Abstract
Gold-copper alloys have rich forms. Here we report an atomically resolved [Au52Cu72(p-MBT)55]+Cl− nanoalloy (p-MBT = SPh-p-CH3). This nanoalloy exhibits unusual structural patterns. First, two Cu atoms are located in the inner 7-atom decahedral kernel (M7, M = Au/Cu). The M7 kernel is then enclosed by a second shell of homogold (Au47), giving rise to a two-shelled M54 (i.e. Au52Cu2) full decahedron. A comparison of the non-truncated M54 decahedron with the truncated homogold Au49 kernel in similar-sized gold nanoparticles provides for the first time an explanation for Marks decahedron truncation. Second, a Cu70(SR)55 exterior cage resembling a 3D Penrose tiling protects the M54 decahedral kernel. Compared to the discrete staple motifs in gold:thiolate nanoparticles, the Cu-thiolate surface of Au52Cu72 forms an extended cage. The Cu-SR Penrose tiling retains the M54 kernel’s high symmetry (D5h). Third, interparticle interactions in the assembly are closely related to the symmetry of the particle, and a “quadruple-gear-like” interlocking pattern is observed.
Similar content being viewed by others
Introduction
Multiply-twinned nanoparticles (MTPs) are particularly important and intriguing in nanoscience research1,2,3, but the formation mechanism has long remained unclear. Historically, Ino and Ogawa first found in the 1960 s MTPs in the early stages of gold film growth4, and such particles could be viewed as 5 or 20 face-centered-cubic (fcc) tetrahedra joined together with twin boundaries, forming decahedra or icosahedra. Marks and Howie reported the presence of such MTPs up to 200 nm in various heterogeneous metal particles (silver in particular)5. In their further work, thermally annealed Au or Ag nanoparticles of 10–50 nm showed re-entrant surfaces on the decahedral MTPs6, and the truncated-decahedron was proposed7,8, which costs less energy than the truncation in the Ino decahedron.
With the advent of the nanotechnology era, metal nanoparticles (NPs) have since drawn significant attention, and capabilities for manipulating the structure and properties of NPs have been achieved to a great extent1,2,3,9,36. The peculiar arrangement of copper atoms at central sites is found to be decisive to form the second, perfect decahedral shell of gold as opposed to a truncated-decahedron for homogold NPs such as Au102, Au103, and Au13016,17,18.
As shown in Fig. 2, we compared the two inner shells, i.e., Au5Cu2@Au47 (Fig. 2a, b) in [Cu72Au52(SR)55]+ and Au7@Au42 in Au102(SR)44 or Au103S2(SR)41 (Fig. 2d, e) to illustrate the difference brought by the copper incorporation. The parts marked by dashed triangles are emphasized in Fig. 2c, f, showing that the C5 axial Au–Cu–Cu–Au length (8.249(2) Å) is contracted by 7% compared to the Au–Au–Au–Au length (8.901(4) Å). In addition, the angle (θ) is also expanded for the Cu-doped kernel (see labels in Fig. 2c, f). Therefore, when extrapolating the two edges of the decahedron to reach a joint point, the Cu-doped structure gives a corner-to-neighbor distance of 2.820(2) Å, which matches with typical Au–Au bond length (2.88 Å) so that the five corner atoms can exist (Fig. 2c), but the homogold structure gives a much shorter corner-to-neighbor distance (2.560(3) Å), being much shorter than any realistic Au–Au bond length (Fig. 2f), thus the corner Au atoms have to be eliminated. This explains why the Au–Cu nanoalloy herein possesses a full decahedral kernel, while Au102 and Au103 structures have the Marks truncation. Taken together, the incorporation of two Cu atoms into the inner M7 kernel in the Cu52Au72 nanoalloy is critical for the formation of the full decahedral kernel. The two Cu atoms along the disclination core is also reasonable from a strain-induced segregation perspective in bimetallic multiply-twinned NPs in which a segregation of smaller atoms to the kernel could be noticeable37. It is worth mentioning that a similar Ag7@Ag47 kernel (Supplementary Fig. 10) was observed in Ag13630, comparing to the Au5Cu2@Au47 in Cu72Au52 herein. The comparison between Ag and Au NPs reinforces the effect that the two do** Cu atoms play a key role in reaching the full decahedral structure of the Au–Cu nanoalloy.
a Top view and b side view of the Au5Cu2@Au47 shells in [Cu72Au52(SR)55]+, and c the triangle formed by twinning edges and apex in the Au47 shell; d Top view and e side view of the Au7@Au42 shells in Au102(SR)44 or Au103S2(SR)4116,17, and f the truncated triangle formed by twinning edges and apex in the Au42 shell. Color labels: magenta/pink = Au, orange = Cu.
Cu-thiolate cage resembling Penrose tiling
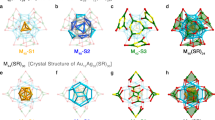
The formation of a full decahedron, Au5Cu2@Au47, further dictates the formation of the outermost Cu70(SR)55 cage with a flower-shaped surface pattern (Fig. 3). It is rationalized that the high symmetry (D5h) of the perfect Cu-thiolate cage is related to the highly coordinated sulfur atoms (μ3 or μ4), note: no lower coordinated thiolate (μ2) is involved in [Au52Cu72(SR)55]+ as in thiolate-protected homogold NPs19. This means that staple motifs or bridging thiolates—which are commonly observed in thiolate-protected gold NPs—are eliminated in the Cu-thiolate cage observed in the current work. It should be noted that the S atoms at the five tips of the flower petals are all coordinated with two Cu atoms and one Au atom inside. As a result, the cage is fixed well on the inner decahedron with much less relaxation, so as to maintain the horizontal mirror plane, but such a plane is missing in all gold cases16,17,18,19,23, thus, the [Au52Cu72(SR)55]+ nanoalloy remains achiral as the kernel is.
The highly regular structure of the Cu70S55 cage along the C5 axis reminds us of a Penrose tiling, an important mathematical and architectural model of aperiodic tessellations exhibiting both reflectional and rotational symmetry but without translational symmetry. The Penrose tiling (Fig. 3 right) has two sets of congruent prototiles, i.e., a fat rhombus (light pink) and a thin rhombus (dark pink). When we mark the joint points with solid circles representing Cu (orange) and S (yellow) atoms, respectively, they match very well with the Cu-thiolate cage except some distortions at the perimeter, probably because the Penrose tiling is a two-dimensional (2D) plane, while the cage on the particle is a 3D structure. The stereoscopic construction of perfection revealed by intrinsic packing pattern of atoms demonstrates how the aperiodic close-packing can evolve from two-dimension to three-dimension.
Assembly of [Cu72Au52(SR)55]+Cl− into single crystals
We further discuss the effect of the high D5h symmetry on the particle assembly. In previous work, intriguing C–H···π interactions were observed between the ligands of neighboring particles, i.e., the interaction of C–H bonds from the phenyl rings or the methyl groups with the π electrons of the phenyl rings19. Specifically, side-by-side and point-to-point stackings of ligands were found in Au246(SR)8019, and the herringbone pattern of ligands led to a zigzag arrangement of particles in the crystal of Au103S2(SR)4117. Such patterns are essential to stabilize the macroscopic crystals because of much closer packing of particles into a regular pattern in the crystal. When the particle has an even higher symmetry than Au246(SR)80, we expect such interactions to be even stronger. Indeed, we found that in the assembly of [Cu72Au52(SR)55]+ particles into macroscopic crystals, the interparticle distances are as short as ~2.6 nm, much smaller than the diameter of the pentagon (~3.0 nm, Supplementary Fig. 11), which implies significant C–H···π interactions.
As shown in Fig. 4a, the four nearest particles are marked by different colors. For the two particles arranged along the x direction, their ligands show a tip-to-edge interaction (green and pink particles, or light orange and blue particles); while for the two particles arranged along the z direction, their ligands show an edge-to-edge interaction (green and blue particles, or light orange and pink particles). The two particles at diagonal positions approach each other in a tip-to-tip manner (green and light orange particles). Figure 4b, c shows the four particles from different views. Such interweaving interactions remind us of the “quadruple-gear” meshing mechanism in which the rotation of any of the gears would lead to an integrated movement. However, the two particles at diagonal positions (green and light orange particles) also show C–H···π interactions between their ligands, making the four gears interlock with each other (Fig. 4d).
To be specific, the two approaching particles in an edge-to-edge assembly show significant intra- and interparticle interactions of the ligands (Supplementary Fig. 12). Interestingly, the intraparticle interactions (indicated by blue dashed lines) are all coming from methyl H to phenyl ring, while the interparticle interactions (indicated by yellow dashed lines) are all originated from phenyl ring H to phenyl ring. On a whole, the six ligands belong to two particles form a triangular mosaic pattern, which is even more compact than the herringbone pattern17. The interparticle interactions between phenyl ring H and phenyl ring for the two tip-to-tip approaching particles are shown in Supplementary Fig. 13. As for the two particles approaching each other in a tip-to-edge way, interparticle and intraparticle interactions can be observed as well, and consistently, the intraparticle interactions are still from phenyl ring H to phenyl ring interaction, while the interparticle interaction is due to the close methyl H to phenyl ring (Supplementary Fig. 14).
Moreover, this interlock pattern can repeat along the x-axis to a long range (Supplementary Fig. 15), but along y- or z-axis (Supplementary Figs. 14 and 15) the particles belonging to different sets have essentially no interaction. Thus, the single crystal can be considered as a bunching of nanowires along the x-axis, and each nanowire (marked in circles) is composed of two lines (x and x’, Supplementary Figs. 16 and 17) of highly connected particles, resembling fibrous construction, which might endow the single crystal with interesting anisotropic electrical transport properties38. It is expected that such twin nanowires with larger cross-sections would increase the conductivity of the material for potential applications.
Discussion
The Au52Cu72(p-MBT)55 nanoalloy offers several important features and implications. The first feature is the unusual behavior that two copper atoms occupy the central positions of this nanoalloy. Such a localization is interesting and differs from the random Au1-xCux alloys as well as the ordered phases of Au3Cu1, Au1Cu1, and Au1Cu3 in bulk and nanoparticles39,40. Localization of the two Cu atoms is critical to realize a perfect decahedral kernel without truncation, i.e., the innermost Au5Cu2 dictating a subsequent decahedral Au47 shell, in contrast with the Au7 kernel dictating a truncated Au42 shell in homogold systems. A scrutiny of local bond distances and a comparison with Marks-decahedra in homogold systems reveal that the two copper atoms shorten the overall length of the central axis so as to give enough space for the five Au atoms at the corners of the pentagon, but this is not the case in homogold NPs. Such atomic-level insights for the truncation of Marks decahedron are unprecedented, which could not be obtained by high-resolution electron microscopy analysis41,42. Future work may explore the Au:Cu ratio to tailor the composition and investigate the potential Cu-atom number dependent structural reorganization predicted by theoretical simulations42.
The Au52Cu72 nanoalloy also exhibits the highest symmetry (D5h) among the reported ones so far. The particle shape is indeed not predicted for Au–Cu alloy nanoparticles, as the most stable shapes were predicted to be dodecahedron, truncated octahedron, and icosahedron regardless of the composition and the size of the nanoalloy28. With respect to the surface protection of Au52Cu72, the discovered Cu surface is also opposite to the earlier prediction of Cu(core)-Au(surface) for all sizes and shapes of nanoparticles43. Previous calculations showed that the lower surface energy of Au should render Au segregation to the surface in AuCu alloys while copper segregates at subsurface sites44. We rationalize that the opposite trend observed in our work should be due to the Cu-S bonding, because the previous prediction considered only the fact that the larger Au atom than Cu requires fewer atoms in covering the particle surface, hence, fewer broken bonds and lower surface energy43. The outmost shell—an extended, rigid Cu70(SR)55 cage—resembles an aesthetic 3D Penrose tiling, which is the key to retain the high symmetry. Compared to the commonly observed discrete staple motifs or bridging thiolates made up of low-coordinated μ2-S in Au-thiolate systems, the Cu-thiolate cage is composed of all highly coordinated sulfur (μ3 or μ4), with very little relaxation, and thus, making this nanoalloy achiral.
Intraparticle and interparticle interactions of ligands are found to be significant in the assembly of [Au52Cu72(p-MBT)55] due to its unique symmetry, and a “quadruple-gear” interlocking pattern is observed among four nearest particles. Such mechanistic insights provide implications on how to tailor the particle symmetry in order to control the macroscopic assembly, and the obtained single crystals may find important applications involving anisotropic electron transport properties.
Alloying of copper with gold also largely improves the stability, which has been widely adopted in nanoscience research43, but atomic-level information of Cu distributions (i.e., kernel vs surface) could not be obtained in earlier work. The atomically resolved Au52Cu72 nanoalloy may provide a model for rationalization of the structure, stability, synergism, and catalytic properties of Au–Cu alloy nanoparticles28,45.
Overall, the Au52Cu72 nanoalloy demonstrates on how to manipulate the kernel shape of Au by heterometal incorporation and emphasizes the relationship between the particle symmetry and particle assembly at the atomic level, which is expected to open up new opportunities in future research on Au–Cu alloys and their applications in catalysis, photonics, antibacterial, and biomedicine fields38,39,40,41,42,43,44,45,46.
Methods
Reagents
All reagents and solvents were commercially available and used as received without further purification, including para-methylphenylthiophenol (C7H7SH, ≥99.9%, Aladdin), 4-tert-butylbenzenethiol (TBBT, 97%, Alfa Aesar), tetrachloroauric(III) acid (HAuCl4·3H2O, ≥99.99% metals basis, Energy Chemical), tetraoctylammonium bromide (TOAB, ≥98%, Aladdin), sodium borohydride (NaBH4, ≥98%, Energy Chemical), dichloromethane (CH2Cl2, ≥98%, Aladdin), toluene (C6H6, HPLC, Aladdin), methanol (CH3OH, ≥98%, Aladdin), ethanol (CH3CH2OH, ≥98%, Aladdin), triphenylphosphine (PPh3, ≥98.8%, Energy Chemical), copper chloride (II) (CuCl2, ≥98.8%, Alfa Aesar), acetonitrile (CH3CN, ≥98%, Aladdin), and pure water.
Synthesis of [Au52Cu72(p-MBT)55]+ nanoalloys
Briefly, 0.10 g HAuCl4·3H2O was dissolved in 5 mL nanopure water, and 0.16 g TOAB was dissolved in 30 mL toluene. The two solutions were combined in a 100 mL tri-neck round bottom flask. The solution was vigorously stirred (~1100 rpm) with a magnetic stir bar to facilitate phase transfer of Au(III) salt into the organic phase. After ~30 min, phase transfer was completed, leaving a clear aqueous phase at the bottom of the flask, which was then removed. After that, 0.10 g PPh3 was added into the dichloromethane solution of Au(III), and the color of the solution changed from orange to colorless. Then, 0.25 g CuCl2 was added to a dichloromethane solution. Subsequently, 15 mL CH3H2OH was added into the solution. After ~30 min, 0.35 mL p-MBT was added to the solution. After ~1 h, 5 mL aqueous solution of NaBH4 (150 mg) was rapidly added. The reaction was allowed to proceed for ~16 h. The product was washed several times with CH3CN to remove the redundant thiol, PPh3 and by-products until the optical absorption spectrum showed distinct peaks, which gave rise to pure [Cu72Au52(p-MBT)55]Cl nanoalloys. It is worth noting that the [Au52Cu72(TBBT)55]+ nanoalloy was obtained with the same method simply by changing the ligand from p-MBT to TBBT. The [Cu72Au52(p-MBT)55]SbF6 nanoalloy with different counterion (SbF6−) was also obtained by adding a methanol solution of NaSbF6 (5 mg) to a CH2Cl2 solution of [Cu72Au52(p-MBT)55]Cl (10 mg), followed by thorough mixing and then washing several times with CH3CN/H2O (v: v = 2: 1) to remove the redundant Na+, Cl−, and excess SbF6− ions.
Characterization
UV-vis spectral measurements were carried out with an Agilent HP8453 diode array spectrometer. The crystals of Au52Cu72 nanoalloys were re-dissolved in dichloromethane (CH2Cl2) for spectral measurements. TGA was carried out on a thermogravimetric analyzer (DTG-60H, Shimadzu Instruments, Inc.) with 4.613 mg of particles in a SiO2 pan at a heating rate of 10 °C min−1 in an Ar atmosphere. Two or three crystals of Au52Cu72 were picked and dissolved 0.5 mL fresh aqua regia. After ultrasonic dissolution for 3 h, the 5 ml ultrapure water was added into above solution, which can be used to test with ICP-MS. Electrospray ionization (ESI) mass spectra were acquired using a Bruker Q-TOF mass spectrometer equipped with ESI source. The sample was dissolved in methylbenzene (~1 mg ∙ ml−1) and then mixed with a dry methanol solution of CsOAc (30 mM) by a 3:1 vol ratio. The sample was infused at 180 μL ∙ h−1 directly. The source temperature was kept at 50 °C with the spray voltage kee** at 4 kV.
X-ray crystallographic determination
Black, rod-like crystals were obtained by diffusing CH3CN into a toluene solution of NPs for 6–10 days at 10 °C. A suitable crystal was selected and performed on a Bruker D8 Venture with GaKα radiation (λ = 1.34139 Å). The crystal was kept at 123 K during data collection. Using Olex247, the structure was solved with the olex2.solve48 structure solution program using Charge Flip** and refined with the olex2.refine49 refinement package using Gauss-Newton minimisation. All the Au, Cu, Cl, and S atoms were found directly. Remaining nonhydrogen atoms were generated via subsequent difference Fourier syntheses. All the nonhydrogen atoms were refined anisotropically. The center was resided by two Cu atoms, and their ellipsoids largely extend and are abnormal when partially occupied by Au atoms. The peripheral atoms possess the same conditions. All the hydrogen atoms were set in geometrically calculated positions and refined isotropically using a riding model. The diffuse electron densities resulting from the residual solvent molecules were removed from the dataset using the SQUEEZE routine of PLATON and refined further using the data generated.
Crystal data
for C385H385Au52ClCu72S55 (M = 21627.80 g mol−1): triclinic, space group P-1 (no. 2), a = 25.8880(19) Å, b = 26.7997(18) Å, c = 45.013(3) Å, α = 79.828(2)°, β = 85.255(3)°, γ = 69.166(2), V = 28722(3) Å3, Z = 2, T = 123(2) K, μ(GaKα) = 32.088 mm−1, Dcalc = 2.501 g cm−3, 356930 reflections measured (1.736° ≤ 2θ ≤ 110.384°), 108535 unique (Rint = 0.0445, Rsigma = 0.0475), which were used in all calculations. The final R1 was 0.0388 (I > 2σ(I)) and wR2 was 0.1206 (all data).
Data availability
The X-ray crystallographic coordinates for structures reported in this study (see Supplementary Table 1 and Supplementary Data 1) have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number CCDC 1875255. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
References
Langille, M. R., Zhang, J., Personick, M. L., Li, S. & Mirkin, C. A. Stepwise evolution of spherical seeds into 20-fold twinned icosahedra. Science 337, 954–957 (2012).
Qi, X., Chen, Z., Yuan, T. & Fichthorn, K. A. Growth mechanism of five-fold twinned Ag nanowires from multiscale theory and simulations. ACS Nano 13, 4647–4656 (2019).
Song, M., Wu, Z., Lu, N. & Li, D. Strain relaxation-induced twin interface migration and morphology evolution of silver nanoparticles. Chem. Mater. 31, 842–850 (2019).
Ino, S. & Ogawa, S. Multiply twinned particles at earlier stage of gold film formation on alkalihalide crystals. J. Phys. Soc. Jpn. 22, 1365–1374 (1967).
Marks, L. D. & Howie, A. Multiply-twinned particles in silver catalysts. Nature 282, 196–198 (1979).
Marks, L. D. Surface structure and energetics of multiply twinned particles. Philos. Mag. A 49, 81–93 (1984).
Marks, L. D. Modified Wulff constructions for twinned particles. J. Cryst. Growth 61, 556–566 (1983).
Marks, L. D. & Peng, L. Nanoparticle shape, thermodynamics and kinetics. J. Phys. Condens. Matter 28, 053001 (2016).
**, R. et al. Photoinduced conversion of silver nanospheres to nanoprisms. Science 294, 1901–1903 (2001).
Zhou, S., Zhao, M., Yang, T.-H. & **a, Y. Decahedral nanocrystals of noble metals: synthesis, characterization, and applications. Mater. Today 22, 108–131 (2019).
Seo, D. et al. Shape adjustment between multiply twinned and single-crystalline polyhedral gold nanocrystals: Decahedra, icosahedra, and truncated tetrahedra. J. Phys. Chem. C. 112, 2469–2475 (2008).
Cathcart, N., Coombs, N., Gourevich, I. & Kitaev, V. Synthesis and sensing properties of D5h pentagonal silver star nanoparticles. Nanoscale 8, 18282–18290 (2016).
Cleveland, C. L. et al. Structural evolution of smaller gold nanocrystals: the truncated decahedral motif. Phys. Rev. Lett. 79, 1873–1876 (1997).
**, R., Zeng, C., Zhou, M. & Chen, Y. Atomically precise colloidal metal nanoclusters and nanoparticles: fundamentals and opportunities. Chem. Rev. 116, 10346–10413 (2016).
Bonacic-Koutecky, V. & Antoine, R. Enhanced two-photon absorption of ligated silver and gold nanoclusters: theoretical and experimental assessments. Nanoscale 11, 12436–12448 (2019).
Jadzinsky, P. D., Calero, G., Ackerson, C. J., Bushnell, D. A. & Kornberg, R. D. Structure of a thiol monolayer-protected gold nanoparticle at 1.1 Å resolution. Science 318, 430–433 (2007).
Higaki, T. et al. Tailoring the structure of 58-electron gold nanoclusters: Au103S2(S-Nap)41 and its implications. J. Am. Chem. Soc. 139, 9994–10001 (2017).
Chen, Y. et al. Crystal structure of barrel-shaped chiral Au130(p-MBT)50 nanocluster. J. Am. Chem. Soc. 137, 10076–10079 (2015).
Zeng, C., Chen, Y., Kirschbaum, K., Lambright, K. J. & **, R. Emergence of hierarchical structural complexities in nanoparticles and their assembly. Science 354, 1580–1584 (2016).
Patala, S., Marks, L. D. & de la Cruz, M. O. Thermodynamic analysis of multiply twinned particles surface stress effects. J. Phys. Chem. Lett. 4, 3089–3094 (2013).
Johnson, C. et al. Effects of elastic anisotropy on strain distributions in decahedral gold nanoparticles. Nat. Mater. 7, 120–124 (2008).
Patala, S., Marks, L. D. & de la Cruz, M. O. Elastic strain energy effects in faceted decahedral nanoparticles. J. Phys. Chem. C 117, 1485–1494 (2013).
Yan, N. et al. Unraveling the long-pursued Au144 structure by x-ray crystallography. Sci. Adv. 4, eaat7259 (2018).
Whetten, R. L. et al. Chiral-icosahedral (I) symmetry in ubiquitous metallic cluster compounds (145A, 60X): structure and bonding principles. Acc. Chem. Res. 52, 34–43 (2019).
Zhou, M. et al. Three-orders-of-magnitude variation of carrier lifetimes with crystal phase of gold nanoclusters. Science 364, 279–282 (2019).
Hu, G., **, R. & Jiang, D. Beyond the staple motif a new order at the thiolate-gold interface. Nanoscale 8, 20103–20110 (2016).
Okamoto, H., Chakrabarti, D. J., Laughlin, D. E. & Massalski, T. B. The Au−Cu (gold-copper) System. J. Phase Equilib. 8, 454–474 (1987).
Guisbiers, G. et al. Gold–copper nano-alloy, “Tumbaga”, in the era of nano: Phase diagram and segregation. Nano Lett. 14, 6718–6726 (2014).
Dass, A., Nimmala, P. R., Jupally, V. R. & Kothalawala, N. Au103(SR)45, Au104(SR)45, Au104(SR)46 and Au105(SR)46 nanoclusters. Nanoscale 5, 12082–12085 (2013).
Yang, H. et al. Plasmonic twinned silver nanoparticles with molecular precision. Nat. Commun. 7, 12809 (2016).
Song, Y. et al. Large-scale synthesis, crystal structure, and optical properties of the Ag146Br2(SR)80 nanocluster. ACS Nano 12, 9318–9325 (2018).
Nair, L. V. et al. Hetero-biicosahedral [Au24Pd(PPh3)10(SC2H4Ph)5Cl2]+ nanocluster: selective synthesis and optical and electrochemical properties. Nanoscale 10, 18969–18979 (2018).
Walter, M. et al. A unified view of ligand-protected gold clusters as superatom complexes. PNAS 105, 9157–9162 (2008).
Ringe, E., Van Duyne, R. P. & Marks, L. D. Wulff construction for alloy nanoparticles. Nano Lett. 11, 3399–3403 (2011).
Yang, H. et al. Structural evolution of atomically precise thiolated bimetallic [Au12+nCu32(SR)30+n]4− (N = 0, 2, 4, 6) nanoclusters. J. Am. Chem. Soc. 136, 7197–7200 (2014).
Negishi, Y., Munakata, K., Ohgake, W. & Nobusada, K. Effect of copper do** on electronic structure, geometric structure, and stability of thiolate-protected Au25 nanoclusters. J. Phys. Chem. Lett. 3, 2209–2214 (2012).
Peng, L., Van Duyne, R. P., Laurence, D. & Marks, L. D. Strain-induced segregation in bimetallic multiply twinned particles. J. Phy. Chem. Lett. 6, 1930–1934 (2015).
Li, Q. et al. Modulating the hierarchical fibrous assembly of Au nanoparticles with atomic precision. Nat. Commun. 9, 3871 (2018).
Ferrando, R., Jellinek, J. & Johnston, R. L. Nanoalloys: From theory to applications of alloy clusters and nanoparticles. Chem. Rev. 108, 845–910 (2008).
Prunier, H. et al. New insights into the mixing of gold and copper in a nanoparticle from a structural study of Au–Cu nanoalloys synthesized via a wet chemistry method and pulsed laser deposition. Phys. Chem. Chem. Phys. 17, 28339–28346 (2015).
Casillas, G., Velázquez-Salazar, J. J. & Jose-Yacaman, M. A new mechanism of stabilization of large decahedral nanoparticles. J. Phys. Chem. C 116, 8844–8848 (2012).
Pauwels, B., Tendeloo, G. V., Zhurkin, E. & Hou, M. Transmission electron microscopy and monte carlo simulations of ordering in Au-Cu clusters produced in a laser vaporization source. Phys. Rev. B 63, 165406 (2001).
Thota, S., Wang, Y. & Zhao, J. Colloidal Au–Cu alloy nanoparticles: synthesis, optical properties and applications. Mater. Chem. Front. 2, 1074–1089 (2018).
Völker, E., Williams, F. J., Calvo, E. J., Jacob, T. & Schiffrin, D. J. O2 induced Cu surface segregation in Au–Cu alloys studied by angle resolved XPS and DFT modelling. Phys. Chem. Chem. Phys. 14, 7448–7455 (2012).
Xu, Z., Lai, E., Shao-Horn, Y. & Hamad-Schifferli, K. Compositional dependence of the stability of AuCu alloy nanoparticles. Chem. Commun. 48, 5626–5628 (2012).
Bracey, C. L., Ellis, P. R. & Hutchings, G. J. Application of copper–gold alloys in catalysis: current status and future perspectives. Chem. Soc. Rev. 38, 2231–2243 (2009).
Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Cryst. 42, 339–341 (2009).
George, M. S. SHELXT – Integrated space-group and crystalstructure determination. Acta Cryst. A71, 3–8 (2015).
George, M. S. Crystal structure refinement with SHELXL. Acta Cryst. C71, 3–8 (2015).
Acknowledgements
Y.S. acknowledges financial support by the NSFC (Grant 21801001). M.Z. acknowledges financial support by the NSFC (Grants 21871001 and 21631001), the Ministry of Education, and the Education Department of Anhui Province.
Author information
Authors and Affiliations
Contributions
Y.S. conceived and carried out experiments. Y.S. and Y.L. analyzed the data and wrote the paper. H.L., F.K. and C.Z. assisted in the synthesis and optical/mass spectral measurements. J.X. and P.L. carried out the TGA and ICP analyses. M.Z. and R.J. designed the project, analyzed the data, and wrote the paper. All authors contributed to the writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer Review Information Nature Communications thanks Laurence Marks, Bo Li and the other anonymous reviewer for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Song, Y., Li, Y., Li, H. et al. Atomically resolved Au52Cu72(SR)55 nanoalloy reveals Marks decahedron truncation and Penrose tiling surface. Nat Commun 11, 478 (2020). https://doi.org/10.1038/s41467-020-14400-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-020-14400-2
- Springer Nature Limited
This article is cited by
-
Atomically precise copper dopants in metal clusters boost up stability, fluorescence, and photocatalytic activity
Communications Chemistry (2023)
-
Layer-by-layer alloying of NIR-II emissive M50 (Au/Ag/Cu) superatomic nanocluster
Nano Research (2022)