Abstract
Rapid industrialization, modern farming practices, and other human activities are contributing significant amounts of harmful heavy metals to the environment. These metals can accumulate and magnify through food chains, posing substantial risks to human health. Recognizing the global environmental threat and its health implications, researchers have developed cutting-edge methods to address heavy metal contamination. Phytoremediation stands out as the foremost method, offering effectiveness and environmental suitability. Combining plant growth-promoting rhizobacteria (PGPR) with phytoremediation can be a viable option for minimizing contamination. PGPR enhances plant growth and aids in metal cleanup through chemical synthesis, the secretion of chelating agents, redox reactions, and acidification. This review conducted a comprehensive online search across peer-reviewed electronic databases using specific keywords related to PGPR in heavy metal phytoremediation. This review included 129 relevant articles out of the initially identified 187 articles and outcomes were represented with schematic sketches and in-depth tables. The articles selected were focused on the potential of PGPR in phytoremediation, with emphasis on the contribution of rhizo and endophytic bacteria in accelerating the benefits of phytoremediation. There is little information available about the mechanisms involved in plant-PGPR relationships for metal accumulation. The causes and effects of heavy metal toxicity in the environment were examined in this review, along with the usage of PGPR as a different biological strategy to reduce metal contamination and prevent metals from migrating into edible plant parts. Finally, these prospects will provide some perspectives for future studies on these bacteria in agriculture and offer the possibility of major breakthroughs through knowledge expansion and the allocation of trial sites for the transfer of phytoremediation technology to the farmers in a better way.
Article highlights
-
A systematic review of the use of PGPR as an alternative biological approach to reduce metal pressure and its translocation into the edible parts of plants.
-
A basis for develo** an integrated approach to phytoremediation of heavy metals.
-
A guideline for future studies on these bacteria in agriculture and offer the possibility of major breakthroughs in phytoremediation technology so that we can offer this to farmers in a better way.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
It is foreseen that by 2050, the global total population will be approximately 9 billion [1]. Because of the projected increase, providing food to the entire population during the next decade will be a significant challenge. In recent years, industrialization is increasing the amount of heavy metal contamination in the ecosystem, which poses possible ecological hazards. Unfixed heavy metals are abundant in wastewater produced by businesses (paint, fertilizer, textile, electrochemical, etc.) and are released partially or untreated, which allows them to penetrate the aquatic and terrestrial environments [2, 3]. The non-degradable nature of heavy metal pollutants in sewage leads to their progressive build up in the ecosystem [4, 5]. Heavy metals such as mercury (Hg), nickel (Ni), lead (Pb), arsenic (As), zinc (Zn), chromium (Cr), cadmium (Cd), cobalt (Co) and copper (Cu) pose a significant health risk due to their cytotoxic, mutagenic, and carcinogenic nature [6, 7]. Such elements enter the soil through both anthropogenic and geological sources, such as agrochemical usage, industrial and domestic wastewater, mining activities, and atmospheric deposition [8,9,10].
The excessive use of biosolids, livestock manure, composts, and sewage sludge as fertilizers also leads to elevated levels of heavy metals in soil, which can further leach into groundwater [11, 12]. Micronutrient-deficient soils are often supplemented with heavy metals like Fe, Co, Cu), Zn, Mn, and Ni for plant growth [13]. Moreover, the excessive use of potassium, phosphorus, and nitrogen fertilizers, particularly phosphate fertilizers, can lead to an increase in the levels of heavy metals such as Hg, Cd, and Pb in the soil. Pesticides that are commonly employed in agriculture contain heavy metals, including substances like copper sulfate and lead arsenate, which are used for pest management [10]. Additionally, the long-term application of wastewater effluents for irrigation purposes, even when they contain low levels of heavy metals, contributes to elevated accumulation in the soil [14, 15]. The magnitude of heavy metal contamination in the soil is dependent on the specific type and location of the compounds, ranging from minimal traces to high concentrations [16].
Based on the United States Environmental Protection Agency (USEPA) [17] estimates, soil heavy metal contamination has affected the health of approximately 10 million people worldwide. Hence, to address this issue, remediation measures are necessary to prevent heavy metal contamination in terrestrial, aquatic, and atmospheric environments and to mitigate the impact on polluted land [18]. Various remediation approaches, including physio-chemical and mechanical techniques like soil excavation, washing, incineration, and to some extent solidification, landfilling, and electric field application, have been developed. Nevertheless, these approaches have limitations, such as high cost, irreversible changes to soil properties, inefficiency at low contaminant concentrations, and the potential for introducing secondary pollution [19]. As an alternative, phytoremediation, a plant-based approach, offers a promising solution. This method involves the use of plants to extract and reduce the bioavailability of elemental contaminants in soil [19]. Plants can take up ionic compounds, even at low concentrations, and translocate them through their root systems. They establish a rhizosphere ecosystem by extending their roots into the soil matrix, facilitating the accumulation of heavy metals and modulating their bioavailability [20]. Whereas, bioremediation that uses living organisms, mainly microorganisms (bacteria, fungi and microalgae) or their processes to degrade or detoxify environmental contaminants, which leads to decontaminate polluted soil and water [21]. Recently, it has become increasingly evident that soil microbes, principally plant growth promoting rhizobacteria (PGPR), have a profound effect on soil remediation of natural and metallic toxins and plant growth through a range of strategies [22]. Metals are mobilized by the PGPR in a variety of ways, including acidifying rhizospheric conditions, solubilizing metallic minerals, improving the discharge of root exudates, and increasing the root surface for uptake of the metals [23].
For the degradation of inorganic and organic impurities, PGPR employ numerous mechanisms, such as transformation, volatilization, and rhizodegradation [24]. By enzymatic detoxification, metal complexation, toxic metal efflux, and volatilization of metals, these PGPR can counterattack the toxicity of metals [64]. Furthermore, PGPR can resist and detoxify metalloids and metals, such as Co2+, Ag2+, Cu2+, Hg2+, Zn2+, Ni2+ Cd2+, and Sb2+ [25]. There is significant evidence that PGPR assuage the integration of metals by plants (immobilization) [23]. Many physiochemical methods have been used in the past to remove metal pollution. The majority of these methods are expensive, change the biological makeup of the soil, and create secondary pollutants. Consequently, it is vitally desirable to adopt an alternate and environmentally beneficial technique. In this case, employing biological resources to remediate heavy metal contamination is a viable and economical method with benefits over traditional physicochemical procedures. Because it is both environmentally friendly and economically viable, phytoremediation is one of the most extensively used biological techniques to bioremediation. However, a number of issues, including the plants' inadequate shallow root structure, sensitivity to several metals, low biomass production at higher metal concentrations, and so on, hampered the plant’s ability to perform phytoremediation. Therefore, using PGPR to increase the plant's phytoremediation efficacy at greater metal concentrations is a potential strategy. Utilizing PGPR as a bioinoculant enhanced the plant's biomass and root development by recycling nutrients, stabilizing the soil's structure, and adjusting the toxicity and bioavailability of heavy metals. The mechanisms employed in plants-PGPR associations on the buildup of metals are scantly documented. In this review, we reviewed the sources and impacts of heavy metal toxicity in the environment; besides, we have discussed the use of PGPR as an alternative biological approach to reduce the metal pressure and their translocation into the edible parts of the plants.
2 A recurring change in the research studies
We conducted a comprehensive online search using reputable sources such as PubMed, Scopus, Google Scholar, Web of Science, ResearchGate and Science Direct for the literature survey. Other government sources such as the USEPA are also utilized for this study. The search employed specific key terms, including “phytoremediation”, “hyperaccumulator plants”, “heavy metal toxicity and decontamination”, "plant growth promoting rhizobacteria or PGPR", "microbial strains for bioremediation", "phytostabilization", “phytoextraction” and "future prospects". The articles included in this review were primarily chosen based on the relevance of their titles and abstracts of the research topic.
The inclusion criteria for this review encompassed studies that specifically investigated the role of PGPR in heavy metal decontamination through plants to maintain environmental sustainability. Papers that did not have importance of PGPR in phytoremediation in their abstract, introduction or conclusion were eliminated in the eligibility stage. Conversely, the exclusion criteria entailed articles that provided other use of PGPR in reducing environmental pollution rather than main focus on phytoremediation, articles not in the English language, those with irrelevant or insufficient data, and articles for which full-text access was unattainable.
We conducted a comprehensive literature search to identify studies that elucidate the potential of PGPR to accelerate the process of phytoremediation. This work presents extensive research on the latest application of PGPR in phytoremediation to maintain agricultural and environmental sustainability. Out of the initially identified 187 articles, a total of 129 relevant articles were included in this review. The objective of this review is to consolidate and present all available information on the discussed topic in one accessible article, facilitating future research efforts by other scholars. To address the existing knowledge gap in this field, we included studies spanning from 1994 to 2024, encompassing both recent and historical research.
3 Heavy metals and their properties
Generally speaking, metals with atomic weights higher than iron are referred to as heavy metals. Heavy metals are defined as metallic elements with a density > 5 g/cm3, which have a relatively high density compared with water. In addition, some other heavy metals, including lead (Pb), mercury (Hg), and cadmium (Cd), which are called non-essential elements, are considered exogenous, and they are able to induce toxicity at low levels of exposure. In the natural world, heavy metals are classified into two classes and are typically non-biodegradable. The first category includes toxic metals, such as Pb, Cd, and As, among others, which are harmful in all quantities and undesirable. They also don't have any biological advantages for human health. The second category consists of important metals, such as Cu, Zn, Mn, Fe, Ni, and Cr, among others. Properties of heavy metal are given in Table 1. These elements are beneficial to human health biologically at low concentrations, but turn hazardous at large ones [26].
4 Sources of heavy metals
Several studies have found that anthropogenic activities including fossil refinement burning, mining and smelting, urban waste disposal, irrigation, pesticides, and fertilization all contribute considerably to the increase in soil heavy metal concentrations. Figure 1 displays the main sources of metals [28,29,30,31]. Inorganic fertilizers, sludge, liming, and drainage are primarily responsible for the presence of heavy metals in soils [32]. According to Toth et al. [32], inorganic fertilizers, phosphate fertilization, and fungicides contribute to the varied concentrations of Cr, Ni, Pb, Cd, and Zn in soil. Negrete et al. [33] found that contamination by heavy metals is not restricted to one pesticide.
Farmers are generally not concerned about environmental hazards or benefits but rather are more concerned with exploiting the land to get maximum yield and profits [34]. The concentrations of metals in wastewater discharges are typically low, however, irrigation of farms for long periods with such water can result in the accumulation of heavy metals in the soil [35, 36]. It has been demonstrated that sewage irrigation promotes heavy metal buildup and mobility in soils. The widespread mining and smelting of lead and zinc ore has resulted in severe soil pollution [37]. Aerosols are formed when heavy metal vapors such as Sn, Cu, As, Pb, Zn, and Cd interact with water [31, 32, 38].
Soil contamination is mainly caused by two types of depositing methods; wet depositing (precipitation) and dry depositing (deposited by wind). Smith et al. [39] noted that heavy metals transform into oxides after which they condense as fine particulates in the absence of a reducing atmosphere. Natural air currents frequently disseminate stack emissions across a wide region until they are eliminated from the gas stream by wet and/or dry precipitation processes. Heavy metals are predominantly released by wear on tires, brake linings, road surfaces, and other road-building materials along transportation routes, in addition to exhaust gases [40]. Heavy metal contamination can be found in incinerator effluent, industrial discharges, open pits, landfills, and transportation or traffic emissions [41,42,43,44].
5 Toxicity profile of heavy metals on living cells and environment
Ecosystems and the environment are at risk due to the discharge of multiple hazardous substances into natural resources as a result of increased anthropogenic activities. The most significant cause of environmental pollution is heavy metal ions, which are extremely toxic, non-degradable, and have a propensity to bioaccumulate and biomagnify. There are almost always toxic contaminants in the environment, whether they come from manmade industries like mining or unsustainable farming practices, or from natural events like storms, earthquakes, and volcanoes. These days, one of the biggest environmental risks is heavy metal contamination [45].
Contamination of soil with heavy metals would lead to two major problems: a decrease in soil value and an increase in health risks for people living near the affected areas. Soil poisoned by heavy metals loses at least part of its function. If the concentration of heavy metals is within the permissible limits, the soil can continue to function. However, more attention should be paid to the absence of heavy metals in the soil and the target values. Considering the phytotoxicity and biological importance of metal species regulating various plant processes, the term "heavy metal" was introduced. Few metals like Zn, Fe, Cu, Mn, Ni and Co are essential trace elements for plants but others like Hg, Al, Cd, Pb, As, Ga, Ag and Cr are unnecessary for plants and unknown. physiological function. HM critical thresholds and responses at the cellular and whole plant levels are summarized. The overall visual toxicity response varies between heavy metals due to their different sites of action within the plant. The most common visual sign of heavy metal toxicity is impairment of plant development, including leaf chlorosis, necrosis, loss of turgidity, reduced seed germination rate and damage to the photosynthetic machinery, usually associated with senescence or plant disease. The concentration of this element in food varies depending on where it comes from, how it is stored and how it is processed. These metals have several special properties, including (1) they do not degrade over time, (2) they may be necessary or beneficial to plants in certain amounts but may be toxic if levels exceed a certain threshold, (3) they are always present at background levels of non-anthropogenic origin and their entry into soil is related to weathering and pedogenesis of source rocks, and (4) soil heavy metals can become mobile as a result of changing environmental conditions, since they are often cations that interact strongly with soil [45, 46].
They do not break down and accumulate in living beings, causing various diseases and disorders in the neurological, immunological, reproductive and digestive systems. Because these heavy metal ions (HMI) are not biodegradable, they can persist for decades or even centuries after being released into the environment. Pb, Hg, Cd, Cr and As are the most toxic heavy metals. Melanin can protect tissues by filtering or scavenging heavy metals from the surrounding neuronal retina and photoreceptor cells. The choroid plexus of the brain, like the retinal pigment epithelium, binds lead and acts as a protective barrier against harmful substances entering the brain. During pregnancy, potentially dangerous impurities in the blood of a pregnant woman can enter the fetus and cause a threat to the health of the child. Cd, Pb, Mn, and Hg have received much attention due to their ubiquity [47]. Exposure to heavy metals and other pollutants has caused a variety of problems for humans and wildlife, including carcinogenic, mutagenic, and teratogenic effects [48]. Structural abnormalities, nutritional imbalances, metabolic disorders and low levels have been observed in plants grown in polluted areas [49]. In addition, exposure to these hazardous metals has been linked to several serious diseases, including Alzheimer's disease. Because heavy metal exposure is difficult to completely avoid, chemoprevention is an important method to protect humans and animals from serious health problems caused by exposure to toxic metals.
The utility of several antioxidants, including the vitamin taurine, in reducing heavy metal-induced oxidative DNA damage has been investigated [50]. Liquid pollutants can harm human health and the environment. Landfill leachate and mine effluent, along with other sources of toxins, cause serious health and environmental damage. Pb, Cd, Hg and As are the most common heavy metals that can cause health problems with contaminated food. Heavy metals have been used in various situations in human culture for thousands of years. Although the serious health effects of heavy metals have been known for a long time, exposure to heavy metals continues and even increases in some countries. Unfortunately, food and foodstuffs are one of the most common causes of heavy metal contamination in the population [47]. These hazardous heavy metals need to be removed from the environment in order to protect both people and the ecosystem.
6 Plant response to heavy metal toxicity
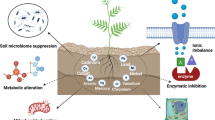
Depending on the degree of oxidation of heavy metals, plant cells are harmed differently. The two ways in which they induce growth inhibition are either by decreasing the uptake of vital micro-and macro-elements into germinating seeds and interfering with various metabolic pathways, or by downregulating protein synthesis and influencing the activities of key enzymes mobilizing food reserves (protease and a-and b-amylases) from the endosperm of cotyledons. It changes several plant physiological processes at the molecular and cellular levels, including enzyme deactivation and protein and DNA denaturation. It substitutes important metal ions from functional and biomolecule units, causing conformational variations and damage to cell membranes, reducing plant metabolism, respiration, photosynthesis, and key enzymes [44, 51] (Fig. 2).
Furthermore, it disrupts redox homeostasis by producing reactive oxygen species (ROS) and free radicals [52]. As a result of interrelated physiological and molecular mechanisms, some plants have established tolerance mechanisms against heavy metals including minimizing on uptake of heavy metals within the cell [3, 53], immobilization in the wall of the cell, production of antioxidants [54], and phytochelation mechanisms and by sequestration in the cell.
The natural ecosystem contains a number of elements, but due to higher contamination or bioaccumulation than allowed limits, species-specific tolerance (plant, animal, and human) and their interactions with one another in the functioning of various species' living cells determine whether an element is toxic or nontoxic [55]. Both heavy metals and metalloids must be immobilized or physically removed from the soil since they cannot undergo chemical degradation in the soil [37]. Heavy metal-contaminated soils are traditionally excavated and disposed of in landfills. As a result of this method of remediation, heavy metals were removed from one place and disposed of in the adjacent environment. Additionally, soil washing was employed to remove the pollutants, although this method is quite expensive and requires further treatment to remove them. According to Gaur and Adholeya [56], the use of physiochemical methods in soil remediation alters biological activities of the soil, resulting in land becoming unsuitable for crop production.
During environmental stress, plant antioxidant systems are dramatically altered by the production of ROS molecules, including hydrogen peroxide, hydroxyl radicals, and superoxide radicals, which cause oxidative damage to biomolecules [57]. A variety of non-enzymatic and enzymatic antioxidants are produced by PGPR strains in this situation, thereby reducing ROS formation and protecting the host plant from ROS-induced oxidative effects [58]. For instance, under Ni and Zn stress, Plants inoculated with RP5 exhibit increased glutathione reductase activity [59]. Similarly, superoxide dismutase and catalase production were boosted after P. sp. infection. Helianthus annuus and Solanum lycopersicum were injected with CPSB21, which reduced ROS production [58, 60]. Plant growth can be enhanced by PGPR strains, which have non-specific mechanisms for promoting plant growth. For example, they make heavy metals less toxic by modulating their bioavailability, regulating phytopathogens, reducing their tolerance to metal stress, and increasing the antioxidant system of plants.
7 Phytoremediation
The term phytoremediation denotes the use of green plants to remove contaminants from polluted environments. Generally, phytoremediation has certain advantages, such as being an autotrophic system with large biomass that only requires a very small amount of fertilizer, being simple to manage, and being generally accepted by society due to its aesthetic appeal and environmental sustainability [61, 62]. The vegetative and reproductive parts of many plant species can accumulate various toxic metals. There are several characteristics of suitable phytoremediation plants, including fast growth with extensive root systems, great biomass production, adaptability, high tolerance, and the capacity to amass pollutants. Plants can be categorized into three classes according to their metal accumulating efficiency and metal tolerance: (1) accumulators (higher metal uptakers and accumulators), (2) indicators (poor metal uptake and transport), and (3) excluders (metal sensitive plants) [63]. Hyperaccumulator plants do accumulate high levels of heavy metals due to their capability to tolerate higher levels of heavy metal concentrations [64]. Nonetheless, the efficacy of a hyperaccumulator is affected by several edaphic factors that influence the rate at which the plant grows, produces biomass, and absorbs metals. In terms of edaphic factors, cation exchange capacity (CEC), metal bioavailability, pH, redox potential, temperature, aeration extent, water content, and organic matter are the most influential [65]. Other biotic factors influence soil nutrients, and reduce phytoremediation efficacy by reducing the growth and uptake of metals [21].
Several processes are involved in the plant’s uptake of metals, based on the type of plant and the amount of heavy metals [10]. These processes include uptake, exclusion, translocation, distribution, accumulation, and osmoregulation [66]. Generally, concentration, accumulation, and translocation occur in the aerial parts of plants. The process of metal uptake is initiated by the extraction of metal from soil solutions and their mobilization towards the roots. Additionally, roots can absorb metals by adjoining metal ions to their cell walls and transporting them to the aerial part of the plant [10, 62, 67]. Transporter proteins and the plant vascular system are involved in the transport process. Under aquatic conditions, phytoremediation occurs through direct absorption of wastewater, which is largely determined by the type of hyperaccumulator plants and wastewater level. Aquatic plants absorb metal from contaminated wastewater through passive metal transport, involving adsorption, resulting in the accumulation of metals in aerial parts [10, 68]. Phytoremediation processes are categorized into phytostabilization (reducing metal mobilization by plant activities), phytoextraction (metal assimilation into plant biomass), phytofiltration (absorption and/or adsorption from the aqueous environment of metal pollutants by plant parts) and phytovolatilization (converting some heavy metals into gaseous state that vaporize through the leaves into the atmosphere) [56, 68]. Some plant species demonstrated maximum remediation effectiveness when exposed to slight metal contamination. Heavy metal exposure will result in slow growth and a reduction in biomass production in plants. To minimize these disadvantages, different biological resources could be utilized [69].
In addition to recycling nutrients, controlling disease, maintaining soil structure, and reducing metal toxicity levels, the rhizosphere microbes are among the potential biological resources that can help plants endure elevated metal toxicity and increase the effectiveness of phytoremediation. Additionally, rhizospheric microorganisms possess a unique ability to uptake/reduce/transform heavy metals through the use of various strategies, including biotransformation, biomineralization, biodegradation biovolatilization, bioaccumulation, biosorption, and bioleaching, thereby reducing the toxicity of heavy metals in plants [70].
8 Role of PGPR in phytoremediation
The use of eco-friendly farming techniques can replace the application of chemicals. Using non-environmentally harmful sources of synthetic pesticides and fertilizers allows for the use of reasonable farming techniques [71]. A viable alternative strategy to increase plant growth and yield in a controlled fashion is the introduction of associative bacteria with the capacity to boost plant development [72]. Rhizobacteria that promote plant growth comprise free-living, associative symbioses that coexist with plants in symbiotic relationships (Frankia spp. and Rhizobium spp.). In the symbiotic relationship between microflora and plants, bacteria consume carbon from the host plant as food and then break down or saturate the pollutant to make it easier for the plant to absorb the released elements by the plants [73, 74]. A surprising fact about symbiosis is that the majority of its energy comes from photosynthesis, which is discharged as exudates from plant roots [75, 76].
Due to the diversity of PGPR, their population may alter as physicochemical conditions in the soil change. According to Zafar-ul-hye et al. [77] and Lynch et al. [76] these bacteria help to lessen the impact of pollution on plants that have fertilizer given to them. The PGPR biofertilizers upsurge the accessibility of nutrients that directly enhance plant development and remediate soils contaminated with trace metals, or they indirectly lessen the inhibitory effects of certain diseases on plants by functioning as tiny biological control entities [78]. Some specific metal peptides that bind to metals are involved in metal chelation or buildup. Nitrogen fixation, phytohormone production (e.g., auxin and cytokinin), and phosphorus solubilization are examples of direct mechanisms used by microorganisms to promote plant growth. Indirect mechanisms comprise the reduction of diseases and the prevention of harmful effects of plant pathogens, besides the removal of toxic metals from soils [79,80,81,82].
The ability of PGPR to reproduce and survive in the soil depends on a variety of soil parameters including temperature, pH, texture, nutritional condition, and trace metal concentration. All these factors cause changes in plant development. The inoculation of PGPR is, however, countered by several other factors, such as limited application effectiveness, cell suitability, and the detrimental effects of numerous soil conditions [83]. Besides enhancing plant development, PGPR also affect soil parameters through a variety of methods to reduce soil metal contamination [84]. According to Ma et al. [64] and Sharma and Archana [85], PGPR decreases metal pollution by lowering metal bioaccumulation, precipitation, biosorption, oxidation, complexation, and reduction as well as the change of enzymatic metals. This decreases the metal ions toxicity to not only plants but also to animals. Effects of some PGPR on plants not only in the case of phytoremediation of metals but also in the case of growth and development are summed up in Table 2.
9 Mechanism of phytoremediation by PGPR
The efficiency of PGPR-assisted phytoremediation depends greatly on several factors, which include plant development, rhizospheric activity, heavy metal bioavailability, and metal tolerance. Among these, metal bioavailability and rhizospheric activity have a significant impact on how effectively plants do phytoremediation. Typically, heavy metals found in contaminated environments are bound to various inorganic and organic components, and their bioavailability is directly associated with their chemical speciation [10]. A variety of metabolic compounds are produced by PGPR strains to modify the nature of metals i.e. oxidation–reduction and acidification reactions) and their mobility i.e. chelation, immobilization, and precipitation) at the rhizospheric environment. This increased the effectiveness of phytoextraction, phytostabilization, and phytovolatilization of the plant (Fig. 3).
9.1 PGPR-assisted phytoextraction
Phytoextraction, or the capability of plants to take contaminants up into their roots and transport them to the surface, can be upgraded by changing heavy metal solubility, mobility, bioavailability, and transport by lowering soil pH, demineralization, producing chelators, redox state, and so on [94]. The above parameters are significantly affected by PGPR inoculations due to the production of many metabolic compounds that promote heavy metal buildup. The principal metal chelating agents, known as microbial siderophores, are critical for heavy metal solubilization and conversion into plant-available forms [94]. Mousavi et al. [95] investigated the effect of inoculating H. annuus with the P. fluorescens and siderophore-producing bacterial strains B. safensis FO-036b (T) on growth and metal accumulation. In this case, inoculation with microbes solubilizes and upsurges Pb and Zn accumulation. Mishra et al. [96] and Ali et al. [97] discovered that inoculating PGPR strains that generate siderophores dramatically boosted the buildup of Fe, Pb, and Zn in the corresponding host plants. In addition, PGPR strains generated a variety of organic acids of low molecular weight including oxalic, 2-ketoglutaric, succinic, gluconic, citric, and malic. Among these, oxalic and citric acids stand out for their significant contributions to the complexation reduction process, which leads to metal solubilization and mobility [98]. Microbial biosurfactants have an important role in controlling the bioavailability and mobility of heavy metals to plant roots. Acinetobacter strains (apoemulson, alasan, emulsan, and biodispersan), Serratia (a cyclic lipopeptide biosurfactant serrawettin), Bacillus (surfactin), and Pseudomonas (glycolipid biosurfactant) are the most important PGPR species for biosurfactant production. The interaction and complex formation of secreted biosurfactants with insoluble metals at the interface of rhizosphere soil particles causes a change in the mobility of metals in soil solution [84, 98]. In addition to biosurfactants, bioleaching is an essential feature of PGPR strains because it aids in the mobilization of heavy metals from mineral resources. As a result, rhizobacterial inoculations increase metal bioavailability in contaminated soils for plant uptake. Table 3 shows the effects of PGPR inoculation on plant heavy metal phytoextraction efficacy.
9.2 PGPR-assisted phytostabilization
Some PGPR strains can inhibit the accumulation and mobilization of heavy metals by develo** several distinct processes, including bioaccumulation, biotransformation, adsorption, biosorption, complexation, precipitation, and alkalization. According to Sessitsch et al. [108], extracellular capsules (EPS), carboxyl, sulfhydryl, hydroxyl, amide, sulfonate, and amine groups on the surface of bacterial cells have a momentous role in the adsorption and immobilization of heavy metals. Heavy metals with cationic charges could be easily captured by anionic-charged bacterial EPS molecules. The microbial EPS has a general negative charge thanks to functional groups like phosphate, amine, carboxyl, sulfhydryl, and hydroxyl [109]. As pointed out by Wang et al. [110], these functional groups have a significant part in the adsorption of cationic-charged heavy metals, reducing heavy metal availability and mobility in the rhizosphere. As observed by Mukherjee and Sinha [111], adsorption of heavy metals by bacterial cell wall components in Hordeum vulgare was associated with reduced Cd accumulation.
PGPR might decrease metal mobility by employing bioaccumulation and biosorption approaches [22, 109]. Microorganisms can absorb heavy metals via metabolism-independent passive transport or metabolism-dependent active transport [109, 112]. Metals are immobilized by several microbial mechanisms following biosorption, including transformation, accumulation, precipitation, sequestration, and accumulation [64]. Furthermore, microbial reduction and/or oxidation processes change the ionic state of heavy metals (Mn, Cr, Fe, Hg, and Se), transforming the hazardous mobile form to a less hazardous immobile form [22, 64, 113]. Karthik et al. [109] reported the biotransformation and immobilization of Cr(VI) by rhizobacterial strain. The four primary processes in this immobilization and biotransformation are Cr (VI) biosorption and interaction by cell surface functional groups, Cr(VI) transport to cytosolic solution by phosphate/sulfate transporters, Cr(III) immobilization, and Cr(VI) reduction in cytosolic solution. Serratia sp uses a variety of processes to decrease Cr(VI) and immobilize reduced organo-Cr(III), according to Srivastava and Thakur [54]. These mechanisms include precipitation, ion exchange, complexation, and immobilization. Stenotrophomonas maltophilia strain has been revealed to have the ability to reduce and immobilize Se [114]. These microbial activities have a significant influence on metal and semi-metal toxicity and mobilization, transforming dangerous metals into lesser toxic forms and rendering them inaccessible to the plant root. According to Chatterjee et al. [115], inoculating the chili plant with the Cellulosimicrobium cellulans KUCr3 strain decreased Cr buildup by 57 and 37% in the root and shoot tissues, respectively. Table 4 summarizes the influence of heavy metal-resistant PGPR strains on heavy metal buildup in plants.
9.3 PGPR-assisted phytovolatilization
The phytovolatilization of heavy metals is greatly affected by PGPR inoculation, much like phytoextraction and phytostabilization. For example, a PGPR strain that was resistant to mercury produced the organomercurial lyase enzymes that are encoded by the MerB gene and cleave organomercurials into mercury-based (HgII) ions [127, 128]. Silver and Hobman [129] discovered in a separate investigation that the MerA gene in microorganisms encodes mercuric reductase, an intracellular flavoenzyme that is responsible for converting harmful ionic mercury dioxide (Hg) into less dangerous volatile mercury (0). These microbial mechanisms considerably reduce the toxicity of Hg and boost the efficacy of phytovolatilization. Various studies put such microbial genes into the plant system and then analyzed the improved phytovolatilization efficacy of Hg [105].
10 Disadvantages of PGPR-assisted phytoremediation
There are some disadvantages associated with using PGPR for heavy metal decontamination:
-
a.
Where the concentration of metals is significantly elevated, the effectiveness of bioremediation from heavy metal-contaminated soil is limited.
-
b.
Because it is impossible to destroy every metal completely and quickly, this procedure is susceptible. Only biodegradable metals can be treated using the bioremediation technique.
-
c.
Compared to other physical and chemical methods, bioremediation procedures require a lot more time.
-
d.
Not all contaminants are eliminated from the contaminated sites.
-
e.
Bioremediation procedures are often accurate; they require the right amount of pollutants and nutrients, the right kind of habitat, and a capable microbial population.
-
f.
The byproduct of heavy metal biodegradation may be more dangerous than the original contaminants.
-
g.
Nevertheless, because microorganisms need energy to survive in the soil, the breakdown of contaminants by them can also result in complex processes. Simultaneously, the chemicals are required for microbial activity, which promotes the growth of some germs but may disturb other native microorganisms that are already present.
11 Key issues and challenges of phytoremediation
Phytoremediation is an attractive option for soil heavy metals removal but it also has some issues and challenges, like:
-
I.
A long time (several years) is required for soil remediation.
-
II.
The majority of metal hyperaccumulator plant’s low biomass and sluggish development rate typically limit their ability to collect metals by phytoextraction.
-
III.
After phytoextraction, the contaminated biomass must be properly disposed of (as hazardous waste).
-
IV.
In tropical and sub-tropical places where the climate is influenced, pests and disease attacks may weaken and render some plants' ability for accumulation useless.
-
V.
Limited bioavailability of the pollutants in the soil, or difficulty mobilizing a more firmly bound percentage of metal ions from the soil.
-
VI.
As a hyperaccumulator, invasive plant species introduction must be avoided as it may reduce native floral variety.
-
VII.
Soil amendments and agronomic techniques may have a detrimental effect on the mobility of pollutants.
-
VIII.
The weather and climate have a major influence on the sustainability of bioremediation.
-
IX.
When plants grow sustainably in heavily contaminated soil, bioremediation is a viable strategy for areas with low to moderate metal pollution levels.
-
X.
Mishandling of biomass can result in the transmission of accumulated metals into the food chain.
12 Future prospects
According to the explanation above, microbial-aided phytoremediation is a reliable procedure. In addition, numerous topics need greater inquiry because of a lack of knowledge or a lack of clarity. These are as follows:
-
i.
In metal-polluted soils, interactions between microbes and plants have been the subject of a very small number of published investigations. The functional properties of bacterial isolates (i.e., production of several metabolites, biosorption, adapting strategies, metal-resistant, and immobilization/mobilization mechanisms, etc.) can be used to identify the factors influencing bacteria's ability to colonize the interior tissues of the plant and/or rhizosphere, promote plant growth, and facilitate metal uptake. For this strategy to work, the inoculums must colonize effectively and endure harsh conditions.
-
ii.
It is difficult to say if changes in heavy metal speciation mediated by bacteria influenced plant development and metal accumulation/distribution both inside plants and in the rhizosphere.
-
iii.
Additional research is necessary to investigate the mechanisms that are primarily in charge of bacteria-assisted phytoremediation, including the plant root-mediated processes and interactive effects of the PGPR on the mobilization and solubilization of metal ions in soils, because plant-mediated processes, such as phytosiderophores or the exudation of organic acids, may also aid in the sequestration and solubilization of metal ions from soil.
-
iv.
Future studies must focus on understanding the ecology and diversity of plant-associated PGPR in various metal-polluted soils to put these PGPB-assisted phytoremediation into practice. Certainly, a deeper comprehension of the impact of naturally adapted native microorganisms that have been grown and enhanced in the lab on the phytoremediation capacity of different plants in diverse metal-polluted soils will yield better results for develo** this technique.
It is still regarded as a relatively new alternative with few long-term field trials, despite having been extensively researched over the past few decades. To fully understand the interactions between microbes and host plants in environments contaminated by metals, more research is necessary.
13 Conclusions
The harmful effects of heavy metals on the biosphere have drawn attention from all around the world to the need to create appropriate methods for eliminating these contaminants from the environment. Despite the development of numerous physiochemical and biological techniques, phytoremediation remains a remarkably dependable and effective method for the removal of metals. The best option for raising the plant's metal removal efficiency is PGPR. Reclamation of metal-contaminated soils using plants and potential PGPR is a practical, low-cost, and develo** method that has made significant advancements in in-situ remediation. Through a variety of metabolism-dependent and/or independent actions, PGPR modify the nature of heavy metals, promoting the plant's development and phytoremediation efficacy. Moreover, more research is required to determine how well phytoremediation works in response to abiotic climate change-related challenges like salinity and drought. Delivering "real" results and giving a deeper understanding of the processes involved without moderated factors are two reasons why field trials are so important. Analysis of factors including the host plant species' and inoculant's compatibility as well as the microorganisms' capacity to function and multiply in contaminated soils over extended periods of time must be done in natural field condition. However, inoculation with PGPR and metal-solubilizing microorganisms portends enormous promise for improving the health, biomass yield, and metal accumulation capability of hyperaccumulators as well as raising the availability of target elements.
Data availability
No datasets were generated or analysed during the current study.
References
Singh SK, Singh PP, Gupta A. Tolerance of Heavy Metal Toxicity Using PGPR Strains of Pseudomonas Species. In: PGPR Amelioration in Sustainable Agriculture. Elsevier Inc. 2019. https://doi.org/10.1016/B978-0-12-815879-1.00012-4
Kumar A, Song HW, Mishra S, Zhang W, Zhang YL, Zhang QR, et al. Application of microbial-induced carbonate precipitation (MICP) techniques to remove heavy metal in the natural environment: a critical review. Chemosphere. 2023;318:137894. https://doi.org/10.1016/j.chemosphere.2023.137894.
Zhang H, Xu W, Guo J, He Z, Ma M. Coordinated responses of phytochelatins and metallothioneins to heavy metals in garlic seedlings. Plant Sci. 2005;169(6):1059–65. https://doi.org/10.1016/j.plantsci.2005.07.010.
Song H, Kumar A, Ding Y, Wang J, Zhang Y. Removal of Cd2+ from wastewater by microorganism induced carbonate precipitation (MICP): An economic bioremediation approach. Sep Purif Technol. 2022;297: 121540. https://doi.org/10.1016/j.seppur.2022.121540.
Pusz A, Wisniewska M, Rogalski D. Assessment of the accumulation ability of Festuca rubra L. and Alyssum saxatile L. Tested on soils contaminated with Zn, Cd, Ni, Pb, Cr, and Cu. Resources. 2021;10:46. https://doi.org/10.3390/resources10050046.
Jan AT, Azam M, Siddiqui K, Ali A, Choi I, Haq QMR. Heavy metals and human health: mechanistic insight into toxicity and counter defense system of antioxidants. Int J Mol Sci. 2015;16:29592–630. https://doi.org/10.3390/ijms161226183.
Shao Z, Mwakidoshi ER, Muindi EM, Soratto RP, Ranjan S, Padhan SR, et al. Synthetic fertilizer application coupled with bioslurry optimizes potato (Solanum tuberosum) growth and yield. Agron. 2023;13(8):2162. https://doi.org/10.3390/agronomy13082162.
Chen J, Lü S, Zhang Z, Zhao X, Li X, Ning P, Liu M. Environmentally friendly fertilizers: a review of materials used and their effects on the environment. Sci Total Environ. 2018;613–614:829–39. https://doi.org/10.1016/j.scitotenv.2017.09.186.
Ngugi MM, Gitari HI, Muui CW, Gweyi-Onyango JP. Growth tolerance, concentration, and uptake of heavy metals as ameliorated by silicon application in vegetables. Int J Phytoremed. 2022;24(14):1543–56. https://doi.org/10.1080/15226514.2022.2045251.
Sairaam M, Maitra S, Praharaj S, Nath S, Shankar T, Sahoo U, et al. An insight into the consequences of emerging contaminants in soil and water and plant responses. In: Aftab T, editor., et al., Emerging contaminants and plants. Cham: Springer; 2023. https://doi.org/10.1007/978-3-031-22269-6_1.
Ashraf S, Ali Q, Zahir ZA, Ashraf S, Asghar HN. Phytoremediation: environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotoxicol Environ Saf. 2019;174:714–27. https://doi.org/10.1016/j.ecoenv.2019.02.068.
Mwakidoshi ER, Gitari HI, Muindi EM, Wamukota AW, Seleiman MF, Maitra S. Smallholder farmers’ knowledge of the use of bioslurry as a soil fertility amendment for potato production in Kenya. Land Degrad Dev. 2023;34(8):2214–27. https://doi.org/10.1002/ldr.4601.
Banerjee R, Goswami P, Lavania S, Mukherjee A, Lavania UC. Vetiver grass is a potential candidate for phytoremediation of iron ore mine spoil dumps. Ecol Eng. 2019;132:120–36. https://doi.org/10.1016/j.ecoleng.2018.10.012.
Jaiswal A, Verma A, Jaiswal P. Detrimental effects of heavy metals in soil, plants, aquatic ecosystem as well as in humans. J Environ Pathol Toxicol. 2018;37(3):183–97. https://doi.org/10.1615/JEnvironPatholToxicolOncol.2018025348.
Morcillo P, Esteban MÁ, Cuesta A. Heavy metals produce toxicity, oxidative stress and apoptosis in the marine teleost fish SAF-1 cell line. Chemosphere. 2016;144:225–33. https://doi.org/10.1016/j.chemosphere.2015.08.020.
Bitew Y, Alemayehu M. Impact of crop production inputs on soil health: a review. Asian J Plant Sci. 2017;16:109–31.
Ullah I, Mateen A, Ahmad MA, Munir I, Iqbal A, Alghamdi KM, et al. Heavy metal ATPase genes (HMAs) expression induced by endophytic bacteria, “AI001, and AI002” mediate cadmium translocation and phytoremediation. Environ Pollut. 2022;293:118508. https://doi.org/10.1016/j.envpol.2021.118508.
Yan X, Liu M, Zhong J, Guo J, Wu W. How human activities affect heavy metal contamination of soil and sediments in a long-term reclaimed area of the Liaohe River Delta, North China. Sustainability. 2018;10(2):1–19. https://doi.org/10.3390/su10020338.
Missimer T, Teaf C, Beeson W, Maliva R, Woolschlager J, Covert D. Natural background and anthropogenic arsenic enrichment in florida soils, surface water, and groundwater: a review with a discussion on public health risk. Int J Environ Res Public Health. 2018;15(10):2278. https://doi.org/10.3390/ijerph15102278.
Ghori NH, Ghori T, Hayat MQ, Imadi SR, Gul A, Altay V, Ozturk M. Heavy metal stress and responses in plants. Int J Environ Sci Technol. 2019;16(3):1807–28. https://doi.org/10.1007/s13762-019-02215-8.
Jacob JM, Karthik C, Saratale RG, Kumar SS, Prabakar D, Kadirvelu K, et al. Biological approaches to tackle heavy metal pollution: a survey of literature. J Environ Manag. 2018;217:56–70. https://doi.org/10.1016/j.jenvman.2018.03.077.
Pratush A, Kumar A, Hu Z. Adverse effect of heavy metals (As, Pb, Hg, and Cr) on health and their bioremediation strategies: a review. Int Microbiol. 2018;21:97–106. https://doi.org/10.1007/s10123-018-0012-3.
Guo J, Muhammad H, Lv X, Wei T, Ren X, Jia H, Atif S, Hua L. Prospects and applications of plant growth promoting rhizobacteria to mitigate soil metal contamination: a review. Chemosphere. 2020;246:125823. https://doi.org/10.1016/j.chemosphere.2020.125823.
USEPA-United States Environmental Protection Agency. Health Effects Assessment Summary Tables (HEAST); Annual Update. United States Environmental Protection Agency, Washington, DC, USA. 2016.
Asad SA, Farooq M, Afzal A, West H. Integrated phytobial heavy metal remediation strategies for a sustainable clean environment—a review. Chemosphere. 2019;217:925–41. https://doi.org/10.1016/j.chemosphere.2018.11.021.
Abdel-Rahman GN. Heavy metals definition sources of food contamination, incidence, impacts and remediation: a literature review with recent updates. Egypt J Chem. 2022;65(1):419–37. https://doi.org/10.21608/EJCHEM.2021.80825.4004.
Chawla N, Chawla KK. Metal matrix composites. 2nd ed. New York: Springer; 2013. https://doi.org/10.1007/978-1-4614-9548-2.
Chen H, Teng Y, Lu S, Wang Y, Wang J. Contamination features and health risk of soil heavy metals in China. Sci Tot Environ. 2015;512–513:143–53. https://doi.org/10.1016/j.scitotenv.2015.01.025.
Khan K, Lu H, Khan H, Ishtiaq M, Khan S, Waqas M, et al. Heavy metals in agricultural soils and crops and their health risks in Swat District, northern Pakistan. Food Chem Toxicol. 2013;58:449–58. https://doi.org/10.1016/j.fct.2013.05.014.
Muradoglu F, Gundogdu M, Ercisli S, Encu T, Balta F, Jaafar HZE, Zia-Ul-Haq M. Cadmium toxicity affects chlorophyll a and b content, antioxidant enzyme activities and mineral nutrient accumulation in strawberry. Biol Res. 2015;48(1):11. https://doi.org/10.1186/s40659-015-0001-3.
Sun C, Liu J, Wang Y, Sun L, Yu H. Multivariate and geostatistical analyses of the spatial distribution and sources of heavy metals in agricultural soil in Dehui, Northeast China. Chemosphere. 2013;92(5):517–23. https://doi.org/10.1016/j.chemosphere.2013.02.063.
Toth G, Hermann T, Da Silva M. Montanarella L. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ Int. 2016;88:299–309. https://doi.org/10.1016/j.envint.2015.12.017.
Negrete J, Pinedo-Hernández J, Díez S. Assessment of heavy metal pollution, spatial distribution and origin in agricultural soils along the Sinú River Basin, Colombia. Environ Res. 2017;154:380–8. https://doi.org/10.1016/j.envres.2017.01.021.
Mugo NJ, Karanja NN, Gachene CK, Dittert K, Gitari HI, Schulte-Geldermann E. Response of potato crop to selected nutrients in Central and Eastern highlands of Kenya. Cogent Food Agric. 2021;7:1898762. https://doi.org/10.1080/23311932.2021.1898762.
Akensous FZ, Anli M, Meddich A. Biostimulants as innovative tools to boost date palm (Phoenix dactylifera L.) performance under drought, salinity, and heavy metal (Oid) s’ stresses: a concise review. Sustainability. 2022;14(23):15984. https://doi.org/10.3390/su142315984.
Marshall FM, Holden J, Ghose C, Chisala B, Kapungwe E, Volk J, et al. Contaminated irrigation water and food safety for the urban and periurban poor: appropriate measures for monitoring and control from field research in India and Zambia, Incpetion Report DFID Enkar R8160. Flamer: University of Sussex, SPRU; 2007.
Kumar SS, Kumar A, Singh S, Malyan SK, Baram S, Sharma J, et al. Industrial wastes: fly ash, steel slag and phosphogypsum-potential candidates to mitigate greenhouse gas emissions from paddy fields. Chemosphere. 2020;241:124824. https://doi.org/10.1016/j.chemosphere.2019.124824.
Nagajyoti PC, Lee KD, Sreekanth TVM. Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett. 2010;8:199–216. https://doi.org/10.1007/s10311-010-0297-8.
Smith LA, Means JL, Chen A. Remedial options for metals contaminated sites. Boca Raton: Lewis Publishers; 1995.
Rolka E, Zolnowski AC, Kozlowska KA. Assessment of the content of trace elements in soils and roadside vegetation in the vicinity of some gasoline stations in Olsztyn (Poland). J Elem. 2020;25(2):549–63. https://doi.org/10.5601/jelem.2019.24.4.1914.
Aryal R, Beecham S, Sarkar B, Chong MN, Kinsela A, Kandasamy J. Readily wash-off road dust and associated heavy metals on motorways. Water Air Soil Pollut. 2017;228:1–12. https://doi.org/10.1007/s11270-016-3178-3.
Dubey B, Pal AK, Singh G. Airborne particulate matter: source scenario and their impact on human health and environment. In: Singh RP, Singh A, Srivastava V, editors. Environmental issues surrounding human overpopulation. Pennsylvania: IGI Global; 2017. p. 202–23. https://doi.org/10.4018/978-1-5225-5487-5.ch023.
Jain N, Johnson TA, Kumar A, Mishra S, Gupta N. Biosorption of Cd(II) on jatropha fruit coat and seed coat. Environ Monit Assess. 2015;187:411. https://doi.org/10.1007/s10661-015-4658-4.
Mohapatra PP, Seleiman MF, Mandal R, Pramanik K, Maity TK, Tarafdar J, et al. Efficiency of RAPD and SSR markers in assessing genetic diversity in summer onion (Allium cepa L.) genotypes. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2023;51(3):13369. https://doi.org/10.15835/nbha51313369.
You M, Wang L, Zhou G, Wang Y, Wang K, Zou R, et al. Effects of microbial agents on cadmium uptake in Solanum nigrum L. and rhizosphere microbial communities in cadmium-contaminated soil. Front Microbiol. 2023;13:1106254. https://doi.org/10.3389/fmicb.2022.1106254.
Mushtaq Z, Liaquat M, Nazir A, Liaquat R, Iftikhar H, Anwar W, Itrat N. Potential of plant growth promoting rhizobacteria to mitigate chromium contamination. Environ Technol Innov. 2022;28:102826. https://doi.org/10.1016/j.eti.2022.102826.
Nungula EZ, Raza MA, Nasar J, Maitra S, Seleiman MF, Ranjan S, et al. Cadmium in soil and plants: a review. In: Jha AK, Kumar N, editors., et al., Cadmium toxicity in Water: challenges and solutions. Cham: Springer; 2024. https://doi.org/10.1007/978-3-031-54005-9_2.
Chaitanya MVNL, Arora S, Pal RS, Ali HS, El Haj BM, Logesh R. Assessment of environmental pollutants for their toxicological effects of human and animal health. In: Organic micropollutants in aquatic and terrestrial environments. 2024: 67–85. Cham: Springer
Ghorbani A, Emamverdian A, Pehlivan N, Zargar M, Razavi SM, Chen M. Nano-enabled agrochemicals: mitigating heavy metal toxicity and enhancing crop adaptability for sustainable crop production. J Nanobiotechnol. 2024;22(1):91.
Santhosh K, Kamala K, Ramasamy P, Musthafa MS, Almujri SS, Asdaq SMB, Sivaperumal P. Unveiling the silent threat: heavy metal toxicity devastating impact on aquatic organisms and DNA damage. Mar Pollut Bull. 2024;200:116139.
Villiers F, Ducruix C, Hugouvieux V, Ezan NJE, Garin J, Junot C, et al. Investigating the plant response to cadmium exposure by proteomic and metabolomics approaches. Proteomics. 2011;11:1650–63. https://doi.org/10.1002/pmic.201000645.
Hossain MA, Piyatida P, Teixeira Da Silva JA, Fujita M. Molecular mechanism of heavy metal toxicity and tolerance in plants: central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J Bot. 2012;2012:872875. https://doi.org/10.1155/2012/872875.
Zhu XF, Zheng C, Hu YT, Jiang A, Liu Y, Dong NY, et al. Cadmium-induced oxalate secretion from root apex is associated with cadmium exclusion and resistance in Lycopersicon esulentum. Plant, Cell Environ. 2011;34:1055–64. https://doi.org/10.1111/j.1365-3040.2011.02304.x.
Srivastava S, Thakur IS. Biosorption and biotransformation of chromium by Serratia sp. isolated from tannery effluent. Environ Technol. 2012;33:113–22. https://doi.org/10.1080/09593330.2011.551842.
Kroopnick PM. Vapor abatement cost analysis methodology for calculating life cycle costs for hydrocarbon vapor extracted during soil venting. In: Wise DL, Trantolo DJ, editors. Remediation of hazardous waste. New York: Marcel Dekker; 1994. p. 779–90.
Gaur A, Adholeya A. Prospects of arbuscular mycorrhizal fungi in phytoremediation of heavy metal contaminated soils. Curr Sci. 2004;86:528–34.
Del Rio LA, Sandalio LM, Altomare DA, Zilinskas BA. Mitochondrial and peroxisomal manganese superoxide dismutase: differential expression during leaf senescence. J Exp Bot. 2003;54:923–33. https://doi.org/10.1093/jxb/erg091.
Bumunang EW, Babalola OO. Characterization of rhizobacteria from field-grown genetically modified (GM) and non-GM maizes. Braz Arch Biol Technol. 2014;57(1):1–8. https://doi.org/10.1590/S1516-89132014000100001.
Wani PA, Khan MS, Zaidi A. Effect of heavy metal toxicity on growth, symbiosis, seed yield and metal uptake in pea grown in metal amended soil. Bull Environ Contam Toxicol. 2008;81:152–8. https://doi.org/10.1007/s00128-008-9383-z.
Gupta P, Rani R, Chandra A, Kumar V. Potential applications of Pseudomonas sp. (strain CPSB21) to ameliorate Cr6þ stress and phytoremediation of tannery effluent contaminated agricultural soils. Sci Rep. 2018;8:4860. https://doi.org/10.1038/s41598-018-23322-5.
Heydarzadeh S, Arena C, Vitale E, Rahimi A, Mirzapour M, Nasar J, et al. Impact of different fertilizer sources under supplemental irrigation and rain-fed conditions on eco-physiological responses and yield characteristics of dragon’s head (Lallemantia iberica). Plants. 2023;12:1693. https://doi.org/10.3390/plants12081693.
Mahajan P, Kaushal J. Role of phytoremediation in reducing cadmium toxicity in soil and water. J Toxicol. 2018;4864365:1–16. https://doi.org/10.1155/2018/4864365.
Khan MS, Zaidi A, Ahmad WP, Oves M. Role of plant growth promoting rhizobacteria in the remediation of metal contaminated soils: a review. Sustain Agric Rev. 2009. https://doi.org/10.1007/978-1-4020-9654-915.
Ma Y, Oliveira RS, Freitas H, Zhang C. Biochemical and molecular mechanisms of plant-microbe-metal interactions: relevance for phytoremediation. Front Plant Sci. 2016;7:1–19. https://doi.org/10.3389/fpls.2016.00918.
Andrea MME, Carolina TEA, Jose CBT, Luis MNJ, Carlos GML. Evaluation of contaminants in agricultural soils in an Irrigation District in Colombia. Heliyon. 2019;5:e02217. https://doi.org/10.1016/j.heliyon.2019.e02217.
Qin D, Pei XX, Zhao LW. Differential cadmium distribution and translocation in roots and shoots related to hyper-tolerance between tall fescue and Kentucky bluegrass. Front Plant Sci. 2017;8:113. https://doi.org/10.3389/fpls.2017.00113.
Suman J, Uhlik O, Viktorova J, Macek T. Phytoextraction of heavy metals: a promising tool for clean-up of polluted environment. Front Plant Sci. 2018;9:1476. https://doi.org/10.3389/fpls.2018.01476.
Rezania S, Taib SM, Din MFM, Dahalan FA, Kamyab H. Comprehensive review on phytotechnology: heavy metals removal by diverse aquatic plants species from wastewater: review. J Hazard Mater. 2016;318:587–99. https://doi.org/10.1016/j.jhazmat.2016.07.053.
DalCorso G, Fasani E, Manara A, Visioli G, Furini A. Heavy metal pollutions: state of the art and innovation in phytoremediation. Int J Mol Sci. 2019;20(14):3412. https://doi.org/10.3390/ijms20143412.
Karthik C, Arulselvi PI. Biotoxic effect of chromium (VI) on plant growth-promoting traits of novel Cellulosimicrobium funkei strain AR8 isolated from Phaseolus vulgaris rhizosphere. Geomicrobiol J. 2017;34:434–42. https://doi.org/10.1080/01490451.2016.1219429.
Zhao Y, Yao J, Li H, Sunahara G, Li M, Tang C, et al. Effects of three plant growth-promoting bacterial symbiosis with ryegrass for remediation of Cd, Pb, and Zn soil in a mining area. J Environ Manage. 2024;353:120167. https://doi.org/10.1016/j.jenvman.2024.120167.
Joradon P, Poolpak T, Kruatrachue M, Yang KM, Saengwilai P, Upatham S, et al. Phytoremediation technology for recovery of Ni by Acacia plants in association with Bacillus amyloliquefaciens isolated from E-waste contaminated site. Int J Phytorem. 2023;28:1–10. https://doi.org/10.1080/15226514.2023.2282043.
Jena J, Maitra S, Hossain A, Pramanick B, Gitari HI, Praharaj S, et al. Role of legumes in crop** system for soil ecosystem improvement. In: Jatav HS, Rajput VD, editors., et al., Ecosystem services: types, management and benefits. New York: Nova Science Publishers Inc.; 2022. p. 11788. https://doi.org/10.52305/PFZA6988.
Mirriam A, Mugwe J, Raza MF, Seleiman MA, Maitra S, et al. Aggrandizing soybean yield, phosphorus use efficiency and economic returns under phosphatic fertilizer application and inoculation with Bradyrhizobium. J Soil Sci Plant Nut. 2022;22:5086–98. https://doi.org/10.1007/s42729-022-00985-8.
Audet P, Charest C. Heavy metal phytoremediation from a meta-analytical perspective. Environ Pollut. 2007;147:231–7. https://doi.org/10.1016/j.envpol.2006.08.011.
Lynch JM, Moffat AJ, Lodge AH, Gu S. Bioremediation e prospects for the future application of innovative applied biological research. Ann Appl Biol. 2005;146(2):217–21. https://doi.org/10.1111/j.1744-7348.2005.040115.x.
Zafar-ul-hye M, Ahmad M, Shahzad SM. Synergistic effect of rhizobia and plant growth promoting rhizobacteria on the growth and nodulation of lentil seedlings under axenic conditions. Proc R Soc B. 2013;32:79–86.
Faridvand S, Rezaei-Chiyaneh E, Battaglia M, Gitari H, Raza MA, Siddique KHM. Application of bio and chemical fertilizers improves yield, and essential oil quantity and quality of Moldavian balm (Dracocephalum moldavica L.) intercropped with mung bean (Vigna radiata L.). Food Energy Sec. 2021;11:319. https://doi.org/10.1002/fes3.319.
Delshadi S, Ebrahimi M, Shirmohammadi E. Influence of plant-growth promoting bacteria on germination, growth and nutrients uptake of Onobrychis sativa L. Under drought stress. J Plant Interact. 2017;12(1):200–8. https://doi.org/10.1080/17429145.2017.1316527.
Tariq M, Noman M, Ahmed T, Manzoor N, Zafar M. Antagonistic features displayed by plant growth promoting rhizobacteria (PGPR). A review. J Plant Sci Phytopathol. 2017;1:38–43. https://doi.org/10.29328/journal.jpsp.1001004.
Thijs S, Sillen W, Rineau F, Weyens N, Vangronsveld J. Towards an enhanced understanding of plantemicrobiome interactions to improve phytoremediation. Eng Metaorganism Front Microbiol. 2016;7:341. https://doi.org/10.3389/fmicb.2016.00341.
Timmusk S, Behers L, Muthoni J, Muraya A, Aronsson AC. Perspectives and challenges of microbial application for crop improvement. Front Plant Sci. 2017;8:49. https://doi.org/10.3389/fpls.2017.00049.
Marschner P, Crowley D, Yang CH. Development of specific rhizosphere bacterial communities in relation to plant species, nutrition and soil type. Plant Soil. 2004;261:199–208. https://doi.org/10.1023/B:PLSO.0000035569.80747.c5.
Rajkumar M, Sandhya S, Prasad MNV, Freitas H. Perspectives of plant-associated microbes in heavy metal phytoremediation. Biotechnol Adv. 2012;30:1562–74. https://doi.org/10.1016/j.biotechadv.2012.04.011.
Sharma RK, Archana G. Cadmium minimization in food crops by cadmium-resistant plant growth promoting rhizobacteria. Appl Soil Ecol. 2016;107:66–78. https://doi.org/10.1016/j.apsoil.2016.05.009.
Liu A, Wang W, Chen X, Zheng X, Fu W, Wang G, et al. Phytoremediation of DEHP and heavy metals co-contaminated soil by rice assisted with a PGPR consortium: Insights into the regulation of ion homeostasis, improvement of photosynthesis and enrichment of beneficial bacteria in rhizosphere soil. Environ Pollut. 2022;314:120303. https://doi.org/10.1016/j.envpol.2022.120303.
Liu A, Wang W, Zheng X, Chen X, Fu W, Wang G, et al. Improvement of the Cd and Zn phytoremediation efficiency of rice (Oryza sativa) through the inoculation of a metal-resistant PGPR strain. Chemosphere. 2022;302:134900. https://doi.org/10.1016/j.chemosphere.2022a.134900.
Jha PN, Gupta G, Jha P. Association of rhizospheric/endophytic bacteria with plants. A potential gateway to sustainable agriculture. Greener J Agric Sci. 2017;3:73–84.
Zhang Y, Wu X, Tao Y, Ke T, Wu W, Liao K, et al. Effect of plant growth–promoting rhizobacteria on oilseed rape Brassica juncea and phytoextraction of cadmium. J Soils Sediments. 2023;23(9):1–13. https://doi.org/10.1007/s11368-023-03559-y.
Sharma S, Saraf M. Biofilm-forming plant growth-promoting rhizobacterial consortia isolated from mines and dumpsites assist green remediation of toxic metal (Ni and Pb) using Brassica juncea. Biologia Futura. 2023;74(3):309–25. https://doi.org/10.1007/s42977-023-00179-y.
Khan WU, Yasin NA, Ahmad SR, Nazir A, Naeem K, Nadeem QUA, et al. Burkholderia cepacia CS8 improves phytoremediation potential of Calendula officinalis for tannery solid waste polluted soil. Int J Phytorem. 2023;25(12):1656–68. https://doi.org/10.1080/15226514.2023.2183717.
Koohkan H, Mortazavi MS, Golchin A, Saraji F, Akbarzadeh-Chomachaei G. Comparison of native bacterial and fungal bioaugmentation in the removal of petroleum from soil in the presence of sorghum. Water Air Soil Pollut. 2023;234(5):309. https://doi.org/10.1007/s11270-023-06298-5.
Chen X, Li Z, Zhang X, Chen F, Zhu Y, Guan C, Li Q. The role of microplastics in the process of Paraclostridium sp. DLY7-assisted phytoremediation of phenanthrene contaminated soil. J Clean Prod. 2024;449:141845.
Mohammadzadeh A, Tavakoli M, Motesharezadeh B, Reza CM. Effects of plant growth-promoting bacteria on the phytoremediation of cadmium-contaminated soil by sunflower. Arch Agron Soil Sci. 2017;63(6):807–16. https://doi.org/10.1080/03650340.2016.1235781.
Mousavi SM, Motesharezadeh B, Hosseini HM, Alikhani H, Zolfaghari AA. Root-induced changes of Zn and Pb dynamics in the rhizosphere of sunflower with different plant growth promoting treatments in a heavily contaminated soil. Ecotoxicol Environ Saf. 2018;147:206–16. https://doi.org/10.1016/j.ecoenv.2017.08.045.
Mishra V, Gupta A, Kaur P, Singh S, Singh N, Gehlot P, et al. Synergistic effects of Arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria in bioremediation of iron contaminated soils. Int J Phytoremediation. 2016;18:697–703. https://doi.org/10.1080/15226514.2015.1131231.
Ali A, Guo D, Mahar A, Ma F, Li R, Shen F, et al. Streptomyces pactum assisted phytoremediation in Zn/Pb smelter-contaminated soil of Feng County and its impact on enzymatic activities. Sci Rep. 2017;7:46087. https://doi.org/10.1038/srep46087.
Ullah A, Heng S, Munis MFH, Fahad S, Yang X. Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria: a review. Environ Exp Bot. 2015;117:28–40.
Shi G, Hu J, Ding F, Li S, Shi W, Chen Y. Exogenous Pseudomonas aeruginosa application improved the phytoremediation efficiency of Lolium multiflorum Lam on Cu–Cd co-contaminated soil. Environ Technol Innov. 2022;27:102489. https://doi.org/10.1016/j.eti.2022.102489.
Abeed AH, Mahdy RE, Alshehri D, Hammami I, Eissa MA, Abdel Latef AAH, et al. Induction of resilience strategies against biochemical deteriorations prompted by severe cadmium stress in sunflower plant when Trichoderma and bacterial inoculation were used as biofertilizers. Front Plant Sci. 2022;13:1004173. https://doi.org/10.3389/fpls.2022.1004173.
Kriti Kumari B, Singh G, Gautam A, Sinam G, Pal S, et al. Enhancement in Ni–Cd phytoremediation efficiency of Helianthus annuus L. from battery waste contaminated soil by bacterial augmentation, isolated from e-waste contaminated sites. Int J Environ Res. 2023;17(1):18. https://doi.org/10.1007/s41742-023-00508-y.
Shahzad A, Siddique A, Ferdous S, Amin MA, Qin M, Aslam U, et al. Heavy metals mitigation and growth promoting effect of endophytic Agrococcus terreus (MW 979614) in maize plants under zinc and nickel contaminated soil. Front Microbiol. 2023;14:1255921. https://doi.org/10.3389/fmicb.2023.1255921.
Qadir M, Hussain A, Shah M, Hamayun M, Iqbal A, Irshad M, et al. Pantoea conspicua promoted sunflower growth and engulfed rhizospheric arsenate by secreting exopolysaccharide. Plant Physiol Biochem. 2023;201:107826. https://doi.org/10.1016/j.plaphy.2023.107826.
Anbuganesan V, Vishnupradeep R, Mehnaz N, Kumar A, Freitas H, Rajkumar M. Synergistic effect of biochar and plant growth promoting bacteria improve the growth and phytostabilization potential of Sorghum bicolor in Cd and Zn contaminated soils. Rhizosphere. 2024;29:100844. https://doi.org/10.1016/j.rhisph.2023.100844.
Zulfiqar U, Yasmin A, Fariq A. Metabolites produced by inoculated Vigna radiata during bacterial assisted phytoremediation of Pb, Ni and Cr polluted soil. PLoS ONE. 2022;17(11):e0277101. https://doi.org/10.1371/journal.pone.0277101.
Guo J, Yang H, Wang C, Liu Z, Huang Y, Zhang C, et al. Inhibitory effects of Pseudomonas sp. W112 on cadmium accumulation in wheat grains: reduced the bioavailability in soil and enhanced the interception by plant organs. Chemosphere. 2024;355:141828.
Direk A, Arikan B, Ozfidan-Konakci C, Yildiztugay E, Uysal A. Effects of Bacillus cereus on physiological and biochemical characteristics of wheat under arsenic and cadmium stress: a biological agent to reduce heavy metal stress. Plant Stress. 2024;12:100458.
Sessitsch A, Kuffner M, Kidd P, Vangronsveld J, Wenzel WW, Fallmann K, et al. The role of plant-associated bacteria in the mobilization and phytoextraction of trace elements in contaminated soils. Soil Biol Biochem. 2013;60:182–94. https://doi.org/10.1016/j.soilbio.2013.01.012.
Karthik C, Elangovana N, Senthil Kumara T, Govindharajua S, Barathia S, Ovesc M, et al. Characterization of multifarious plant growth promoting traits of rhizobacterial strain AR6 under Chromium (VI) stress. Microbiol Res. 2017;204:65–71. https://doi.org/10.1016/j.micres.2017.07.008.
Wang S, Teng S, Fan M. Interaction between Heavy Metals and Aerobic Granular Sludge. In: Sarkar Santosh Kumar, editor. Environmental Management. Croatia: Sciyo; 2010. p. 173–88. https://doi.org/10.5772/10106.
Mukherjee SK, Sinha S. Cadmium-induced siderophore production by a high Cd-resistant bacterial strain relieved Cd toxicity in plants through root colonization. Curr Microbiol. 2008;56:55–60.
Hassan TU, Bano A, Naz I. Alleviation of heavy metals toxicity by the application of plant growth promoting rhizobacteria and effects on wheat grown in saline sodic field. Int J Phytoremediation. 2017;19:522–9.
Hassan MJ, Raza MA, Rehman SU, Ansar M, Gitari H, Khan I, et al. Effect of cadmium toxicity on growth, oxidative damage, antioxidant defense system and cadmium accumulation in two sorghum cultivars. Plants. 2020;9(11):1575. https://doi.org/10.3390/plants9111575.
Gregorio SD, Lampis S, Vallini G. Selenite precipitation by a rhizospheric strain of Stenotrophomonas sp. isolated from the root system of Astragalus bisulcatus: a biotechnological perspective. Environ Int. 2005;31:233–41. https://doi.org/10.1016/j.envint.2004.09.021.
Chatterjee S, Sau GB, Mukherjee SK. Plant growth promotion by a hexavalent chromium reducing bacterial strain, Cellulosimicrobium cellulans KUCr3. World J Microbiol Biotechnol. 2009;25(10):1829–36. https://doi.org/10.1007/s11274-009-0084-5.
Li Q, **ng Y, Huang B, Chen X, Ji L, Fu X, et al. Rhizospheric mechanisms of Bacillus subtilis bioaugmentation-assisted phytostabilization of cadmium-contaminated soil. Sci Total Environ. 2022;825:154136. https://doi.org/10.1016/j.scitotenv.2022.154136.
Saha J, Pal A. Cadmium biosorption and plant growth promotion efficacy of a metalloresistant Pseudomonas sp. unveils augmented growth with reduced metal accumulation in Brassica napus L. Vegetos. 2023; 1–9.
Tirry N, Kouchou A, El Omari B, Ferioun M, El Ghachtouli N. Improved chromium tolerance of Medicago sativa by plant growth-promoting rhizobacteria (PGPR). J Genet Eng Biotechnol. 2021;19:1–14. https://doi.org/10.1186/s43141-021-00254-8.
Ke T, Guo G, Liu J, Zhang C, Tao Y, Wang P, et al. Improvement of the Cu and Cd phytostabilization efficiency of perennial ryegrass through the inoculation of three metal-resistant PGPR strains. Environ Pollut. 2021;271:116314. https://doi.org/10.1016/j.envpol.2020.116314.
Hayat K, Menhas S, Bundschuh J, Zhou P, Niazi NK, Amna, et al. Plant growth promotion and enhanced uptake of Cd by combinatorial application of Bacillus pumilus and EDTA on Zea mays L. Int J Phytoremediation. 2020;22(13):1372–84. https://doi.org/10.1080/15226514.2020.1780410.
Ustiatik R, Nuraini Y, Suharjono S, Jeyakumar P, Anderson CW, Handayanto E. Mercury resistance and plant growth promoting traits of endophytic bacteria isolated from mercury-contaminated soil. Bioremediat J. 2022;26(3):208–27. https://doi.org/10.1080/10889868.2021.1973950.
Tang L, Hamid Y, Zehra A, Sahito ZA, He Z, Beri WT, et al. Fava bean intercrop** with Sedum alfredii inoculated with endophytes enhances phytoremediation of cadmium and lead co-contaminated field. Environ Pollut. 2020;265:114861. https://doi.org/10.1016/j.envpol.2020.114861.
Chi NTL, Hương ĐTT, Đạo P, Lapcik V. Phytoremediation proficiency of Jatropha gossypifolia under the influence of Pseudomonas aeruginosa on metal contaminated soil. Environ Res. 2023;232:116295. https://doi.org/10.1016/j.envres.2023.116295.
Singh K, Tripathi S, Chandra R. Bacterial assisted phytoremediation of heavy metals and organic pollutants by Cannabis sativa as accumulator plants growing on distillery sludge for ecorestoration of polluted site. J Environ Manage. 2023;332:117294. https://doi.org/10.1016/j.jenvman.2023.117294.
Virk ZA, Al Farraj DA, Iqbal M, Lewińska K, Hussain S. Inoculation with the pH lowering plant growth promoting bacterium Bacillus sp. ZV6 enhances Ni Phytoextraction by Salix alba from a Ni-polluted soil receiving effluents from Ni Electroplating Industry. Sustainability. 2022;14(12):6975. https://doi.org/10.3390/su14126975.
Ayub A, Shabaan M, Malik M, Asghar HN, Zulfiqar U, Ejaz M, et al. Synergistic application of Pseudomonas strains and compost mitigates lead (Pb) stress in sunflower (Helianthus annuus L.) via improved nutrient uptake, antioxidant defense and physiology. Ecotoxicol Environ Saf. 2024;274:116194.
Matsui K, Yoshinami S, Narita M, Chien MF. Mercury resistance transposons in Bacilli strains from different geographical regions. FEMS Microbiol Lett. 2016;363:fnw013.
Abii TA, Okorie DO. Assessment of the level of heavy metals (Cu, Pb, Cd and Cr) contamination in four popular vegetables sold in urban and rural markets of Abia State Nigeria. Cont J Water Air Soil Pollut. 2011;2(1):42–7.
Silver S, Hobman JL. Mercury microbiology: resistance systems, environmental aspects, methylation, and human health. In: Nies DH, Silver S, editors. Molecular microbiology of heavy metals. Microbiology monographs, vol. 6. Berlin: Springer; 2007. p. 357–70. https://doi.org/10.1007/7171_2006_085.
Acknowledgements
The support and guidance of all the peer-reviewed electronic databases are very much appreciated. The invaluable anonymous review comments and helpful suggestions provided by the Editors are gratefully acknowledged.
Funding
NA.
Author information
Authors and Affiliations
Contributions
Ritwik Sahoo, Sumit Sow, Shivani Ranjan: Conceptualization, Ritwik Sahoo, Sumit Sow, Shivani Ranjan, Dharminder, Rajan Kumar, Dhirendra Kumar Roy: investigation, Ritwik Sahoo, Sumit Sow, Shivani Ranjan, Sunil Kumar, Amrendra Kumar: writing-original draft preparation, Rajeev Kumar Srivastava, Rajendra Prasad, Smruti Ranjan Padhan, Dibyajyoti Nath: writing, review and editing. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sahoo, R., Sow, S., Ranjan, S. et al. Unveiling the potential of plant growth promoting rhizobacteria (PGPR) in phytoremediation of heavy metal. Discov Appl Sci 6, 324 (2024). https://doi.org/10.1007/s42452-024-06024-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-06024-8