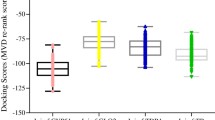
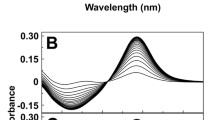
Abstract
The cytochrome P540 (CYP) superfamily currently includes about 9,000 proteins forming more than 800 families. The enzymes catalyze monooxygenation of a vast array of compounds and play essentially two roles. They provide biodefense (detoxification of xenobiotics, antibiotic production) and participate in biosynthesis of important endogenous molecules, particularly steroids. Based on these two roles, sterol 14|*alpha*|-demethylases (CYP51) belong to the second group of P450s. The CYP51 family, however, is very special as its members preserve strict functional conservation in enzyme activity in all biological kingdoms. At amino acid identity across the kingdoms as low as 25–30%, they all catalyze essentially the same three-step reaction of oxidative removal of the 14|*alpha*|-methyl group from the lanostane frame. This reaction is the required step in sterol biosynthesis of pathogenic microbes. We have shown that specific inhibition of protozoan CYP51 can potentially provide treatment for human trypanosomiases. Three sets of CYP51 inhibitors tested in vitro and in trypanosomal cells in this study include azoles [best results being 50% cell growth inhibition at <1 and at 1.3 μM for Trypanosoma cruzi (TC) and Trypanosoma brucei (TB), respectively], non-azole compounds (50% TC cell growth inhibition at 5 μM) and substrate analogs of the 14|*alpha*|-demethylase reaction. 32-Methylene cyclopropyl lanost-7-enol exhibited selectivity toward TC with 50% cell growth inhibition at 3 μM.




Similar content being viewed by others
Abbreviations
- CYP:
-
Cytochrome P450
- CYP51:
-
Sterol 14α-demethylase
- TC:
-
Trypanosoma cruzi
- TB:
-
Trypanosoma brucei
- MCP:
-
32-Methylene cyclopropyl-lanost-7-enol
- YNE:
-
32-Yne-lanost-7-enol
References
Volkman JK (2005) Sterols and other triterpenoids: source specificity and evolution of biosynthetic pathways. Org Geochem 36:139–159
Nes WR, McKean MR (1977) Biochemistry of steroids and other isopentenoids. University Park Press, Baltimore
Ourisson G, Rohmer M, Poralla K (1987) Prokaryotic hopanoids and other polyterpenoid sterol surrogates. Annu Rev Microbiol 41:301–333
Nes WR (1974) Role of sterols in membranes. Lipids 9:596–612
Nes WR, Nes WD (1980) Lipids in evolution. Plenum, New York, pp 71–81
Lepesheva GI, Waterman MR (2007) Sterol 14alpha-demethylase cytochrome P450 (CYP51), a P450 in all biological kingdoms. Biochim Biophys Acta 1770:467–477
Nes WD, Norton RA, Crumley FG, Madigan SJ, Katz ER (1990) Sterol phylogenesis and algal evolution. Proc Natl Acad Sci USA 87:7565–7569
Docampo R, Moreno SN, Turrens JF, Katzin AM, Gonzalez-Cappa SM, Stoppani AO (1981) Biochemical and ultrastructural alterations produced by miconazole and econazole in Trypanosoma cruzi. Mol Biochem Parasitol 3:169–180
Lazardi K, Urbina JA, de Souza W (1990) Ultrastructural alterations induced by two ergosterol biosynthesis inhibitors, ketoconazole and terbinafine, on epimastigotes and amastigotes of Trypanosoma (Schizotrypanum) cruzi. Antimicrob Agents Chemother 34:2097–2105
Urbina JA (2001) Specific treatment of Chagas disease: current status and new developments. Curr Opin Infect Dis 6:733–741
Croft SL, Barrett MP, Urbina JA (2005) Chemotherapy of trypanosomiases and leishmaniasis. Trends Parasitol 21:508–512
Roberts CW, McLeod R, Rice DW, Ginger M, Chance ML, Goad LJ (2003) Fatty acid and sterol metabolism: potential antimicrobial targets in apicomplexan and trypanosomatid parasitic protozoa. Mol Biochem Parasitol 126:129–142
Lepesheva GI, Virus C, Waterman MR (2003) Conservation in the CYP51 family. Role of the B’ helix/BC loop and helices F and G in enzymatic function. Biochemistry 42:9091–9101
Lepesheva GI, Nes WD, Zhou W, Hill GC, Waterman MR (2004) CYP51 from Trypanosoma brucei is obtusifoliol-specific. Biochemistry 43:10789–10799
Lepesheva GI, Zaitseva NG, Nes WD, Zhou W, Arase M, Liu J, Hill GC, Waterman MR (2006) CYP51 from Trypanosoma cruzi: a phyla-specific residue in the B’ helix defines substrate preferences of sterol 14α-demethylase. J Biol Chem 281:3577–3585
Lepesheva GI, Ott RD, Hargrove TY, Kleshchenko YY, Schuster I, Nes WD, Hill GC, Villalta F, Waterman MR (2007) Sterol 14α-demethylase as a potential target for antitrypanosomal therapy: enzyme inhibition and parasite cell growth. Chem Biol 11:1283–1293
Villalta F, Kierszenbaum F (1982) Growth of isolated amastigotes of Trypanosoma cruzi in cell-free medium. J. Protozool 29:570–576
Madison MN, Kleshchenko YY, Nde PN, Simmons KJ, Lima MF, Villalta F (2007) Human defensin α–1 causes Trypanosoma cruzi membrane pore formation and induces DNA fragmentation, which leads to trypanosome destruction. Infect Immun 75:4780–4791
Buckner FS, Griffin JH, Wilson AJ, Van Voorhis WC (2001) Potent anti-Trypanosoma cruzi activities of oxidosqualene cyclase inhibitors. Antimicrob Agents Chemother 45:1210–1215
Joubert BM, Buckner FS, Matsuda SP (2001) Trypanosome and animal lanosterol synthases use different catalytic motifs. Org Lett 3:1957–1960
Lepesheva GI, Hargrove TY, Ott RD, Nes WD, Waterman MR (2006) Biodiversity of CYP51 in trypanosomes. Biochem Soc Trans 34(Pt 6):1161–1164
Zhou W, Lepesheva GI, Waterman MR, Nes WD (2006) Mechanistic analysis of a multiple product sterol methyltransferase implicated in ergosterol biosynthesis in Trypanosoma brucei. J Biol Chem 281:6290–6296
Zhou W, Cross GA, Nes WD (2007) Cholesterol import fails to prevent catalyst-based inhibition of ergosterol synthesis and cell proliferation of Trypanosoma brucei. J Lipid Res 48:665–673
Faundez M, Pino L, Letelier P, Ortiz C, López R, Seguel C, Ferreira J, Pavani M, Morello A, Maya JD (2005) Buthionine sulfoximine increases the toxicity of nifurtimox and benznidazole to Trypanosoma cruzi. Antimicrob Agents Chemother 49:126–130
de Castro SL, de Meirelles Mde N (1987) Effect of drugs on Trypanosoma cruzi and on its interaction with heart muscle cell “in vitro”. Mem Inst Oswaldo Cruz 82:209–218
Saraiva J, Vega C, Rolon M, da Silva R, E Silva ML, Donate PM, Bastos JK, Gomez-Barrio A, de Albuquerque S (2007) In vitro and in vivo activity of lignan lactones derivatives against Trypanosoma cruzi. Parasitol Res 100:791–795
Docampo R, Mason RP, Mottley C, Muniz RP (1981) Generation of free radicals induced by nifurtimox in mammalian tissues. J Biol Chem 256:10930–10933
Docampo R (1990) Sensitivity of parasites to free radical damage by antiparasitic drugs. Chem Biol Interact 73:1–27
Viodé C, Bettache N, Cenas N, Krauth-Siegel RL, Chauvière G, Bakalara N, Périé J (1999) Enzymatic reduction studies of nitroheterocycles. Biochem Pharmacol 57:549–557
Revelli S, Le Page C, Piaggio E, Wietzerbin J, Bottasso O (1999) Benznidazole, a drug employed in the treatment of Chagas’ disease, down-regulates the synthesis of nitrite and cytokines by murine stimulated macrophages. Clin Exp Immunol 118:271–277
Diaz de Toranzo EG, Castro JA, Franke de Cazzulo BM, Cazzulo JJ (1988) Interaction of benznidazole reactive metabolites with nuclear and kinetoplastic DNA, proteins and lipids from Trypanosoma cruzi. Experientia 44:880–881
Wilkinson SR, Taylor MC, Horn D, Kelly JM, Cheeseman I (2008) A mechanism for cross-resistance to nifurtimox and benznidazole in trypanosomes. Proc Natl Acad Sci USA 105:5022–5027
Viodé C, Bettache N, Cenas N, Krauth-Siegel RL, Chauvière G, Bakalara N, Périé J (1999) Enzymatic reduction studies of nitroheterocycles. Biochem Pharmacol 57:549–557
Lupetti A, Danesi R, Campa M, Del Tacca M, Kelly S (2002) Molecular basis of resistance to azole antifungals. Trends Mol Med 8:76–81
Aoyama Y, Yoshida Y, Sonoda Y, Sato Y (1987) 7-Oxo-24, 25-dihydrolanosterol: a novel lanosterol 14 α-demethylase (P-45014DM) inhibitor which blocks electron transfer to the oxyferro intermediate. Biochim Biophys Acta 922:270–277
Frye LL, Leonard DA (1999) Lanosterol analogs: dual-action inhibitors of cholesterol biosynthesis. Crit Rev Biochem Mol Biol 34:123–140
Trzaskos JM, Ko SS, Magolda RL, Favata MF, Fischer RT, Stam SH, Johnson PR, Gaylor JL (1995) Substrate-based inhibitors of lanosterol 14α-methyl demethylase: I. Assessment of inhibitor structure-activity relationship and cholesterol biosynthesis inhibition properties. Biochemistry 34:9670–9676
Acknowledgments
This work was supported by grants from the American Heart Association (0535121 N to G.I.L), the National Institutes of Health (GM067871 to M.R.W and G.I.L., GM 081168 and AI 080580 to F.V. and GM63477 to W.D.N), and from the Robert A. Welch Foundation (D-1276 to W.D.N.).
We thank Dr. Inge Schuster (Institute of Pharmaceutical Chemistry, University Vienna, Oesterreich, Austria) and Novartis Research Institute (Vienna, Austria) for imidazole derivatives and Dr. Jens P. von Kries (Unit and Department of Medicinal Chemistry, Leibniz Institute for Molecular Pharmacology, Berlin, Germany) or HTS measurements.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Lepesheva, G.I., Hargrove, T.Y., Kleshchenko, Y. et al. CYP51: A Major Drug Target in the Cytochrome P450 Superfamily. Lipids 43, 1117–1125 (2008). https://doi.org/10.1007/s11745-008-3225-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-008-3225-y