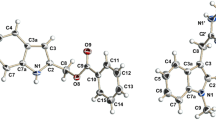
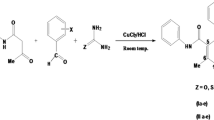
Abstract
Background
To tackle the menace of multi-resistant bacteria which pose challenges to scientist, four benzotriazole derivatives and three benzimidazole derivatives were synthesized via two routes due to the importance of heterocyclic compound in medicinal chemistry. Two protein target DNA gyrase with PDB ID 2XCT and PDB ID 3ILW were docked with the synthesized compound and compared with the two standard drugs sparfloxacin and ciprofloxacin.
Results
For benzotriazole derivatives, the reaction in acetonitrile resulted in lower yields (18–25%) than with dimethylformamide/potassium hydroxide (DMF/KOH), which generated higher yields (68–92%) in shorter reaction time. Those of benzimidazole were comparable. The products were characterized by FTIR, 1H, and 13C NMR, while the association between atoms was shown in 2D NMR. The synthesized compounds were docked against two DNA gyrase with PDB ID 2XCT and PDB ID 3ILW. It was observed that 1-butyl-1H-benzotriazole (− 13.716) and 1-butyl-3-octyl-1H-benzotriazolium (− 12.324) were the most active against PDB ID 2XCT and PDB ID 3ILW, respectively. However, they were both less active than the standard drugs used. Using the agar diffusion technique, the synthesized compounds demonstrated broad-spectrum antibacterial activity against gram-positive and gram-negative pathogenic bacteria strains. Notably, all the compounds were of varying potency; with inhibition zones ranging from 24 to 30 mm and MIC ranging from 25 to 50 μg/mL.
Conclusions
The benzotriazole and benzimidazole derivatives were synthesized successfully. This research revealed that the synthesized compounds were active against organisms with activity, the docking results revealed that the synthesized compounds have relatively low binding energy when compared with the standard drug used as well as those reported earlier. Thus, it will give better interaction on the binding site of the protein. These synthesized compounds could be a source of new drugs that could be used to treat multi-resistance bacteria.










Similar content being viewed by others
Data availability
Not applicable.
Abbreviations
- DNA:
-
Deoxyribonucleic acid
- PDB ID:
-
Protein Data Bank Identity
- DMF:
-
Dimethylformamide
- KOH:
-
Potassium hydroxide
- FTIR:
-
Fourier transform infrared spectroscopy
- GCMS:
-
Gas chromatography-mass spectroscopy
- TLC:
-
Thin layer chromatography
- DMSO:
-
Dimethyl sulfoxide
References
Zagotto G, Bortoli M (2021) Drug design: where we are and future prospects. Molecules 26(22):7061
Pinzi L, Rastelli G (2019) Molecular docking: shifting paradigms in drug discovery. Int J Mol Sci. https://doi.org/10.3390/ijms20184331
Ardebili A, Pouriayevali MH, Aleshikh S, Zahani M (2021) Antiviral therapeutic potential of curcumin: an update. Molecules 26:1–23
Rajasekaran S, Rao G (2012) Synthesis, antibacterial and antioxidant activity of some 2,3-susbtituted quinazolin-4(3H)-ones. Pharm Lett 4(2):470–474
Khayyat AN, Mohamed KO, Malebari AM, El-malah A (2021) Substituted imidazole-thione linked. Molecules 26:5983
Datta A, Alpana A, Shrikant M, Pratyush K, Abhibnav B, Ruchita T (2020) Review on synthetic study of benzotriazole. GSC Biol Pharm Sci 11(2):215–225. https://doi.org/10.30574/gscbps.2020.11.2.0137
Marinescu M (2021) Synthesis of antimicrobial benzimidazole–pyrazole compounds and their biological activities. Antibiotics (Basel, Switzerland) 10(8):1–29. https://doi.org/10.3390/antibiotics10081002
Katritzky AR, Lan X, Yang JZ, Denisko OV (1998). Properties and synthetic utility of N-substituted benzotriazoles. 2665(94): 409–548
Guruvelli PVS, Wagmare P, Harinath BC, Jamullamudi RN, Kurre PN, Muthyala MKK (2018) Synthesis, screening and docking analysis of novel benzimidazolium and benzotriazolium compounds as potent anti tubercular agents. Anti-Infect Agents 17(1):20–27. https://doi.org/10.2174/2211352516666180810101327
Lino A, Deogracious O (2006) The in-vitro antibacterial activity of Annona senegalensis, Securidacca longipendiculata and Steganotaenia araliacea—Ugandan medicinal plants. Afr Health Sci 6(1):31–35
Sochorova R (2001) Isoquinoline alkaloids from mahonia aquifolium stem bark are active against Malassezia spp. Folia Microbiol 46(2):107–111
Evans DA (2014) History of the Harvard ChemDraw Project. Angew Chemie Int Ed 53(42):11140–11145. https://doi.org/10.1002/anie.201405820
Li Z, Wan H, Shi Y, Ouyang P (2004) Personal experience with four kinds of chemical structure drawing software: review on chemdraw, chemwindow, ISIS/draw, and chemsketch. J Chem Inf Comput Sci 44(5):1886–1890. https://doi.org/10.1021/ci049794h
Ruben A, Maxim T (1994) Biased probability Monte Carlo conformational searches and electrostatic calculations for peptides and protein. J Mol Biol 983–1002
An J, Totrov M, Abagyan R (2005) Pocketome via comprehensive identification and classification of ligand binding envelopes. Molecular and Cellular Proteomics, 4(6):752–761. https://doi.org/10.1074/mcp.M400159-MCP200
Viswanadhan VN, Ghose AK, Reyankar GR, Robins RK, Al ET (1989) Atomic Physicochemical Parameters for Three Dimensional Structure Directed Quantitative Structure-Activity Relationships . 4 . Additional Parameters for Hydrophobic and Dispersive Interactions and Their Application for an Automated Superposition of Certai. Eur J Med Chem 183:163–172
Tretter EM, Schoeffler AJ, Weisfield SR, Berger JM (2009) Crystal structure of the DNA Gyrase. August, pp, 492–495. https://doi.org/10.1002/prot.22600
Bax BD, Chan PF, Eggleston DS, Fosberry A, Gentry DR, Gorrec F, Giordano I, Hann MM, Hennessy A., Hibbs M, Huang J, Jones E, Jones J, Brown K K, Lewis CJ, May EW, Saunders MR, Singh O, Spitzfaden CE, Gwynn MN (2010) Type IIA topoisomerase inhibition by a new class of antibacterial agents. Nature, 466(7309): 935–940.https://doi.org/10.1038/nature09197
Capurso M, Gette R, Radivoy G, Dorn V (2019) The Sn2 Reaction: A Theoretical-Computational Analysis of a Simple and Very Interesting Mechanism. Proceedings, 41(1):81. https://doi.org/10.3390/ecsoc-23-06514
Swamy SN, Sarala G, Priya BS, Gaonkar SL, Prasad JS, Rangappa KS (2006) Microwave-assisted synthesis of N -alkylated benzotriazole derivatives : Antimicrobial studies. Bioorganic & Medicinal Chemistry Letters, 16:999–1004. https://doi.org/10.1016/j.bmcl.2005.10.084
Abbas H, Niazi M, Makker J (2017) Mucosa-Associated Lymphoid Tissue (MALT) Lymphoma of the Colon: A Case Report and a Literature Review. Am J Case 18:491–497. https://doi.org/10.12659/AJCR.902843.
Bairam R, Muppavarapu SM, Sreekanth S (2017) SYNTHESIS , CHARACTERIZATION , BIOLOGICAL EVALUATION AND DOCKING OF SOME NOVEL SUBSTITUTED 1 , 3-THIAZINE DERIVATIVES. International Journal of Pharmacy and Pharmaceutical Sciences, 9(3):233–242.
Hameed S, Seraj F, Ra R, Chigurupati S (2019) Synthesis of benzotriazoles derivatives and their dual potential as a-amylase and a-glucosidase inhibitors in vitro : Structure-activity relationship, molecular docking, and kineti studies. Eur. J Med Chem 183. https://doi.org/10.1016/j.ejmech.2019.111677
Acknowledgements
The authors are grateful to Mall. Abdullahi Mikaila of Nigeria Institute of Leather Technology (NILET) Zaria, Kaduna State, Nigeria for the antimicrobial activity studies and Mall. Jafar of Multi-User Laboratory, Ahmadu Bello University, Zaria Kduna State, Nigeria for the spectroscopic analysis. Finally, I will like to acknowledge Dr. Abdulfatai Usman, Mall. Abduljelil Ajala and Mall. Zakari Yau Ibrahim for their advice.
Funding
No any form of fund was received from any organization.
Author information
Authors and Affiliations
Contributions
TAJ. and Prof. AOO.: design the experiment and carry out the synthesis of the compounds. Dr. HI. and Dr. JDH.: write the manuscript and proofread the manuscript. Dr. DEA.: docked the synthesized compound against the target protein.
Corresponding author
Ethics declarations
Conflict of interest
No competing interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jimoh, T.A., Oyewale, A.O., Ibrahim, H. et al. Biological Evaluation and Docking Study of Synthesized Derivatives of Benzotriazole and Benzimidazole as Antibacterial Agents. Chemistry Africa 5, 509–523 (2022). https://doi.org/10.1007/s42250-022-00348-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-022-00348-x