Abstract
Purpose of Review
In this review, we will explore the clinical and commercial focus on amniotic membranes (AMs) and their use in tissue engineering (TE). We will showcase the therapeutic potential of AM-isolated epithelial cells (hAECs) and the prospective use of their secreted factors, extracellular vesicles (EVs), in cell and non-cell therapies.
Recent Findings
The potential of the hAECs as a therapeutic source has been investigated in various preclinical models with some progressing into phase I clinical trials to evaluate their safety. Additionally, multiple animal studies showcase the therapeutic potential of EVs as non-cellular treatments.
Summary
The amniotic membrane (AM) has been used as a form of regenerative medicine in wound healing for burns and ulcerated surfaces and in ophthalmology for over a century. In the last few decades, research has looked to the use of the various stem cells that can be isolated from the AM. The use of AM-isolated hAECs has proven rather promising with phase I clinical trials currently underway across life-threatening diseases in both pediatric and adult populations. However, due to limitations of using cell-based therapies (e.g., cost of production, delivery restricted to major hospitals, etc.), attention has turned to investigating EVs secreted by the cells.



Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Young SM, Benyshek DC. In search of human placentophagy: a cross-cultural survey of human placenta consumption, disposal practices, and cultural beliefs. Ecol Food Nutr. 2010;49(6):467–84.
Davis J. Skin transplantation with a review of 550 cases at the Johns Hopkins Hospital. Johns Hopkins Med J. 1910;15.
Stern M. The grafting of preserved amniotic membrane to burned and ulcerated surfaces, substituting skin grafts. J Am Med Assoc. 1913;60(13):973–4.
Sabella N. Use of fetal membranes in skin grafting. Med Rec. 1913;83:478–80.
Silini AR, et al. The long path of human placenta, and its derivatives, in regenerative medicine. Front Bioeng Biotechnol. 2015;3:162.
De Rotth A. Plastic repair of conjunctival defects with fetal membranes. Arch Ophthalmol. 1940;23:522–5.
Sorsby A, Symons HM. Amniotic membrane grafts in caustic burns of the eye (burns of the second degree). Br J Ophthalmol. 1946;30:337–45.
Sorsby A, Haythorne J, Reed H. Further experience with amniotic membrane grafts in caustic burns of the eye. Br J Ophthalmol. 1947;31(7):409–18.
Trelford-Sauder M, Trelford JD, Matolo NM. Replacement of the peritoneum with amnion following pelvic exenteration. Surg Gynecol Obstet. 1977;145(5):699–701.
Trelford-Sauder M, Dawe EJ, Trelford JD. Use of allograft amniotic membrane for control of intra-abdominal adhesions. J Med. 1978;9(4):273–84.
Silverton JS, Trelford JD, Roussere JT, Wolfe BM, Conti S. The use of amniotic membrane in acute massive full-thickness loss of the abdominal wall from clostridial myonecrosis. Ann Plast Surg. 1979;3(6):558–66.
Dhall K. Amnion graft for treatment of congenital absence of the vagina. Br J Obstet Gynaecol. 1984;91(3):279–82.
Nisolle M, Donnez J. Vaginoplasty using amniotic membranes in cases of vaginal agenesis or after vaginectomy. J Gynecol Surg. 1992;8(1):25–30.
Georgy M, Aziz N. Vaginoplasty using amnion graft: new surgical technique using the laparoscopic transillumination light. J Obstet Gynaecol. 1996;16:262–4.
Gharib M, Ure BM, Klose M. Use of amniotic grafts in the repair of gastroschisis. Pediatr Surg Int. 1996;11(2-3):96–9.
Kubanyi A. Prevention of peritoneal adhesions by transplantation of amnion. Br Med J. 1947;2(4514):55–6.
Muralidharan S, Gu J, Laub GW, Cichon R, Daloisio C, McGrath LB. A new biological membrane for pericardial closure. J Biomed Mater Res. 1991;25(10):1201–9.
Chao YC, Humphreys S, Penfield W. A new method of preventing adhesions. The use of amnioplastin after craniotomy. Br Med J. 1940;1(4134):517–538.1.
Troensegaard-Hansen E. Amniotic grafts in chronic skin ulceration. Lancet. 1950;1(6610):859–60.
Bennett JP, Matthews R, Faulk WP. Treatment of chronic ulceration of the legs with human amnion. Lancet. 1980;1(8179):1153–6.
Subrahmanyam M. Amniotic membrane as a cover for microskin grafts. Br J Plast Surg. 1995;48(7):477–8.
Gruss JS, Jirsch DW. Human amniotic membrane: a versatile wound dressing. Can Med Assoc J. 1978;118(10):1237–46.
Bose B. Burn wound dressing with human amniotic membrane. Ann R Coll Surg Engl. 1979;61(6):444–7.
Dua HS, Gomes JAP, King AJ, Maharajan VS. The amniotic membrane in ophthalmology. Surv Ophthalmol. 2004;49(1):51–77.
Fetterolf DE, Snyder RJ. Scientific and clinical support for the use of dehydrated amniotic membrane in wound management. Wounds. 2012;24(10):299–307.
Morandi F, et al. Human amnion epithelial cells impair T cell proliferation: the role of HLA-G and HLA-E molecules. Cells. 2020;9(9):2123.
Glat P, Orgill DP, Galiano R, Armstrong D, Serena T, DiDomenico LA, et al. Placental membrane provides improved healing efficacy and lower cost versus a tissue-engineered human skin in the treatment of diabetic foot ulcerations. Plast Reconstr Surg Glob Open. 2019;7(8):e2371.
Snyder RJ, et al. A prospective, randomized, multicenter, controlled evaluation of the use of dehydrated amniotic membrane allograft compared to standard of care for the closure of chronic diabetic foot ulcer. Wounds. 2016;28(3):70–7.
Doucette M, et al. Early advanced therapy for diabetic foot ulcers in high amputation risk veterans: a cohort study. Int J Low Extrem Wounds. 2020;22:1534734620928151.
Zelen CM, Serena TE, Snyder RJ. A prospective, randomised comparative study of weekly versus biweekly application of dehydrated human amnion/chorion membrane allograft in the management of diabetic foot ulcers. Int Wound J. 2014;11(2):122–8.
Serena TE, Carter MJ, le LT, Sabo MJ, DiMarco DT, EpiFix VLU Study Group. A multicenter, randomized, controlled clinical trial evaluating the use of dehydrated human amnion/chorion membrane allografts and multilayer compression therapy vs. multilayer compression therapy alone in the treatment of venous leg ulcers. Wound Repair Regen. 2014;22(6):688–93.
Tettelbach W, Cazzell S, Reyzelman AM, Sigal F, Caporusso JM, Agnew PS. A confirmatory study on the efficacy of dehydrated human amnion/chorion membrane dHACM allograft in the management of diabetic foot ulcers: a prospective, multicentre, randomised, controlled study of 110 patients from 14 wound clinics. Int Wound J. 2019;16(1):19–29.
Zelen CM, Serena TE, Denoziere G, Fetterolf DE. A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J. 2013;10(5):502–7.
Zelen CM. An evaluation of dehydrated human amniotic membrane allografts in patients with DFUs. J Wound Care. 2013;22(7):347–8 350-1.
Bianchi C, Cazzell S, Vayser D, Reyzelman AM, Dosluoglu H, Tovmassian G, et al. A multicentre randomised controlled trial evaluating the efficacy of dehydrated human amnion/chorion membrane (EpiFix((R))) allograft for the treatment of venous leg ulcers. Int Wound J. 2018;15(1):114–22.
Zelen CM, Gould L, Serena TE, Carter MJ, Keller J, Li WW. A prospective, randomised, controlled, multi-centre comparative effectiveness study of healing using dehydrated human amnion/chorion membrane allograft, bioengineered skin substitute or standard of care for treatment of chronic lower extremity diabetic ulcers. Int Wound J. 2015;12(6):724–32.
Lavery L, Fulmer J, Shebetka KA, Regulski M, Vayser D, Fried D, et al. Open-label extension phase of a chronic diabetic foot ulcer multicenter, controlled, randomized clinical trial using cryopreserved placental membrane. Wounds. 2018;30(9):283–9.
Ananian CE, Dhillon YS, van Gils CC, Lindsey DC, Otto RJ, Dove CR, et al. A multicenter, randomized, single-blind trial comparing the efficacy of viable cryopreserved placental membrane to human fibroblast-derived dermal substitute for the treatment of chronic diabetic foot ulcers. Wound Repair Regen. 2018;26(3):274–83.
Mligiliche N, Endo K, Okamoto K, Fujimoto E, Ide C. Extracellular matrix of human amnion manufactured into tubes as conduits for peripheral nerve regeneration. J Biomed Mater Res. 2002;63(5):591–600.
Mohammad J, Shenaq J, Rabinovsky E, Shenaq S. Modulation of peripheral nerve regeneration: a tissue-engineering approach. The role of amnion tube nerve conduit across a 1-centimeter nerve gap. Plast Reconstr Surg. 2000;105(2):660–6.
Miyamoto K, Hayashi K, Suzuki T, Ichihara S, Yamada T, Kano Y, et al. Human placenta feeder layers support undifferentiated growth of primate embryonic stem cells. Stem Cells. 2004;22(4):433–40.
Ueno M, Matsumura M, Watanabe K, Nakamura T, Osakada F, Takahashi M, et al. Neural conversion of ES cells by an inductive activity on human amniotic membrane matrix. Proc Natl Acad Sci U S A. 2006;103(25):9554–9.
** CZ, Park SR, Choi BH, Lee KY, Kang CK, Min BH. Human amniotic membrane as a delivery matrix for articular cartilage repair. Tissue Eng. 2007;13(4):693–702.
Portmann-Lanz CB, Ochsenbein-Kölble N, Marquardt K, Lüthi U, Zisch A, Zimmermann R. Manufacture of a cell-free amnion matrix scaffold that supports amnion cell outgrowth in vitro. Placenta. 2007;28(1):6–13.
Yang L, Shirakata Y, Shudou M, Dai X, Tokumaru S, Hirakawa S, et al. New skin-equivalent model from de-epithelialized amnion membrane. Cell Tissue Res. 2006;326(1):69–77.
Ishino Y, Sano Y, Nakamura T, Connon CJ, Rigby H, Fullwood NJ, et al. Amniotic membrane as a carrier for cultivated human corneal endothelial cell transplantation. Invest Ophthalmol Vis Sci. 2004;45(3):800–6.
Farhadihosseinabadi B, et al. Amniotic membrane and its epithelial and mesenchymal stem cells as an appropriate source for skin tissue engineering and regenerative medicine. Artif Cells Nanomed Biotechnol. 2018;46(sup2):431–40.
Tsai SH, Liu YW, Tang WC, Zhou ZW, Hwang CY, Hwang GY, et al. Characterization of porcine arterial endothelial cells cultured on amniotic membrane, a potential matrix for vascular tissue engineering. Biochem Biophys Res Commun. 2007;357(4):984–90.
Xu H, et al. Therapeutic potential of human amniotic epithelial cells on injuries and disorders in the central nervous system. Stem Cells Int. 2019;2019:5432301.
Magatti M, Vertua E, Cargnoni A, Silini A, Parolini O. The immunomodulatory properties of amniotic cells: the two sides of the coin. Cell Transplant. 2018;27(1):31–44.
Krause M, Lozano J, Lim R. The regenerative and reparative potential of amniotic membrane stem cells: biology, manufacturing and translational medicine. In: Perital Stem Cells; 2019. p. 9–26.
Fatimah SS, Tan GC, Chua KH, Tan AE, Hayati AR. Effects of epidermal growth factor on the proliferation and cell cycle regulation of cultured human amnion epithelial cells. J Biosci Bioeng. 2012;114(2):220–7.
Insausti CL, Blanquer M, García-Hernández AM, Castellanos G, Moraleda JM. Amniotic membrane-derived stem cells: immunomodulatory properties and potential clinical application. Stem Cells Cloning. 2014;7:53–63.
McDonald CA, Payne NL, Sun G, Moussa L, Siatskas C, Lim R, et al. Immunosuppressive potential of human amnion epithelial cells in the treatment of experimental autoimmune encephalomyelitis. J Neuroinflammation. 2015;12(1):112.
Lim R. Concise review: fetal membranes in regenerative medicine: new tricks from an old dog? Stem Cells Transl Med. 2017;6(9):1767–76.
Tan JL, Chan ST, Wallace EM, Lim R. Human amnion epithelial cells mediate lung repair by directly modulating macrophage recruitment and polarization. Cell Transplant. 2014;23(3):319–28.
Vosdoganes P, et al. Human amnion epithelial cells as a treatment for inflammation-induced fetal lung injury in sheep.(Report). Am J Obstet Gynecol. 2011;205(2):156.e26–33.
• Lim R, et al. First-in-human administration of allogeneic amnion cells in premature infants with bronchopulmonary dysplasia: a safety study. Stem Cells Transl Med. 2018;7(9):628–35 Demonstrates the safety of hAECS with no adverse effects in this first-in-human clinical trial of hAECs in babies with BPD.
Zhu D, Tan J, Maleken AS, Muljadi R, Chan ST, Lau SN, et al. Human amnion cells reverse acute and chronic pulmonary damage in experimental neonatal lung injury. Stem Cell Res Ther. 2017;8(1):257.
• Hodge A, et al. Soluble factors derived from human amniotic epithelial cells suppress collagen production in human hepatic stellate cells. Cytotherapy. 2014;16(8):1132–44 Provides evidence that factors secreted by hAECs have anti-fibrotic effects in liver fibrosis in vivo, significantly decreasing the expression of pro-fibrotic cytokine TGF-β1 and reducing HSC collagen production.
Kuk N, Hodge A, Sun Y, Correia J, Alhomrani M, Samuel C, et al. Human amnion epithelial cells and their soluble factors reduce liver fibrosis in murine non-alcoholic steatohepatitis. J Gastroenterol Hepatol. 2019;34(8):1441–9.
Manuelpillai U, Lourensz D, Vaghjiani V, Tchongue J, Lacey D, Tee JY, et al. Human amniotic epithelial cell transplantation induces markers of alternative macrophage activation and reduces established hepatic fibrosis. PLoS One. 2012;7(6):e38631.
Kakishita K, Nakao N, Sakuragawa N, Itakura T. Implantation of human amniotic epithelial cells prevents the degeneration of nigral dopamine neurons in rats with 6-hydroxydopamine lesions. Brain Res. 2003;980:48–56.
Roh D-H, Seo MS, Choi HS, Park SB, Han HJ, Beitz AJ, et al. Transplantation of human umbilical cord blood or amniotic epithelial stem cells alleviates mechanical allodynia after spinal cord injury in rats. Cell Transplant. 2013;22(9):1577–90.
Dong W, Chen H, Yang X, Guo L, Hui G. Treatment of intracerebral haemorrhage in rats with intraventricular transplantation of human amniotic epithelial cells. Cell Biol Int. 2010;34(6):573–7.
Roy R, et al. Epithelial-to-mesenchymal transition enhances the cardioprotective capacity of human amniotic epithelial cells. Cell Transplant. 2013;24(6):985–1002.
Fournel S, Aguerre-Girr M, Huc X, Lenfant F, Alam A, Toubert A, et al. Cutting edge: soluble HLA-G1 triggers CD95/CD95 ligand-mediated apoptosis in activated CD8+ cells by interacting with CD8. J Immunol. 2000;164(12):6100–4.
Marchal-Bras-Goncalves R, Rouas-Freiss N, Connan F, Choppin J, Dausset J, Carosella ED, et al. A soluble HLA-G protein that inhibits natural killer cell-mediated cytotoxicity. Transplant Proc. 2001;33(3):2355–9.
Kapasi K, Albert SE, Yie SM, Zavazava N, Librach CL. HLA-G has a concentration-dependent effect on the generation of an allo-CTL response. Immunology. 2000;101(2):191–200.
Riteau B, Rouas-Freiss N, Menier C, Paul P, Dausset J, Carosella ED. HLA-G2, -G3, and -G4 isoforms expressed as nonmature cell surface glycoproteins inhibit NK and antigen-specific CTL cytolysis. J Immunol. 2001;166(8):5018–26.
Riteau B, Menier C, Khalil-Daher I, Sedlik C, Dausset J, Rouas-Freiss N, et al. HLA-G inhibits the allogeneic proliferative response. J Reprod Immunol. 1999;43(2):203–11.
Bainbridge DR, Ellis SA, Sargent IL. HLA-G suppresses proliferation of CD4(+) T-lymphocytes. J Reprod Immunol. 2000;48(1):17–26.
Kanai T, Fujii T, Kozuma S, Yamashita T, Miki A, Kikuchi A, et al. Soluble HLA-G influences the release of cytokines from allogeneic peripheral blood mononuclear cells in culture. Mol Hum Reprod. 2001;7(2):195–200.
Kanai T, et al. Human leukocyte antigen-G-expressing cells differently modulate the release of cytokines from mononuclear cells present in the decidua versus peripheral blood. Am J Reprod Immunol. 2001;45(2):94–9.
Li H, Niederkorn JY, Neelam S, Mayhew E, Word RA, McCulley JP, et al. Immunosuppressive factors secreted by human amniotic epithelial cells. Invest Ophthalmol Vis Sci. 2005;46(3):900–7.
Miki T, Lehmann T, Cai H, Stolz DB, Strom SC. Stem cell characteristics of amniotic epithelial cells. Stem Cells. 2005;23(10):1549–59.
Parolini O, et al. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells. 2008;26(2):300–11.
Miki T, Strom SC. Amnion-derived pluripotent/multipotent stem cells. Stem Cell Rev. 2006;2(2):133–42.
Atala A. Perinatal stem cells: research and therapy. Cambridge: Academic Press; 2018.
Moodley Y, Ilancheran S, Samuel C, Vaghjiani V, Atienza D, Williams ED, et al. Human amnion epithelial cell transplantation abrogates lung fibrosis and augments repair. Am J Respir Crit Care Med. 2010;182(5):643–51.
Murphy S, Lim R, Dickinson H, Acharya R, Rosli S, Jenkin G, et al. Human amnion epithelial cells prevent bleomycin-induced lung injury and preserve lung function. Cell Transplant. 2011;20(6):909–24.
Murphy SV, Shiyun SC, Tan JL, Chan S, Jenkin G, Wallace EM, et al. Human amnion epithelial cells do not abrogate pulmonary fibrosis in mice with impaired macrophage function. Cell Transplant. 2012;21(7):1477–92.
Vosdoganes P, Wallace EM, Chan ST, Acharya R, Moss TJM, Lim R. Human amnion epithelial cells repair established lung injury. Cell Transplant. 2013;22(8):1337–49.
Tan JL, et al. Amnion cell-mediated immune modulation following bleomycin challenge: controlling the regulatory T cell response. Stem Cell Res Ther. 2015;6(1):1–12.
Royce SG, Patel KP, Mao W, Zhu D, Lim R, Samuel CS. Serelaxin enhances the therapeutic effects of human amnion epithelial cell-derived exosomes in experimental models of lung disease. Br J Pharmacol. 2019;176(13):2195–208.
Geng L, Chen Z, Ren H, Niu X, Yu X, Yan H. Effects of an early intervention using human amniotic epithelial cells in a COPD rat model. Pathol-Res Prac. 2016;212(11):1027–33.
Vosdoganes P, et al. Human amnion epithelial cells as a treatment for inflammation-induced fetal lung injury in sheep. Am J Obstet Gynecol. 2011;205(2):156.e26–33.
Hodges RJ, et al. Human amnion epithelial cells reduce ventilation-induced preterm lung injury in fetal sheep. Am J Obstet Gynecol. 2012;206(5):448.e8–448.e15.
Vosdoganes P, Lim R, Koulaeva E, Chan ST, Acharya R, Moss TJM, et al. Human amnion epithelial cells modulate hyperoxia-induced neonatal lung injury in mice. Cytotherapy. 2013;15(8):1021–9.
Zhu D, Tan J, Maleken AS, Muljadi R, Chan ST, Lau SN, et al. Human amnion cells reverse acute and chronic pulmonary damage in experimental neonatal lung injury. Stem Cell Res Ther. 2017;8(1):257.
Fang C-H, ** J, Joe JH, Song YS, So BI, Lim SM, et al. In vivo differentiation of human amniotic epithelial cells into cardiomyocyte-like cells and cell transplantation effect on myocardial infarction in rats: comparison with cord blood and adipose tissue-derived mesenchymal stem cells. Cell Transplant. 2012;21(8):1687–96.
Song Y-S, Joo HW, Park IH, Shen GY, Lee Y, Shin JH, et al. Transplanted human amniotic epithelial cells secrete paracrine proangiogenic cytokines in rat model of myocardial infarctio. Cell Transplant. 2015;24(10):2055–64.
Yawno T, Schuilwerve J, Moss TJM, Vosdoganes P, Westover AJ, Afandi E, et al. Human amnion epithelial cells reduce fetal brain injury in response to intrauterine inflammation. Dev Neurosci. 2013;35(2-3):272–82.
Yawno T, Sabaretnam T, Li J, Mcdonald C, Lim R, Jenkin G, et al. Human amnion epithelial cells protect against white matter brain injury after repeated endotoxin exposure in the preterm ovine fetus. Cell Transplant. 2017;26(4):541–53.
Barton SK, Melville JM, Tolcos M, Polglase GR, McDougall ARA, Azhan A, et al. Human amnion epithelial cells modulate ventilation-induced white matter pathology in preterm lambs. Dev Neurosci. 2015;37(4-5):338–48.
Leaw B, et al. Human amnion epithelial cells rescue cell death via immunomodulation of microglia in a mouse model of perinatal brain injury. Stem Cell Res Ther. 2017;8(1):1–17.
van den Heuij LG, Fraser M, Miller SL, Jenkin G, Wallace EM, Davidson JO, et al. Delayed intranasal infusion of human amnion epithelial cells improves white matter maturation after asphyxia in preterm fetal sheep. J Cereb Blood Flow Metab. 2019;39(2):223–39.
Evans MA, Lim R, Kim HA, Chu HX, Gardiner-Mann CV, Taylor KWE, et al. Acute or delayed systemic administration of human amnion epithelial cells improves outcomes in experimental stroke. Stroke. 2018;49(3):700–9.
Liu T, Wu J, Huang Q, Hou Y, Jiang Z, Zang S, et al. Human amniotic epithelial cells ameliorate behavioral dysfunction and reduce infarct size in the rat middle cerebral artery occlusion model. Shock. 2008;29(5):603–11.
Zhou H, et al. HAEC in the treatment of brain hemorrhage: a preliminary observation in rabbits. Int J Clin Exp Pathol. 2015;8(6):6772.
Liang H, Guan D, Gao A, Yin Y, **g M, Yang L, et al. Human amniotic epithelial stem cells inhibit microglia activation through downregulation of tumor necrosis factor-α, interleukin-1β and matrix metalloproteinase-12 in vitro and in a rat model of intracerebral hemorrhage. Cytotherapy. 2014;16(4):523–34.
Kim HA, et al. Systemic treatment with human amnion epithelial cells after experimental traumatic brain injury. Brain Behav Immun Health. 2020;5:100072.
Wu Z-Y, et al. Transplantation of human amniotic epithelial cells improves hindlimb function in rats with spinal cord injury. Chin Med J. 2006;119(24):2101–7.
Meng XT, et al. Co-transplantation of bFGF-expressing amniotic epithelial cells and neural stem cells promotes functional recovery in spinal cord-injured rats. Cell Biol Int. 2008;32(12):1546–58.
Xue H, et al. Development of a chemically extracted acellular muscle scaffold seeded with amniotic epithelial cells to promote spinal cord repair. J Biomed Mater Res A. 2013;101(1):145–56.
Wang T-G, et al. Human amniotic epithelial cells combined with silk fibroin scaffold in the repair of spinal cord injury. Neural Regen Res. 2016;11(10):1670.
Sankar V, Muthusamy R. Role of human amniotic epithelial cell transplantation in spinal cord injury repair research. Neuroscience. 2003;118(1):11–7.
Kim KY, Suh Y-H, Chang K-A. Therapeutic effects of human amniotic epithelial stem cells in a transgenic mouse model of Alzheimer’s disease. Int J Mol Sci. 2020;21(7):2658.
Yang X, Song L, Wu N, Liu Z, Xue S, Hui G. An experimental study on intracerebroventricular transplantation of human amniotic epithelial cells in a rat model of Parkinson’s disease. Neurol Res. 2010;32(10):1054–9.
Kakishita K, Nakao N, Sakuragawa N, Itakura T. Implantation of human amniotic epithelial cells prevents the degeneration of nigral dopamine neurons in rats with 6-hydroxydopamine lesions. Brain Res. 2003;980(1):48–56.
Kuk N, Hodge A, Sun Y, Correia J, Alhomrani M, Samuel C, et al. Human amnion epithelial cells and their soluble factors reduce liver fibrosis in murine non-alcoholic steatohepatitis. J Gastroenterol Hepatol. 2019;34(8):1441–9.
Manuelpillai U, Tchongue J, Lourensz D, Vaghjiani V, Samuel CS, Liu A, et al. Transplantation of human amnion epithelial cells reduces hepatic fibrosis in immunocompetent CCl4-treated mice. Cell Transplant. 2010;19(9):1157–68.
Hong S-B, Seo MS, Park SB, Seo YJ, Kim JS, Kang KS. Therapeutic effects of human amniotic epithelial stem cells in Niemann–Pick type C1 mice. Cytotherapy. 2012;14(5):630–8.
Skvorak KJ, Dorko K, Marongiu F, Tahan V, Hansel MC, Gramignoli R, et al. Placental stem cell correction of murine intermediate maple syrup urine disease. Hepatology. 2013;57(3):1017–23.
Skvorak KJ, Dorko K, Marongiu F, Tahan V, Hansel MC, Gramignoli R, et al. Improved amino acid, bioenergetic metabolite and neurotransmitter profiles following human amnion epithelial cell transplant in intermediate maple syrup urine disease mice. Mol Genet Metab. 2013;109(2):132–8.
Liu YH, Vaghjiani V, Tee JY, To K, Cui P, Oh DY, et al. Amniotic epithelial cells from the human placenta potently suppress a mouse model of multiple sclerosis. PLoS One. 2012;7(4):e35758.
Tan B, Yuan W, Li J, Yang P, Ge Z, Liu J, et al. Therapeutic effect of human amniotic epithelial cells in murine models of Hashimoto’s thyroiditis and systemic lupus erythematosus. Cytotherapy. 2018;20(10):1247–58.
Zhang Q, Huang Y, Sun J, Gu T, Shao X, Lai D. Immunomodulatory effect of human amniotic epithelial cells on restoration of ovarian function in mice with autoimmune ovarian disease. Acta Biochim Biophys Sin. 2019;51(8):845–55.
Lim R, Malhotra A, Tan J, Chan ST, Lau S, Zhu D, et al. First-in-human administration of allogeneic amnion cells in premature infants with bronchopulmonary dysplasia: a safety study. Stem Cells Transl Med. 2018;7(9):628–35.
Baker EK, Malhotra A, Lim R, Jacobs SE, Hooper SB, Davis PG, et al. Human amnion cells for the prevention of bronchopulmonary dysplasia: a protocol for a phase I dose escalation study. BMJ Open. 2019;9(2):e026265.
Lim R, Hodge A, Moore G, Wallace EM, Sievert W. A pilot study evaluating the safety of intravenously administered human amnion epithelial cells for the treatment of hepatic fibrosis. Front Pharmacol. 2017;8:549.
Phan TG, Ma H, Lim R, Sobey CG, Wallace EM. Phase 1 trial of amnion cell therapy for ischemic stroke. Front Neurol. 2018;9:198.
Akle CA, Adinolfi M, Welsh KI, Leibowitz S, McColl I. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet. 1981;2(8254):1003–5.
Adinolfi M, Akle CA, McColl I, Fensom AH, Tansley L, Connolly P, et al. Expression of HLA antigens, beta 2-microglobulin and enzymes by human amniotic epithelial cells. Nature. 1982;295(5847):325–7.
Scaggiante B, et al. Successful therapy of Niemann-Pick disease by implantation of human amniotic membrane. Transplantation. 1987;44(1):59–61.
Bembi B, Comelli M, Scaggiante B, Pineschi A, Rapelli S, Gornati R, et al. Treatment of sphingomyelinase deficiency by repeated implantations of amniotic epithelial cells. Am J Med Genet. 1992;44(4):527–33.
• Niknejad H, et al. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater. 2008;15:88–99 Discusses the biological properties of the amniotic membrane that makes it an ideal candidate for creating scaffolds that can be used in tissue engineering.
Benson-Martin J, Zammaretti P, Bilic G, Schweizer T, Portmann-Lanz B, Burkhardt T, et al. The Young’s modulus of fetal preterm and term amniotic membranes. Eur J Obstet Gynecol Reprod Biol. 2006;128(1-2):103–7.
Gobinathan S, Zainol SS, Azizi SF, Iman NM, Muniandy R, Hasmad HN, et al. Decellularization and genipin crosslinking of amniotic membrane suitable for tissue engineering applications. J Biomater Sci Polym Ed. 2018;29(17):2051–67.
Wang F, Liu T, Yang L, Zhang G, Liu H, Yi X, et al. Urethral reconstruction with tissue-engineered human amniotic scaffold in rabbit urethral injury models. Med Sci Monit. 2014;20:2430–8.
Lai JY. Photo-cross-linking of amniotic membranes for limbal epithelial cell cultivation. Mater Sci Eng C Mater Biol Appl. 2014;45:313–9.
Murphy SV, Skardal A, Nelson RA Jr, Sunnon K, Reid T, Clouse C, et al. Amnion membrane hydrogel and amnion membrane powder accelerate wound healing in a full thickness porcine skin wound model. Stem Cells Transl Med. 2020;9(1):80–92.
Murphy SV, Skardal A, Song L, Sutton K, Haug R, Mack DL, et al. Solubilized amnion membrane hyaluronic acid hydrogel accelerates full-thickness wound healing. Stem Cells Transl Med. 2017;6(11):2020–32.
Ramakrishnan R, Krishnan LK, Nair RP, Krishnan KV. Reinforcement of amniotic membrane with fibrin coated poly-[Lactide-co-Glycolide-co-Caprolactone] terpolymer containing silver nanoparticles for potential wound healing applications. Int J Polym Mater Polym Biomater. 2020;69(12):810–9.
Toniato TV, Stocco TD, dos Martins DS, Santanna LB, Tim CR, Marciano FR, et al. Hybrid chitosan/amniotic membrane-based hydrogels for articular cartilage tissue engineering application. Int J Polym Mater Polym Biomater. 2019;69(15):961–70.
Maas SLN, Breakefield XO, Weaver AM. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol. 2017;27(3):172–88.
van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–28.
Kusuma GD, Carthew J, Lim R, Frith JE. Effect of the microenvironment on mesenchymal stem cells paracrine signalling: opportunities to engineer the therapeutic effect. Stem Cells Dev. 2017;26(9):617–31.
Riazifar M, Pone EJ, Lötvall J, Zhao W. Stem cell extracellular vesicles: extended messages of regeneration. Annu Rev Pharmacol Toxicol. 2017;57:125–54.
Kusuma GD, Barabadi M, Tan JL, Morton DAV, Frith JE, Lim R. To protect and to preserve: novel preservation strategies for extracellular vesicles. Front Pharmacol. 2018;9:1199.
Tan JL, Lau SN, Leaw B, Nguyen HPT, Salamonsen LA, Saad MI, et al. Amnion epithelial cell-derived exosomes restrict lung injury and enhance endogenous lung repair. Stem Cells Transl Med. 2018;7(2):180–96.
• Alhomrani M, et al. The human amnion epithelial cell secretome decreases hepatic fibrosis in mice with chronic liver fibrosis. Front Pharmacol. 2017;8:748 Provides novel evidence that extracellular vesicles secreted by hAECs significantly reduces liver fibrosis and macrophage infiltration, further highlighting the therapeutic potential of hAEC-derived EVs.
Broughton B, Tran TN, Lim R, Wallace E, Kemp-Harper B. A8321 Delayed post-stroke administration of human amnion epithelial cell-derived exosomes improve outcomes. J Hypertens. 2018;36:e51.
• Zhao B, et al. Exosomal microRNAs derived from human amniotic epithelial cells accelerate wound healing by promoting the proliferation and migration of fibroblasts. Stem Cells Int. 2018;2018:5420463 Demonstrates the role exosomal microRNA from hAECs play in promoting the wound healing response by reducing collagen deposition in vivo.
Zhao B, Zhang Y, Han S, Zhang W, Zhou Q, Guan H, et al. Exosomes derived from human amniotic epithelial cells accelerate wound healing and inhibit scar formation. J Mol Histol. 2017;48(2):121–32.
Zhang Q, Sun J, Huang Y, Bu S, Guo Y, Gu T, et al. Human amniotic epithelial cell-derived exosomes restore ovarian function by transferring microRNAs against apoptosis. Mol Ther Nucleic Acids. 2019;16:407–18.
Acknowledgements
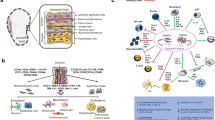
Figures 1 and 2 were created with BioRender.com.
RHMS is supported by NHMRC project grant GNT1144265. MG is supported by an MRFF Stem Cell Mission grant. DZ is supported by NHMRC project grant GNT1141946. GDM is supported by the Rebecca L. Cooper Medical Research Foundation. RL is supported by an NHMRC career development fellowship. This work is supported by the Victorian Government’s Operational Infrastructure Support Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Prenatal Therapies
Renate H. M. Schwab and Mihiri Goonetilleke are joint first authors.
Rights and permissions
About this article
Cite this article
Schwab, R.H.M., Goonetilleke, M., Zhu, D. et al. Amnion Epithelial Cells — a Therapeutic Source. Curr Stem Cell Rep 7, 13–29 (2021). https://doi.org/10.1007/s40778-021-00187-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40778-021-00187-5