development of delayed-release pellets of ibuprofen using Kollicoat® MAE 100P via hot-melt extrusion technology. J. Pharm. Innov. 18, 1827–1837 (2023).
Constantin, C. P., Aflori, M., Damian, R. F. & Rusu, R. D. Biocompatibility of polyimides: a mini-review. Materials 12, 3166 (2019).
Lee, K. et al. Flexible, scalable, high channel count stereo-electrode for recording in the human brain. Nat. Commun. 15, 218 (2024).
Kwan, C. et al. Co-registration of imaging modalities (MRI, CT and PET) to perform frameless stereotaxic robotic injections in the common marmoset. Neuroscience 480, 143–154 (2022).
Alkire, M. T., Hudetz, A. G. & Tononi, G. Consciousness and anesthesia. Science 322, 876–880 (2008).
Lewis, L. D. et al. Rapid fragmentation of neuronal networks at the onset of propofol-induced unconsciousness. Proc. Natl Acad. Sci. USA 109, E3377–E3386 (2012).
Bastos, A. M. et al. Neural effects of propofol-induced unconsciousness and its reversal using thalamic stimulation. eLife 10, e60824 (2021).
Lee, H., Tanabe, S., Wang, S. & Hudetz, A. G. Differential effect of anesthesia on visual cortex neurons with diverse population coupling. Neuroscience 458, 108–119 (2021).
Lee, E. K. et al. Non-linear dimensionality reduction on extracellular waveforms reveals cell type diversity in premotor cortex. eLife 10, e67490 (2021).
Sun, S. H. et al. Analysis of extracellular spike waveforms and associated receptive fields of neurons in cat primary visual cortex. J. Physiol. 599, 2211–2238 (2021).
Paulk, A. C. et al. Large-scale neural recordings with single neuron resolution using Neuropixels probes in human cortex. Nat. Neurosci. 25, 252–263 (2022).
Robbins, A. A., Fox, S. E., Holmes, G. L., Scott, R. C. & Barry, J. M. Short duration waveforms recorded extracellularly from freely moving rats are representative of axonal activity. Front. Neural Circuits 7, 181 (2013).
Calabrese, E. et al. A diffusion tensor MRI atlas of the postmortem rhesus macaque brain. Neuroimage 117, 408–416 (2015).
Yushkevich, P. A. et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31, 1116–1128 (2006).
Quiroga, R. Q., Nadasdy, Z. & Ben-Shaul, Y. Unsupervised spike detection and sorting with wavelets and superparamagnetic clustering. Neural Comput. 16, 1661–1687 (2004).
Tort, A. B. L., Komorowski, R., Eichenbaum, H. & Kopell, N. Measuring phase-amplitude coupling between neuronal oscillations of different frequencies. J. Neurophysiol. 104, 1195–1210 (2010).
Sharf, T. et al. Functional neuronal circuitry and oscillatory dynamics in human brain organoids. Nat. Commun. 13, 4403 (2022).
Lu, L. et al. Soft and MRI compatible neural electrodes from carbon nanotube fibers. Nano Lett. 19, 1577–1586 (2019).
Acknowledgements
We acknowledge the Molecular Materials and Nanofabrication Laboratory in the College of Chemistry at Peking University and Peking Nanofab for the use of fabrication instruments. We thank L. Cheng from the Institute of Automation, Chinese Academy of Sciences, for help on MRI/CT image registration and S. Tang from Peking University and D. **ng from Bei**g Normal University for valuable discussions. This work was supported by grants from the National Natural Science Foundation of China (T2188101 and 21972005), the STI2030-Major Projects (2021ZD0202204 and 2021ZD0202200), the National Key R&D Program of China (2021YFF1200700) and the Natural Science Foundation of Bei**g Municipality (JQ20008). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
X.D. supervised the project. X.D., Y.L., H.J. and H.S. conceived the project and designed the experiments. Y.L., H.J., Z.Y., S.J., A.J., J.Z. and M.L. fabricated and characterized the devices. Y.L. conducted the recordings and MRI on rats. H.S., Y.L., H.J., A.L., Y.N., C.Y., Z.Y., S.J. and S.X. conducted the recordings and MRI/CT on rhesus macaques. X.L. and B.C. provided the 1,024-channel Intan system. S.J., Y.L., H.J., H.S. and C.Z. conducted the data analysis. X.D., Y.L., S.J., H.J. and H.S. wrote the paper, with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Ueli Rutishauser and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1
Schematics showing the stepwise fabrication process of the MEAs used for Neuroscroll probes fabrication.
Extended Data Fig. 2
SEM images of Neuroscroll probes with shank lengths of 10 mm (a), 50 mm (b), and 90 mm (c).
Extended Data Fig. 3 Crosstalk tests of the Neuroscroll probes.
Inset schematically illustrates the measurement setup. Simulated neural action potential pulses were applied to the aggressor channel. Crosstalk signals induced on the victim channel were acquired with an Intan RHD2132 amplifier. Crosstalk was calculated as the ratio between the amplitude values of recorded voltage on the victim channel and the input voltage on the aggressor channel. Same measurements were conducted on the planar MEAs without rolling for a comparison. The box plots show the median and quartile range, and the whiskers denote 1.5× the interquartile range (n = 45 from five Neuroscroll probes and n = 19 from two planar MEAs). Individual data points are overlaid on the box plots. n.s.: not significant, two-tailed Mann-Whitney U test.
Extended Data Fig. 4 Bending tests.
a, A Neuroscroll probe at unstrained state. b, A bent Neuroscroll probe which remained intact after several cycles of bending. c, The 1 kHz-impedance values of Neuroscroll probes before and after 10 cycles of bending to ± 15 degrees. Individual data points are overlaid on the box plots. The box plots show the median and quartile range, and the whiskers denote 1.5× the interquartile range (n = 399 from 4 devices). n.s.: not significant, two-tailed paired sample Wilcoxon signed rank test was used. The Neuroscroll probes remained intact and functional after repeated bending deformation. The tungsten wires were left inside the Neuroscroll probes used in this test.
Extended Data Fig. 5 Spatial footprint of some example single unit activities recorded in a rat.
Each column shows the simultaneously recorded signals from 12 adjacent channels arranged in a column. Average traces were overlaid with 100 randomly selected single event raw traces high-pass filtered at 300 Hz (gray). Each column shows the signal spread of an isolated single unit.
Extended Data Fig. 6 Chronically stable recording in rat 1.
a, Representative AP band traces (high-pass filtered at 300 Hz) from channels 52 to 81 in rat 1 (implanted with a Neuroscroll probe with recording pitch of 36 μm) at week 0, week 16 and week 33 post-implantation. The intraoperative recording at week 0 showed larger noise arising from the anesthesia and surgery monitoring system. b, Representative mean waveforms of single units isolated in channels 49 to 70 in rat 1 at different timepoints post-implantation. c, d, Amplitude (c) and SNR (d) of all sorted single units from rat 1 over 33 weeks. The box plots in c and d show the median and quartile range, and the whiskers denote 1.5× the interquartile range. The sample size at each timepoint in c and d is summarized in Supplementary Table 3. e, Firing rates across all channels normalized by the total firing rate of each recording session over 33 weeks.
Extended Data Fig. 7 Immunohistochemistry (IHC) studies on rats implanted with Neuroscroll probes.
a, Example immunofluorescence images of tissue responses following a 4-week (left) and 8-week (right) implantation of Neuroscroll probes. b, Relative fluorescence intensity profiles as a function of distance from the center of the Neuroscroll probe tracts for 4- and 8-week post-implantation. Circular outlines of 40 μm size were segmented from the center of the Neuroscroll probe tracts (set as 0) to the distant uninjured regions. Fluorescence intensity values were binned into 40 μm of circular outlines and normalized against the intensity on the contralateral sides with no implants. c, Neuron counts within the area with a radius of 150 μm from the center of Neuroscroll probe tracts. Error bars in b, c show SEM (n = 12 from 3 animals in each group). n.s.: not significant, two-tailed t test was used.
Extended Data Fig. 8 SEM images of a Neuroscroll probe after being implanted in a rat brain for 23 weeks.
a, The Neuroscroll probe remained as a tight scroll after being dislodged from the rat brain. b, Some recording sites of the probe.
Extended Data Fig. 9 Spatial footprint of some example single unit activities recorded in the anesthetized rhesus macaque.
The recording channels were distributed in two columns. Each group, as distinguished with colours, shows the simultaneously recorded signals from 6 adjacent channels. Average traces were overlaid with 100 randomly selected single event raw traces high-pass filtered at 300 Hz (gray). Each group shows the signal spread of an isolated single unit.
Extended Data Fig. 10 Spatial footprint of some example single unit activities recorded with probe 2 in the cwm located between 8B and Cd of the anesthetized rhesus macaque.
The recording channels were distributed in two columns. Each group shows the simultaneously recorded signals from 6 adjacent channels. Average traces were overlaid with 100 randomly selected single event raw traces high-pass filtered at 300 Hz (gray). Each group shows the signal spread of an isolated single unit.
Supplementary information
Supplementary Information
Supplementary Figs. 1–5, Supplementary Tables 1–4, Supplementary Methods and References.
Supplementary Video 1
Movies showing the mechanical stability of the Neuroscroll probes. The tungsten wires remain inside the probes except for the left one.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source Data.
Source Data Extended Data Fig. 6
Statistical Source data.
Source Data Extended Data Fig. 7
Statistical Source Data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Y., Jia, H., Sun, H. et al. A high-density 1,024-channel probe for brain-wide recordings in non-human primates. Nat Neurosci (2024). https://doi.org/10.1038/s41593-024-01692-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41593-024-01692-6
- Springer Nature America, Inc.