Abstract
Background
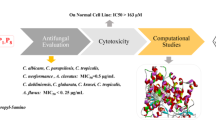
The efficacy of azole drugs has been reduced due to the emergence of drug resistant fungal pathogens. Despite this, heterocyclic compounds are still regarded as a viable lead structure for the synthesis of a more effective antimicrobial agent. The synthesized derivatives were screened in vitro against some fungal organisms. The synthesized compounds were docked to CYP51, a member of the cytochrome P450 family 51 sub-family A. (PDB ID: 5JLC) to determine its most favorable conformation and the energy-maximizing and minimizing orientation.
Results
The efficacy of seven derivatives of benzotriazole and benzimidazole each against some Candida species varied, with inhibition zones ranging from 24 to 37 mm and MICs from 25 to 50 g/mL. The molecular docking study of the synthesized compounds with target CYP51 is a member of the cytochrome P450 family 51 sub-family A. (PDB ID: 5JLC). 1,3-dioctyl-1H-benzimidazol-3-ium gave the best docking score of − 22.79, slightly below the standard drug (fluconazole) used with a docking score of − 22.73 when docked with CYP 51 with PDB ID: 5JLC. The p value obtained using ANOVA analysis of the zone of inhibition for compound 5 was 0.018099 which is less than 0.05, thus confirming it as the most active compound against the Candida species.
Conclusion
According to the study, 1,3-dioctyl-1H-benzimidazol-3-ium (compound 5) showed the highest antifungal activity with maximum zone of inhibition of 37 mm, minimum inhibitory concentration of 6.25 g/mL, and minimum fungicidal concentration of 25 g/mL against Candida stellatoidea. The docking study confirmed that the 1,3-dioctyl-1H-benzimidazol-3-ium had a greater binding affinity of − 22.79 kcal/mol than the other synthesized derivatives as well as the control drug, fluconazole.






Similar content being viewed by others
References
Rajasekaran S, Rao G (2012) Synthesis, antibacterial and antioxidant activity of some 2, 3-susbtituted quinazolin-4(3H)-ones. Der Pharm Lett 4(2):470–474
Lagu SB, Yejella RP, Bhandare RR, Shaik AB (2020) Design, synthesis, and antibacterial and antifungal activities of novel trifluoromethyl and trifluoromethoxy substituted chalcone derivatives. Pharmaceuticals 13(11):1–16
Shojaei P, Mokhtari B, Ghorbanpoor M (2019) Synthesis, in vitro antifungal evaluation and docking studies of novel derivatives of imidazoles and benzimidazoles. Med Chem Res 28(9):1359–1367. https://doi.org/10.1007/s00044-019-02369-7
Zhong Y, Han X, Li S, Qi H, Song Y, Qiao X (2017) Design, synthesis, antifungal activity and molecular docking of thiochroman-4-one derivatives. Chem Pharm Bull 65(10):904–910
Kleshchenko Y, Nes WD, Villalta F, Waterman MR (2008) CYP51: a major drug target in the cytochrome P450 superfamily. Lipids 43:1117–1125
Keniya MV, Sabherwal M, Wilson RK, Woods MA, Sagatova AA, Tyndall JDA et al (2018) Crystal structures of full-length lanosterol 14α-demethylases of prominent fungal pathogens candida albicans and candida glabrata provide tools for antifungal discovery. Antimicrob Agents Chemother 62(11). https://doi.org/10.1128/AAC.01134-18
Khabnadideh S, Rezaei Z, Pakshir K, Zomorodian K, Ghafari N (2012) Synthesis and antifungal activity of benzimidazole, benzotriazole and aminothiazole derivatives. Res Pharm Sci 7(May):65–72
Lage EV, Magalhães J, Pinheiro M, Reis S (2018) Effect of the alkyl group in the piperazine N-substitution on the therapeutic action of rifamycins: a drug-membrane interaction study. Chem-Biol Interact. https://doi.org/10.1016/j.cbi.2018.04.027
Sahariah P, Benediktsdóttir BE, Hjálmarsdóttir MA, Eysteinn O, Sørensen KK, Thygesen MB et al (2015) The impact of chain length on antibacterial activity and hemocompatibility of quaternary N-alkyl and N,N-Dialkyl chitosan derivatives. Biomacromolecules 16:1449–1460
Agarwal S, Mehrotra R (2016) An overview of molecular docking. JSM Chem 4(2):1024
Jimoh TA, Oyewale AO, Ibrahim H, Habila JD, Arthur DE (2022) Biological evaluation and docking study of synthesized derivatives of benzotriazole and benzimidazole as antibacterial agents. Chem Afr 5(0123456789):509–523. https://doi.org/10.1007/s42250-022-00348-x
Amole KL, Bello IA, Oyewale AO (2019) Synthesis, characterization and antifungal study of five new derivatives of E-1-(2-Hydroxyphenyl)chalcone. Chem Afr 2(1):1–14. https://doi.org/10.1007/s42250-018-0027-3
Lino A, Deogracious O (2006) The in-vitro antibacterial activity of Annona senegalensis, Securidacca longipendiculata and Steganotaenia araliacea—Ugandan medicinal plants. Afr Health Sci 6(1):31–35
Vollekova A, Kogtalova D, Sochorova R (2001) Isoquinoline alkaloids from mahonia aquifolium stem bark are active against Malassezia spp. Folia Microbiol 46(2):107–111
Usman H, Abdulrahman FI, Ladan AA (2007) Phytochemical and amtimicrobial evaluation of Tribulus terrestris L. (Zygophyllaceae) growing in Nigeria. Res J Biol Sci 2:244–247
CLSI. Clinical and Laboratory Standards Institute (2015) Performance standards for antimicrobial susceptibility testing; twenty-fifth informational supplement. CLSI Document M100-S25. Vol. 32, Performance Standards for Antimicrobial Susceptibility Testing. 25th ed. CLSI supplement M100. Clinical and Laboratory Standards Institute, Wayne, PA, pp 1–184
Hehre WJ, Huang WW (1995) Chemistry with computation: an introduction to SPARTAN: wavefunction, Inc., pp 79–84
Evans DA (2014) History of the Harvard ChemDraw project. Angew Chem Int Ed 53(42):11140–11145
Li Z, Wan H, Shi Y, Ouyang P (2004) Personal experience with four kinds of chemical structure drawing software: review on chemdraw, chemwindow, ISIS/draw, and chemsketch. J Chem Inf Comput Sci 44(5):1886–1890
An J, Totrov M, Abagyan R (2005) Pocketome via comprehensive identification and classification of ligand binding envelopes. Mol Cell Proteom 4(6):752–761
Bairam R, Muppavarapu SM, Sreekanth S (2017) Synthesis, characterization, biological evaluation and docking of some novel substituted 1, 3-thiazine derivatives. Int J Pharm Pharm Sci 9(3):233–242
Acknowledgements
The authors are grateful to Mall. Abdullahi Mikaila of Nigeria Institute of Leather Technology (NILET) Zaria, Kaduna State, Nigeria for the antimicrobial activity studies and Mall. Jafar of Multi-User Laboratory, Ahmadu Bello University, Zaria Kduna State, Nigeria for the spectroscopic analysis. Finally, I will like to acknowledge Dr. Abdulfatai Usman, Mall. Abduljelil Ajala and Mall. Zakari Yau Ibrahim for their advice.
Funding
No any form of fund was received from any organization.
Author information
Authors and Affiliations
Contributions
T.A.J. and Prof. A.O.O.: Design the experiment and carry out the synthesis of the compounds. Dr. H.I. and Dr. J.D.H.: write the manuscript and proofread the manuscript. Dr. D.E.A.: Docked the synthesized compound against the target protein.
Corresponding author
Ethics declarations
Competing interests
No competing interest.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jimoh, T.A., Oyewale, A.O., Ibrahim, H. et al. Some Benzotriazole and Benzimidazole Derivatives as Antifungal Agents for Candida Species: A Molecular Docking Study. Chemistry Africa 6, 383–391 (2023). https://doi.org/10.1007/s42250-022-00498-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-022-00498-y