Abstract
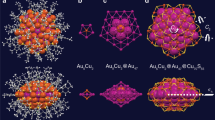
Although the hollow icosahedral M12 kernel has been extensively observed in metal nanoclusters, its origin remains a mystery. Here we report a reasonable avenue for the generation of the hollow icosahedron: the kernel collapse from several small nano-building blocks to an integrated hollow icosahedron. On the basis of the Au alloying processes from Ag28Cu12(SR)24 to the template-maintained AuxAg28-xCu12(SR)24 and then to the template-transformed Au12CuyAg32-y(SR)30, the kernel evolution/collapse from “tetrahedral Ag4 + 4∗Ag3” to “tetrahedral Au4 + 4∗M3 (M = Au/Ag)” and then to “hollow icosahedral Au12” is mapped out. Significantly, the “kernel collapse” from small-sized nano-building blocks to large-sized nanostructures not only unveils the formation of hollow icosahedral M12 in this work, but also might be a very common approach in constructing metallic kernels of nanoclusters and nanoparticles (not limited to the M12 structure).
Similar content being viewed by others
Introduction
Metal nanoclusters are an emerging class of modular nanomaterials1,2,3,4,5,6, and have been sparking great research interests owing to their atomically precise structures and intriguing properties7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27. The physiochemical properties of these nanomaterials, such as chirality, luminescence, catalysis, magnetism, and electrochemistry, can be rationalized in terms of their quantum size effect as well as discrete electronic states28,29,30,31,32,33,34,35,36,37,38. Besides, the atomically precise nature of these modular nanomaterials is of the most interest — indeed, compared with large-sized nanoparticles, nanoclusters (typically <2 nm of the metallic kernel) present more precise compositions/constructions, and thus allow for the atomic-level elucidation of structural evolutions and structure-property correlations1,2,3,4,5,6,39,40,41,42,43,44,45,46.
Of all reported nanoclusters with precise structures, the icosahedral configuration is the most typical, which is frequently observed in both metal kernels and ligand shells of nanoclusters47,48,49,50,51. Interestingly, except for the non-hollow icosahedral M1@M12 kernel (M represents the metal), the hollow icosahedral M12 kernel has also served as a basic nano-building block of nanoclusters (e.g., Ag44(SR)30, Au12+nCu32(SR)30+n, Ag50(dppm)6(SR)30, Au144(SR)60, etc.)10,11,52,53,54,55,56. Structurally, it is accepted that the non-hollow icosahedron might be more energetically favorable than the corresponding hollow one due to the extra 12 metal···metal interactions in M1@M12; accordingly, the hollow icosahedral kernel is unlikely to arise in the initial stage of the nanocluster growth. Besides, the hollow M12 kernel is also less likely to originate from its non-hollow counterpart because the 12 metal···metal interactions make it difficult to extract the innermost metal atom out. In this context, the origin of such hollow icosahedral kernels remains a mystery.
In this work, based on the Au-alloying-induced nanocluster transformation from M40(SR)24 to M44(SR)30 (M = Au/Ag/Cu), a reasonable avenue for the generation of hollow icosahedral M12 kernels has been mapped out, i.e., the kernel collapse from several small nano-building blocks to an integrated hollow icosahedron. The proposed avenue might serve as a common approach in constructing metallic kernels of nanoclusters and nanoparticles (not limited to the M12 structure).
Results
Structural anatomy of M40(SR)24 and M44(SR)30 nanoclusters
For the clarity of the structural transformation and the corresponding kernel collapse, the nanocluster structures involved in this work are first discussed (Fig. 1) —
-
(i)
M40(SR)24 (M = Au/Ag/Cu; SR = SPhCl2): the M40(SR)24 nanoclusters start from the bi-metallic Ag28Cu12(SR)2457. Figure 1a–d depict the structure anatomy of Ag28Cu12(SR)24. Ag28Cu12(SR)24 adopts a three-shell configuration, in a form of Ag4(M40-S1)@Ag24(M40-S2)@4*Cu3(SR)6(M40-S3). The innermost Ag4 is in tetrahedral (Fig. 1a). The 24 Ag atoms on M40-S2 can be divided into two categories, and each of 12 Ag atoms constitute four Ag3 triangles (Fig. 1b). The Ag atoms highlighted in dark blue connect with inward SR ligands on M40-S3 (Fig. 1c, highlighted in yellow); in contrast, the Ag atoms in light blue links outward SR ligands (highlighted in red) on M40-S3.
-
(ii)
M44(SR)30 (M = Au/Ag/Cu; SR = SPhCl2): the Au12Ag32(SR)30 nanocluster is adopted to analyze the structure of M44(SR)3058. Au12Ag32(SR)30 also has a three-shell configuration: Au12(M44-S1)@Ag20(M44-S2)@6*Ag2(SR)5(M44-S3). Of note, the Au12 kernel is a hollow icosahedron (Fig. 1e).
a–d Structure anatomy of M40(SPhCl2)24: a M40-S1: the tetrahedral M4 kernel; b M40-S2: the M24 shell; c M40-S3: the Cu12(SR)24 surface; and d The overall structure of M40(SPhCl2)24. e–h Structure anatomy of M44(SPhCl2)30. e M44-S1: the hollow icosahedral M12 kernel; f M44-S2: the M20 shell; g M40-S3: the M12(SR)30 surface; and h The overall structure of M44(SPhCl2)30. Color labels: orange/light blue/blue/green, Ag or Cu atoms at different positions; red, S. All C and H atoms are omitted for clarity.
Au-alloying-induced transformation from M40(SR)24 to M44(SR)30
The Au-alloying structural transformation started from the bi-metallic Ag28Cu12(SR)24 (Fig. 2). The slight Au alloying on Ag28Cu12(SR)24 resulted in a tri-metallic AuxAg28-xCu12(SR)24 (x = 1.32) nanocluster, wherein the tetrahedral Ag4 kernel of Ag28Cu12(SR)24 was partially alloyed by the incorporated Au (Fig. 2 and Supplementary Fig. 1). When more Au heteroatoms were doped into M40(SR)24 (AuxAg28-xCu12(SR)24, x = 7.56; Fig. 2), all sites of the tetrahedron were entirely occupied by Au; besides, the redundant Au heteroatoms were further arranged onto M40-S2, invading the Ag sites that related to outward SR thiols (light blue triangles in Fig. 1b and Supplementary Figs. 2–3). Of note, throughout the abovementioned Au-alloying processes the M40(SR)24 framework retained. Furthermore, the overdose of Au heteroatom induced the structural transformation from M40(SR)24 to M44(SR)30. Structurally, from the crystal structure of Au12CuyAg32-y(SR)30 (y = 0–6; Avg. 3.74; Fig. 2 and Supplementary Fig. 4), the M44(SR)30 nanocluster reached its stable state when Cu atoms only occupied the M44-S3. The corresponding bond lengths in M40(SR)24 (including Ag28Cu12(SR)24, AuxAg28-xCu12(SR)24 (x = 1.32), AuxAg28-xCu12(SR)24 (x = 7.56), and Au4Ag24Cu12(SR)24) or M44(SR)30 (including Au12Ag32(SR)30 and Au12CuyAg32-y(SR)30, y = 3.74) nanoclusters were compared in detail (Supplementary Figs. 5–6 and Supplementary Tables 1–2).
Step I contains the Au-do** processes from Ag28Cu12(SR)24 to AuxAg28-xCu12(SR)24 (x = 1.32) and then to AuxAg28-xCu12(SR)24 (x = 7.56), in which processes the M40(SR)24 framework retains. Step II is the Au-do** process from AuxAg28-xCu12(SR)24 (x = 7.56) to Au12CuyAg32-y(SR)30 (y = 3.74), in which process the M40(SR)24 framework transforms into M44(SR)30 (M = Au/Ag/Cu). The Au proportion gradually increases with the nanocluater evolution from Ag28Cu12(SR)24 to AuxAg28-xCu12(SR)24 (x = 1.32), AuxAg28-xCu12(SR)24 (x = 7.56), and Au12CuyAg32-y(SR)30 (y = 3.74). Color labels: light blue, Ag; orange, Au; green, Cu; red, S. All C and H atoms are omitted for clarity.
Despite our repeated best efforts to obtain the crystal structure of Au12CuyAg32-y(SR)30, its perfect crystal data remains unavailable. Herein, for acquiring an excellent crystal data of Au12CuyAg32-y(SR)30, we promoted the co-crystallization between the M44(SR)30 and a small-sized M40(SR)24 nanoclusters (Supplementary Figs. 7–9). Fortunately, the structures of both two nanoclusters were excellently determined (Au4Ag24Cu12(SR)24 and Au12CuyAg32-y(SR)30 (y = 3.74)) although both displayed strongly negative valence state (i.e., “−4”). In this context, the presence of (PPh4)+ cations neutralized the electrostatic repulsion between these clusters (Supplementary Fig. 8) and thus promoted the nanocluster co-crystallization, which was different from previously reported co-crystallized nanocluster cases with both “0” or opposite valence states59,60,61,62. In the crystal lattice of the co-crystallized nanoclusters, three types of nanoclusters were observed (Supplementary Fig. 7) — L-Au4Ag24Cu12(SR)24 (Aα, Aβ), R-Au4Ag24Cu12(SR)24 (Bα, Bβ), and Au12CuyAg32-y(SR)30 (y = 3.74; Cα, Cβ), among which the α- and β-nanoclusters were identical, but arranged in different rotation angles (Supplementary Fig. 7a–c). In contrast to the crystallization of homogeneous nanoclusters, which are typically packed into superlattices with simple translational symmetry, such as ABAB or ABCABC packing pattern40, the (AuAgCu)40 and (AuAgCu)44 nanoclusters were packed with a more complex pattern (Supplementary Fig. 7d–f). From the x-axis view, the clusters were packed with an Aα-Bα-Cα#Aβ-Bβ-Cβ pattern. α- and β-nanoclusters were arranged separately along the z-direction, giving rise to α- and β-cluster lines (Supplementary Fig. 7f). In either cluster line, the adjacent three nanoclusters constituted a repetitive unit, Aα-Bα-Cα or Aβ-Bβ-Cβ, which was labeled by red or black frames, respectively. Such repetitive units were also observed from y-axis and z-axis views (Supplementary Fig. 7).
The optical absorptions of the obtained nanocluster crystals (dissolved in CH2Cl2) were compared (Supplementary Fig. 10). Along with the Au-alloying process from Ag28Cu12(SR)24 to AuxAg28-xCu12(SR)24 (x = 1.32) and AuxAg28-xCu12(SR)24 (x = 7.56), there was no significant alteration of the optical absorptions (405, 465, and 555 nm). By contrast, when the nanocluster template transformed from M40(SR)24 to M44(SR)30, these absorptions shifted to 390, 490, and 595 nm immediately (Supplementary Fig. 10), demonstrating the remarkable change over electronic structures with the template transformation. Time-dependent UV-vis of the transformation from Ag28Cu12(SR)24 to AuxAg28-xCu12(SR)24 and then to Au12CuyAg32-y(SR)30 were performed to track the cluster transformation (Supplementary Fig. 11a). The changes of UV-vis contained two stages: (stage 1, from Ag28Cu12(SR)24 to AuxAg28-xCu12(SR)24) eight isoabsorption points at 360, 385, 420, 445, 485, 550, 570, and 640 nm were observed (Supplementary Fig. 11b); (stage 2, from AuxAg28-xCu12(SR)24 to Au12CuyAg32-y(SR)30) three isoabsorption points at 400, 485, and 510 nm were detected (Supplementary Fig. 11c). The observation of these isoabsorption points suggested that the overall cluster transformation was a proportional conversion. Accordingly, both transformations from Ag28Cu12(SR)24 to AuxAg28-xCu12(SR)24 and from AuxAg28-xCu12(SR)24 to Au12CuyAg32-y(SR)30 followed an “intramolecular rearrangement” approach, but not an “intermolecular decomposition-recombination” approach.
Besides, along with the Au-alloying process, the thermal stability of nanoclusters was enhanced. As shown in Supplementary Fig. 12, UV-vis characteristic absorptions of the Ag28Cu12(SR)24 nanocluster (dissolved in CH2Cl2) gradually decreased in intensity after 1 h and completely disappeared in ~4 h, indicating degradation. In contrast, the UV-vis absorptions of AuxAg28-xCu12(SR)24 (x = 1.32) were essentially identical in the first 2 h, and gradually decreased as time went on. Of note, the optical absorptions of AuxAg28-xCu12(SR)24 (x = 7.56) was almost retained within 24 h, suggesting the enhanced thermal stability of AuxAg28-xCu12(SR)24 (x = 7.56) over other two M40(SR)24 nanoclusters. In this context, the sequence of the thermal stability of these three M40(SR)24 nanoclusters was determined as AuxAg28-xCu12(SR)24 (x = 7.56) > AuxAg28-xCu12(SR)24 (x = 1.32) > Ag28Cu12(SR)24; that is, increasing the Au-do** amount in nanoclusters was in favor of preparing M40(SR)24 with higher thermal stability.
Electrospray ionization mass spectrometry (ESI-MS) was then performed on nanocluster crystals (dissolved in CH2Cl2), and the mass results confirmed the compositions of these “−4”-charged M40(SR)24 and M44(SR)30 nanoclusters (Supplementary Fig. 13). Besides, the in-situ Au-alloying process and the nanocluster template transformation were tracked by exploiting the ESI-MS (Supplementary Figs. 14–18). At the very beginning process (1–2 min in Supplementary Fig. 14), only AuxAg28-xCu12(SR)24 nanoclusters were detected (Fig. 2), corresponding to the Au-do** process from Ag28Cu12(SR)24 to AuxAg28-xCu12(SR)24 (x = 1.32) and AuxAg28-xCu12(SR)24 (x = 7.56). The further Au-alloying induced both the Au component growth in M40(SR)24 and the template transformation from M40(SR)24 to M44(SR)30 (3–6 min in Supplementary Fig. 14). Finally, only Au12CuyAg32-y(SR)30 nanoclusters could be observed (7–8 min in Supplementary Fig. 14), which suggested the complete transformation of nanoclusters. Of note, the Au12Ag32-yCuy(SR)30 would stable at y = 3 or 4 once generated, matching with the crystal structure of Au12CuyAg32-y(SR)30 (y = 3.74). Energy-dispersive X-ray spectroscopy (EDS) map** and X-ray photoelectron spectroscopy (XPS) were conducted to confirm the Au-alloying process (Supplementary Figs. 19–28).
Kernel transformation from tetrahedron to hollow icosahedron
Figure 3 depicts the kernel collapse from the “tetrahedral Au4 + 4*M3” to “hollow icosahedral Au12” induced by the Au alloying. Specifically, the initial Au-do** process transported the Au heteroatoms to the tetrahedral kernel, converting the Ag4 kernel to the alloyed AuxAg4-x and the final Au4 (Fig. 3a). The further Au-alloying sites on M40-S2 predominantly located at the four M3 triangles that adhered to a vertex-to-face relationship to the tetrahedral Au4 kernel (Fig. 3b and Supplementary Fig. 3c); in contrast, the other four triangles on M40-S2, following a face-to-face relationship to the Au4 tetrahedral kernel, maintained unalloyed as Ag3 (Supplementary Fig. 3d). For easily distinguishing these M3 positions, we define these Au3 positions as “stable location” (Supplementary Fig. 3c) and “unstable location” (Supplementary Fig. 3d). However, the Au do** on stable locations is simply concluded from the crystallography, and the Au positions may change throughout the crystallization process. From ESI-MS results (Supplementary Fig. 14), a maximum of 18–19 Au heteroatoms could be doped into the M40 cluster framework, >16 positions from the M4 kernel and 4*M3 stable locations; accordingly, there are other Ag positions in M40 that could be occupied by the introduced Au. X-ray absorption fine structure spectroscopy (XAFS) measurements were then performed for gras** the in-situ Au-do** process (Supplementary Figs. 29 and 30 and Supplementary Tables 3–4). The XAFS results demonstrated that the introduced Au occupied the innermost M4 tetrahedron first, and then substituted the Ag atoms in unstable locations, different from the crystal results wherein the unstable locations were maintained as undoped Ag throughout. We further crystallized this cluster sample and the crystal data suggested the Au heteroatoms on stable locations (i.e., AuxAg28-xCu12(SR)24, x = 7.76), demonstrating the intracluster Au–Ag metal exchange throughout the crystallization. In this context, we made some speculations on mass signals (Supplementary Fig. 14): the introduced Au heteroatoms occupied the innermost tetrahedron first, and then substituted Ag atoms on M40-S2 randomly; the mass signals i represented the dominant Au-occupation in stable locations, whereas the signals ii represented the unstable locations, resulting in two groups of signals in the 3-min mass spectrum (Supplementary Fig. 14). In the 3-min sample, the M40 with Au-occupation in unstable locations might be the main product by referring XAFS results. Then, the M40 clusters of signals ii would transform to M44 clusters of signals iii, and then decomposed due to their instability. By comparison, the M40 clusters of signals i were continually doped by Au and transformed to M44 clusters of signals iv finally. In this context, the driving force for the transformation from M40 to M44 was determined as the Au-alloying at unstable locations, which rendered the M40 nanoclusters unstable molecules and triggered the kernel collapse from several small nano-building blocks to an integrated hollow icosahedron.
a Crystal structure of AuxAg28-xCu12(SR)24 (x = 1.32) and its tetrahedral M4 (M = Au/Ag) kernel surrounded by four Ag3 triangles. b Crystal structure of AuxAg28-xCu12(SR)24 (x = 7.56) and its tetrahedral Au4 kernel surrounded by four M3 (M = Au/Ag) triangles. The red arrows represent the trend of kernel collapse from “tetrahedral Au4 + 4∗M3” to “hollow icosahedral Au12”. c Crystal structure of Au12CuyAg32-y(SR)30 (y = 3.74) and its hollow icosahedral Au12 kernel. Color labels: light blue, Ag; orange, Au; green, Cu; red, S. All C and H atoms are omitted for clarity.
Significantly, the further Au-alloying induced the transformation from M40(SR)24 to M44(SR)30, among which process the hollow icosahedral Au12 was generated (Fig. 3b, c). Structurally, the pre-transformed AuxAg28-xCu12(SR)24 possesses a “tetrahedral Au4 + 4*M3” kernel (M = Au/Ag with a high Au proportion). Upon the nanocluster conversion, the M3 triangles collapsed inward to the Au4 tetrahedron, and finally rearranged into the hollow icosahedral Au12 kernel in Au12CuyAg32-y(SR)30 (Fig. 3c). Of note, there are 16 metal atoms in the “tetrahedral M4 + 4*M3” kernel while the icosahedral kernel only contains 12 metal atoms; in this context, a structural rearrangement occurred in this structural and kernel transformation (indeed, the “kernel+surface” configurations between M40(SR)24 and M44(SR)30 nanoclusters are different). However, due to the existence of several isoabsorption points in the UV-vis spectra, the structure transformation from M40(SR)24 to M44(SR)30 should follow an “intramolecular rearrangement” approach, but not an “intermolecular decomposition-recombination” approach. Accordingly, it is reasonable to conjecture the formation of icosahedral M12 in M44(SR)30 as the kernel collapse from “tetrahedral Au4 + 4*M3”. Besides, all sites in the hollow icosahedron are fully occupied by Au (i.e., Au12); in vivid contrast, the non-hollow M1@M12 kernels of previously alloy clusters are always partially occupied by two or more types of metals. We proposed that the complete Au occupation of the hollow icosahedron resulted from the kernel collapse in which process only the collapse of Au atom to Au4 was the most energetically favorable.
This avenue (i.e., kernel collapse) is of great importance since it maps out a reasonable avenue for the generation of the hollow icosahedral M12 kernel in metal nanoclusters. Besides, the kernel collapse might be a very common approach in constructing metallic kernels of nanoclusters and nanoparticles (not limited to the hollow icosahedron, but also compliant to other configurations such as non-hollow icosahedron, FCC/BCC kernels, etc.), because the routine growth of several large-sized nanoclusters shell-by-shell should be not that energetically favorable. We also note that the kernel collapse should not be the unique approach for the generation of hollow icosahedra (or other structures) in metal nanoclusters and nanoparticles; other approaches may also exist and are still worth map** out.
Discussion
In summary, on the basis of the Au-alloying-induced transformation from M40(SR)24 to M44(SR)30 (M = Au/Ag/Cu), a reasonable avenue—kernel collapse—for the generation of the hollow icosahedral M12 kernel in metal nanoclusters has been mapped out. The Au alloying on Ag28Cu12(SR)24 produced template-maintained AuxAg28-xCu12(SR)24 (x = 1.32), AuxAg28-xCu12(SR)24 (x = 7.56), and template-transformed Au12CuyAg32-y(SR)30 (y = 3.74) step by step, accompanying with which processes the cluster kernel stepwisely evolved from “tetrahedral Ag4 + 4*Ag3” to “tetrahedral Au4 + 4*Ag3”, then to “tetrahedral Au4 + 4*Au3”, and finally to “hollow icosahedral Au12”. The entire process was tracked by ESI-MS, and the crystal structures of the key nodes (altogether five crystal structures) have been determined. Overall, this work presents a reasonable avenue for comprehending the generation of hollow icosahedra in metal nanoclusters, and the “structure collapse” might be a very common approach for constructing kernel structures (not limited to the hollow icosahedron) in the size growth of nanoclusters and nanoparticles.
Methods
Materials
All reagents were purchased from Sigma-Aldrich and used without further purification: silver nitrate (AgNO3, 99%, metal basis), tetrachloroauric (III) acid (HAuCl4·3H2O, 99.99% metal basis), copper(II) acetylacetonate (Cu(O2C5H7)2, 99%, metal basis), 2,4-dichlorobenzenethiol (HSPhCl2, 99%), sodium borohydride (NaBH4, 97%), tetraphenylphosphonium bromide ((PPh4)Br, 95%), dichloromethane (CH2Cl2, HPLC, Sigma-Aldrich), methanol (CH3OH, HPLC, Sigma-Aldrich), N,N-dimethylformamide (DMF, HPLC, Sigma-Aldrich), hexane (C6H6, HPLC, Sigma-Aldrich), and ethyl ether ((CH3CH2)O, HPLC, Sigma-Aldrich).
Synthesis of Au(I)-SPhCl2
For the Au(I)-SPhCl2 complexes synthesis, HAuCl4·3H2O (1 mmol) was dissolved in 5 mL CH3OH, and 2,4-dichlorobenzenethiol (500 μL, 4 mmol) was dissolved in 5 mL CH3OH and added drop-wise to the solution under vigorously stirring (~1200 rpm). After reacted for 15 min, the resulting precipitate was washed several times with hexane. Then the final product was used directly.
Synthesis of [Ag28Cu12(SPhCl2)24]4−
The Ag28Cu12(SPhCl2)24 was prepared by a literature method reported by the Zheng group with some modification57. Specifically, 60 mg of Cu(O2C5H7)2 was dissolved in 5 mL of CH3OH and 15 mL of CH2Cl2, to which 60 mg AgNO3 (dissolved in 2 mL of H2O) was added. After stirring for 20 min, 100 μL of HSPhCl2 was added in, and the reaction further processed for 30 min. Then, 30 mg NaBH4 (dissolved in 2 mL of H2O) was added in. The reaction was allowed to proceed for 5 h. After that, the aqueous layer was removed, and the mixture in the organic phase was rotavaporated under vacuum. Then 50 mL of CH3OH was used to extract the Ag28Cu12(SPhCl2)24 nanocluster, to which supernatant 20 mg of (PPh4)Br was added in. The precipitate was then washed three times by CH3OH. Then the final product, i.e., [Ag28Cu12(SPhCl2)24]4−(PPh4)4, was used directly. The yield is 35% based on the Ag element (calculated from the AgNO3).
Syntheses of [AuxAg28-xCu12(SPhCl2)24]4− (x = 1.32), [AuxAg28-xCu12(SPhCl2)24]4− (x = 7.56), and [Au12CuyAg32-y(SPhCl2)30]4− nanoclusters
These three nanoclusters were prepared from parallel Au-alloying reactions (in the same condition but were stopped at different times). Specifically, 20 mg of Ag28Cu12(SPhCl2)24 was first dissolved in 20 mL of CH2Cl2 and then 5 mg of Au(I)-SPhCl2 complexes was added in. After 2 min, 100 mL of hexane was poured in to pause the reaction; the precipitate was then dissolved in 20 mL of CH2Cl2 to yield the AuxAg28-xCu12(SPhCl2)24 (x = 1.32). Expanding the reaction time from 2 min to 3 min would produce the AuxAg28-xCu12(SPhCl2)24 (x = 7.56). Expanding the reaction time from 2 min to 8 min would produce the Au12CuyAg32-y(SPhCl2)30.
Synthesis of [Au12Ag32(SPhCl2)30]4−
The Au12Ag32(SPhCl2)30 nanocluster was prepared by a literature method reported by the Zheng group58.
Preparation of XAFS samples
In all, 10 mg of Ag28Cu12(SPhCl2)24 was dissolved in 10 mL of CH2Cl2 and then 3 mg of Au(I)-SPhCl2 complexes was added in. After 1 min, 200 mL of hexane was poured in to pause the reaction; the precipitate was then dissolved in 5 mL of CH2Cl2 to yield the AuxAg28-xCu12(SPhCl2)24 (Sample 1). In total, 10 mg of Ag28Cu12(SPhCl2)24 was first dissolved in 10 mL of CH2Cl2 and then 3 mg of Au(I)-SPhCl2 complexes was added in. After 2 min, 200 mL of hexane was poured in to pause the reaction; the precipitate was then dissolved in 5 mL of CH2Cl2 to yield the AuxAg28-xCu12(SPhCl2)24 (Sample 2). Single crystals of XAFS Sample 2 (i.e., AuxAg28-xCu12(SPhCl2)24, x = 7.76) were cultivated at room temperature by vapor diffusing the ethyl ether into the DMF solution of nanoclusters.
Crystallization of [Ag28Cu12(SPhCl2)24]1(PPh4)4, [AuxAg28-xCu12(SPhCl2)24]1(PPh4)4 (x = 1.32), [AuxAg28-xCu12(SPhCl2)24]1(PPh4)3 (x = 7.56), [AuxAg28-xCu12(SPhCl2)24]1(PPh4)4 (x = 7.76), [Au12CuyAg32-y(SPhCl2)30]4− and [Au12Ag32(SPhCl2)30]1[N(C4H9)4]4 nanoclusters
Single crystals of these nanoclusters were cultivated at room temperature by vapor diffusing the ethyl ether into the DMF solution of them. After 21 days, black crystals were collected, and the structures of these nanoclusters were determined. The CCDC number of [Ag28Cu12(SPhCl2)24]1(PPh4)4 is 2009375; the CCDC number of [AuxAg28-xCu12(SPhCl2)24]1(PPh4)4 (x = 1.32) is 2009456; the CCDC number of [AuxAg28-xCu12(SPhCl2)24]1(PPh4)3 (x = 7.56) is 2009457; the CCDC number of [AuxAg28-xCu12(SPhCl2)24]1(PPh4)4 (x = 7.76) is 2083130; the CCDC number of [Au12CuyAg32-y(SPhCl2)30]4− is 2009378; and the CCDC number of [Au12Ag32(SPhCl2)30]1[N(C4H9)4]4 is 1936551. Of note, the perfect crystal data of [Au12CuyAg32-y(SPhCl2)30]4− remained unavailable despite our repeated efforts, and we only got its kernel structure (i.e., Au12CuyAg32-yS30) while the peripheral C, H, and Cl atoms were hard to determine.
Co-crystallization between M40(SPhCl2)24 and M44(SPhCl2)30 nanoclusters ([Au4Ag24Cu12(SR)24]2[Au12CuyAg32-y(SR)30]1, y = 3.74)
In all, 20 mg of Au12CuyAg32-y(SPhCl2)30 (8-min sample in Supplementary Fig. 8) and 20 mg of AuxAg28-xCu12(SPhCl2)30 (2-min sample in Supplementary Fig. 14) were dissolved in 5 mL of DMF. Single crystals of the co-crystallized nanoclusters were cultivated at room temperature by vapor diffusing the ethyl ether into the DMF solution. After 21 days, black crystals were collected, and the structure of the co-crystallized nanoclusters was determined. The CCDC number of the co-crystallized [Au4Ag24Cu12(SR)24]2[Au12CuyAg32-y(SR)30]1 (y = 3.74) is 2009377.
Time-dependent ESI-MS of the Au alloying process on Ag28Cu12(SPhCl2)24
In total, 20 mg of Ag28Cu12(SPhCl2)24 was firstly dissolved in 20 mL of CH2Cl2 and then 5 mg of Au(I)-SPhCl2 complexes (powder) was added in. The ESI-MS measurement of the reaction was performed every minute.
X-ray absorption fine structure spectroscopy measurements
XAFS measurements at the Au L3-edge (11919 eV) were performed at the beamline BL14W1 station of the Shanghai Synchrotron Radiation Facility (SSRF), China. The storage ring of the SSRF was working at an energy of 3.5 GeV with an average electron current of 300 mA. The hard X-ray was monochromatized with a Si (311) monochromator. XAFS data were collected in the transmission mode in the energy range from 200 below to 1000 eV above the Au L3-edge. The acquired XAFS data were processed according to the standard procedures using the ARTEMIS module implemented in the IFEFFIT software packages.
X-ray crystallography
For the crystal date of Ag28Cu12(SPhCl2)24, AuxAg28-xCu12(SPhCl2)24 (x = 1.32), Au12CuyAg32-y(SPhCl2)30, AuxAg28-xCu12(SPhCl2)24 (x = 7.76), and the co-crystallized [Au4Ag24Cu12(SR)24]2[Au12CuyAg32-y(SR)30]1 (y = 3.74): the data collection for single-crystal X-ray diffraction was carried out on Stoe Stadivari diffractometer under nitrogen flow, using graphite-monochromatized Cu Kα radiation (λ = 1.54186 Å). For the crystal date of AuxAg28-xCu12(SPhCl2)24 (x = 7.56), Au12Ag32(SPhCl2)30: the data collection for single crystal X-ray diffraction was carried out on a Bruker Smart APEX II CCD diffractometer under liquid nitrogen flow, using graphite-monochromatized Mo Kα radiation (λ = 0.71069 Å). Data reductions and absorption corrections were performed using the SAINT and SADABS programs, respectively63. The structure was solved by direct methods and refined with full-matrix least squares on F2 using the SHELXTL software package64. All non-hydrogen atoms were refined anisotropically, and all the hydrogen atoms were set in geometrically calculated positions and refined isotropically using a riding model. All crystal structures were treated with PLATON SQUEEZE, and the diffuse electron densities from these residual solvent molecules were removed65.
Characterization
The UV-vis absorption spectra of nanoclusters were recorded using an Agilent 8453 diode array spectrometer. Electrospray ionization mass spectrometry (ESI-MS) measurements were performed by MicrOTOF-QIII high-resolution mass spectrometer. The sample was directly infused into the chamber at 5 μL/min. For preparing the ESI samples, nanoclusters were dissolved in CH2Cl2 (1 mg/mL) and diluted (v/v = 1:2) by CH3OH. Energy-dispersive X-ray spectroscopy (EDS) map** of nanoclusters were characterized by SEM (Quanta 400 F). X-ray photoelectron spectroscopy (XPS) measurements were performed on a Thermo ESCALAB 250 configured with a monochromatized Al Kα (1486.8 eV) 150 W X-ray source, 0.5 mm circular spot size, flood gun to counter charging effects, and analysis chamber base pressure lower than 1 × 10−9 mbar.
Data availability
The X-ray crystallographic coordinates for structures reported in this work have been deposited at the Cambridge Crystallographic Data Center (CCDC), under deposition numbers CCDC-2009375, 2009456, 2009457, 2009377, 2009378, and 2083130. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif, which has been mentioned in the article.
References
Chakraborty, I. & Pradeep, T. Atomically precise clusters of noble metals: emerging link between atoms and nanoparticles. Chem. Rev. 117, 8208–8271 (2017).
Hossain, S. et al. Alloy clusters: precise synthesis and mixing effects. Acc. Chem. Res. 51, 3114–3124 (2018).
Sharma, S. et al. Structurally precise dichalcogenolate-protected copper and silver superatomic nanoclusters and their alloys. Acc. Chem. Res. 51, 2475–2483 (2018).
Konishi, K., Iwasaki, M. & Shichibu, Y. Phosphine-ligated gold clusters with core+exo geometries: unique properties and interactions at the ligand-cluster interface. Acc. Chem. Res. 51, 3125–3133 (2018).
Bhattarai, B. et al. Chemistry and structure of silver molecular nanoparticles. Acc. Chem. Res. 51, 3104–3113 (2018).
Chen, T. et al. Electrospray ionization mass spectrometry: a powerful platform for noble-metal nanocluster analysis. Angew. Chem. Int. Ed. 58, 11967–11977 (2019).
Yang, D. et al. Controllable conversion of CO2 on non-metallic gold clusters. Angew. Chem. Int. Ed. 59, 1919–1924 (2020).
Guan, Z.-J. et al. Isomerization in alkynyl-protected gold nanoclusters. J. Am. Chem. Soc. 142, 2995–3001 (2020).
Kenzler, S., Schrenk, C. & Schnepf, A. Au108S24(PPh3)16: a highly symmetric nanoscale gold cluster confirms the general concept of metalloid clusters. Angew. Chem. Int. Ed. 56, 393–396 (2017).
Yuan, S.-F. et al. A ligand-protected golden fullerene: the dipyridylamido Au328+ nanocluster. Angew. Chem. Int. Ed. 58, 5906–5909 (2019).
Kenzler, S. et al. Synthesis and characterization of three multi-shell metalloid gold clusters Au32(R3P)12Cl8. Angew. Chem. Int. Ed. 58, 5902–5905 (2019).
Narouz, M. R. et al. N-heterocyclic carbene-functionalized magic-number gold nanoclusters. Nat. Chem. 11, 419–425 (2019).
Tasaka, Y. et al. Electron binding in a superatom with a repulsive coulomb barrier: the case of [Ag44(SC6H3F2)30]4− in the gas phase. J. Phys. Chem. Lett. 11, 3069–3074 (2020).
Huard, D. J. E. et al. Atomic structure of a fluorescent Ag8 cluster templated by a multistranded DNA scaffold. J. Am. Chem. Soc. 141, 11465–11470 (2018).
Wang, Z. et al. A hierarchically assembled 88-nuclei silver-thiacalix[4]arene nanocluster. Nat. Commun. 11, 308 (2020).
Zhuang, S. et al. Hard-sphere random close-packed Au47Cd2(TBBT)31 nanoclusters with a faradaic efficiency of up to 96% for electrocatalytic CO2 reduction to CO. Angew. Chem. Int. Ed. 59, 3073–3077 (2020).
Qu, M. et al. Observation of non-FCC copper in alkynyl-protected Cu53 nanoclusters. Angew. Chem. Int. Ed. 59, 6507–6512 (2020).
Sun, C. et al. Atomically precise, thiolated copper-hydride nanoclusters as single-site hydrogenation catalysts for ketones in mild conditions. ACS Nano 13, 5975–5986 (2019).
Baghdasaryan, A. et al. Thiolato protected copper sulfide cluster with the tentative composition Cu74S15(2-PET)45. Inorg. Chem. 59, 2200–2208 (2020).
Sakthivel, N. A. et al. Crystal structure of faradaurate-279: Au279(SPh-tBu)84 plasmonic nanocrystal molecules. J. Am. Chem. Soc. 139, 15450–15459 (2017).
Hosier, C. A. & Ackerson, C. J. Regiochemistry of thiolate for selenolate ligand exchange on gold clusters. J. Am. Chem. Soc. 141, 309–314 (2019).
Han, X.-S. et al. Structure determination of alkynyl‐protected gold nanocluster Au22(tBuC≡C)18 and its thermochromic luminescence. Angew. Chem. Int. Ed. 59, 2309–2312 (2020).
Cook, A. W. & Hayton, T. W. Case studies in nanocluster synthesis and characterization: challenges and opportunities. Acc. Chem. Res. 51, 2456–2464 (2018).
Takano, S., Ito, S. & Tsukuda, T. Efficient and selective conversion of phosphine-protected (MAu8)2+(M = Pd, Pt) superatoms to thiolate-protected (MAu12)6+ or alkynyl-protected (MAu12)4+ superatoms via hydride do**. J. Am. Chem. Soc. 141, 15994–16002 (2019).
Fei, W. et al. Metal do** of Au25(SR)18− clusters: insights and hindsights. J. Am. Chem. Soc. 141, 16033–16045 (2019).
Alhilaly, M. J. et al. Assembly of atomically precise silver nanoclusters into nanocluster-based frameworks. J. Am. Chem. Soc. 141, 9585–9592 (2019).
Huang, R.-W. et al. [Cu81(PhS)46(tBuNH2)10(H)32]3+ reveals the coexistence of large planar cores and hemispherical shells in high-nuclearity copper nanoclusters. J. Am. Chem. Soc. 142, 8696–8705 (2020).
Nieto-Ortega, B. & Bürgi, T. Vibrational properties of thiolate-protected gold nanoclusters. Acc. Chem. Res. 51, 2811–2819 (2018).
Zhu, Y. et al. Enantioseparation of Au20(PPh3)4Cl4 Clusters with Intrinsically Chiral Cores. Angew. Chem. Int. Ed. 57, 9059–9063 (2018).
Sugiuchi, M., Shichibu, Y. & Konishi, K. An inherently chiral Au24 framework with double-helical hexagold strands. Angew. Chem. Int. Ed. 57, 7855–7859 (2018).
Lu, J. et al. Giant emission enhancement of solid-state gold nanoclusters by surface engineering. Angew. Chem. Int. Ed. 59, 8270–8276 (2020).
Soldan, G. et al. Gold do** of silver nanoclusters: a 26-fold enhancement in the luminescence quantum yield. Angew. Chem. Int. Ed. 55, 5749–5753 (2016).
Weerawardene, K. L. D. M. et al. Luminescence and electron dynamics in atomically precise nanoclusters with eight superatomic electrons. J. Am. Chem. Soc. 141, 18715–18726 (2019).
Yan, J., Teo, B. K. & Zheng, N. Surface chemistry of atomically precise coinage-metal nanoclusters: from structural control to surface reactivity and catalysis. Acc. Chem. Res. 51, 3084–3093 (2018).
Lee, S. et al. [Cu32(PET)24H8Cl2](PPh4)2: a copper hydride nanocluster with a bisquare antiprismatic core. J. Am. Chem. Soc. 142, 13974–13981 (2020).
Agrachev, M. et al. Nuclear and electron magnetic resonance spectroscopies of atomically precise gold nanoclusters. Acc. Chem. Res. 52, 44–52 (2018).
Shen, H. et al. Highly robust but surface-active: an N-heterocyclic carbene-stabilized Au25 nanocluster. Angew. Chem. Int. Ed. 58, 17731–17735 (2019).
Kwak, K. & Lee, D. Electrochemistry of atomically precise metal nanoclusters. Acc. Chem. Res. 52, 12–22 (2018).
Sakthivel, N. A. & Dass, A. Aromatic thiolate-protected series of gold nanomolecules and a contrary structural trend in size evolution. Acc. Chem. Res. 51, 1774–1783 (2018).
Kang, X. & Zhu, M. Intra-cluster growth meets inter-cluster assembly: the molecular and supramolecular chemistry of atomically precise nanoclusters. Coord. Chem. Rev. 394, 1–38 (2019).
Weerawardene, K. L. D. M., Häkkinen, H. & Aikens, C. M. Connections between theory and experiment for gold and silver nanoclusters. Annu. Rev. Phys. Chem. 69, 205–229 (2018).
Tang, Q. et al. Insights into interfaces, stability, electronic properties, and catalytic activities of atomically precise metal nanoclusters from first principles. Acc. Chem. Res. 51, 2793–2802 (2018).
Cirri, A. et al. Systematically tuning the electronic structure of gold nanoclusters through ligand derivatization. Angew. Chem. Int. Ed. 58, 13818–13822 (2019).
Yonesato, K. et al. Controlled assembly synthesis of atomically precise ultrastable silver nanoclusters with polyoxometalates. J. Am. Chem. Soc. 141, 19550–19554 (2019).
Huang, R.-W. et al. Hypersensitive dual-function luminescence switching of a silver-chalcogenolate cluster-based metal-organic framework. Nat. Chem. 9, 689–697 (2017).
Wang, Q.-Y. et al. o-Carborane-based and atomically precise metal clusters as hypergolic materials. J. Am. Chem. Soc. 142, 12010–12014 (2020).
Zhu, M. et al. Correlating the crystal structure of a thiol-protected Au25 cluster and optical properties. J. Am. Chem. Soc. 130, 5883–5885 (2008).
Joshi, C. P. et al. [Ag25(SR)18]−: the “golden” silver nanoparticle. J. Am. Chem. Soc. 137, 11578–11581 (2015).
Nguyen, T.-A. D. et al. A Cu25 nanocluster with partial Cu(0) character. J. Am. Chem. Soc. 137, 13319–13324 (2015).
Conn, B. E. et al. Confirmation of a de novo structure prediction for an atomically precise monolayer-coated silver nanoparticle. Sci. Adv. 2, e1601609 (2016).
Sun, Q. et al. Synthesis, structures, and photoluminescence of elongated face-centered-cubic Ag14 clusters containing lipoic acid and its amide analogue. Inorg. Chem. 59, 8836–8845 (2020).
Desireddy, A. et al. Ultrastable silver nanoparticles. Nature 501, 399–402 (2013).
Yang, H. et al. Structural evolution of atomically precise thiolated bimetallic [Au12+nCu32(SR)30+n]4− (n = 0, 2, 4, 6) nanoclusters. J. Am. Chem. Soc. 136, 7197–7200 (2014).
Du, W. et al. Ag50(Dppm)6(SR)30 and its homologue AuxAg50-x(Dppm)6(SR)30 alloy nanocluster: seeded growth, structure determination, and differences in properties. J. Am. Chem. Soc. 139, 1618–1624 (2017).
Yan, N. et al. Unraveling the long-pursued Au144 structure by x-ray crystallography. Sci. Adv. 4, eaat7259 (2018).
Barik, S. K. et al. Polyhydrido copper nanoclusters with a hollow icosahedral core: [Cu30H18{E2P(OR)2}12] (E=S or Se; R=nPr, iPr or iBu). Chem. Eur. J. 26, 10471–10479 (2020).
Yan, J. et al. Asymmetric synthesis of chiral bimetallic [Ag28Cu12(SR)24]4- nanoclusters via ion pairing. J. Am. Chem. Soc. 138, 12751–12754 (2016).
Yang, H. et al. All-thiol-stabilized Ag44 and Au12Ag32 nanoparticles with single-crystal structures. Nat. Commun. 4, 2422 (2013).
He, L. et al. Alternative array stacking of Ag26Au and Ag24Au nanoclusters. Angew. Chem. Int. Ed. 58, 9897–9901 (2019).
Bodiuzzaman, M. et al. Camouflaging structural diversity: co-crystallization of two different nanoparticles having different cores but the same shell. Angew. Chem. Int. Ed. 58, 189–194 (2019).
Liu, J.-Y. et al. Different silver nanoparticles in one crystal: Ag210(iPrPhS)71(Ph3P)5Cl and Ag211(iPrPhS)71(Ph3P)6Cl. Angew. Chem. Int. Ed. 58, 195–199 (2019).
Yan, J. et al. Co-crystallization of atomically precise metal nanoparticles driven by magic atomic and electronic shells. Nat. Commun. 9, 3357 (2018).
APEX2 Ver.2014.11-0, SAINT Ver.8.34A, SADABS Ver.2014/15, Bruker AXS, Inc., Madison, Wisconsin, USA, 2014.
Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. A 64, 112–122 (2008).
Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 71, 3–8 (2015).
Acknowledgements
We acknowledge the financial support by NSFC (U1532141, 21631001, and 21871001), the Ministry of Education, the Education Department of Anhui University.
Author information
Authors and Affiliations
Contributions
X.K. and X.W. carried out experiments, analyzed the data and wrote the manuscript. X.L., Si.W. and T.Y. assisted the X-ray absorption fine structure spectroscopy analysis and completed the manuscript. Sh.W. and M.Z. designed the project, analyzed the data, and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kang, X., Wei, X., Liu, X. et al. A reasonable approach for the generation of hollow icosahedral kernels in metal nanoclusters. Nat Commun 12, 6186 (2021). https://doi.org/10.1038/s41467-021-26528-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-021-26528-w
- Springer Nature Limited