Abstract
Purpose
The aim of this mini-review is to discuss the possible role of radioguided surgery in brain tumours and, in particular, in gliomas.
Methods
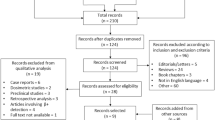
A research in the PubMed/Medline database was carried out to identify relevant studies evaluating radioguided surgery in brain tumours.
Results
Radioguided surgery results using gamma (γ)-emitting tracers and γ-detection probes were summarised. Most importantly, the review included preliminary findings with novel approaches, particularly those relying on the use of beta (β)−emitting isotopes and a dedicated β probe.
Conclusion
Although few data are available in the current literature, the use of β probes could be useful to accurately identify surgical margins in brain tumours. Nevertheless, further in vivo studies are required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Purpose
Surgery is the standard treatment for brain tumours. The extent of surgical resection has an essential role in the management of cerebral gliomas. Indeed, a greater extent of resection leads to an improved overall survival, progression-free survival and higher quality of life [1]. To date, many intraoperative techniques and technological tools, such as neurophysiological monitoring, ultrasound aspirator, awake surgery, intraoperative neuroradiology, magnetic resonance imaging (MRI) and/or computed tomography (CT) scan, are employed to remove the glioma completely, preserving the neurological functions. In recent years, neurosurgeons have experimented techniques to identify the neoplasm and remove the tissue as much as possible. The 5-aminolevulinic acid (5-ALA) for fluorescence-guided surgery (FGS) has proven to be an effective and safe intraoperative adjunct for glioma resection [2]. This prodrug is an optical imaging agent widely employed in patients with high-grade gliomas for the visualisation of malignant tissue during surgery [2]. Administered as an oral solution, 5-ALA is taken up by glioma cells and metabolized into its red fluorescent form, protoporphyrin IX. A specific filter in the microscope allows the visualization of the “red tissue” that can be easily removed. A multicentre, randomized phase III study demonstrated that 5-ALA FGS enables a more complete tumour resection in high-grade gliomas with a better outcome than conventional microsurgery [3].
Nevertheless, there are some drawbacks related to the use of 5-ALA. In particular, increased risks are of neurological deficits, probably due to the more aggressive resection when removing the tissue detected by the technique. Moreover, the risk is much higher where eloquent areas are involved and closely related to the surgeon’s experience and neurophysiological monitoring. Although a failure in 5-ALA uptake by neoplastic tissue has rarely been reported, it may occur in heterogeneous tumours making it difficult to identify the tumour margins. Most importantly, the possible uptake of 5-ALA by non-neoplastic tissues, such as scar tissue or ependymal surface, may influence the surgical procedure with the removal of healthy tissue, thus increasing the risk of neurological sequelae.
Bearing in mind that the greater the removal of glioma, the better the outcome, it is worth evaluating further tools capable of recognising the neoplastic tissue as clearly as possible. In this context, a promising procedure may be radio-guided surgery (RGS). This technique enables to intraoperatively identify lesions or tissues preoperatively marked with a radiotracer employing a detection probe [4]. Established methods currently rely on γ-emitting tracers coupled with γ-detecting probes [5, 6]. However, the deep penetration of γ radiation can reduce the tumour-to-background ratio, a crucial factor in RGS, thereby limiting its efficacy and applicability. Accordingly, in the last few years, there has been an increasing interest in novel approaches relying on β− or β+ emitting radioisotopes and β probes. Despite the growing use of RGS in several tumours [4], very few data are currently available on its application in brain tumours [7,8,9,10].
The aim of this mini-review is to discuss literature results suggesting the potential role of RGS in the removal of brain tumours, particularly gliomas. It also provides an insight into possible future developments in this field.
Methods
A literature search in the PubMed/Medline database was performed to identify relevant studies evaluating the use of RGS in brain tumours. The research strategy was based on the combination of the key words “radioguided surgery” AND “brain tumours” and their synonyms. Only original studies on RGS in brain tumours, published in English and including a minimum of 5 patients, were selected.
Results
γ -based RGS
According to the literature research, few studies investigated the role of RGS in brain tumours using a preoperative single photon emission tomography (SPECT) or SPECT/CT to guide the location of craniotomy and a γ probe to identify intraoperatively the tumour tissue and assess its extensiveness. In particular, Kojima et al. distinguished intraoperatively tumour from normal brain tissue in 13 patients, using a γ probe and a mobile γ camera after the intravenous injection of 99mTc-hexakis-2-methoxy-isobutyl-isonitrile (99mTc-MIBI) immediately before surgery [7]. In 9 out of 13 patients, histologic examination found no residual tumour tissue. Encouraging results were obtained by Bhanot et al. using RGS and a γ probe in 13 patients with high-grade gliomas that showed high 99mTc-MIBI uptake on preoperative SPECT/CT [8]. A gradient of at least 2:1 was employed to differentiate the tumour from the normal tissue intraoperatively. In 9 (69.2%) patients, no residual enhancing area was detected by postoperative CT/MRI scan, whereas a nearly total excision was achieved in the remaining 4 cases. Most importantly, the RGS procedure improved the neurosurgeon’s confidence with the neoplasm located in or close to eloquent brain areas, providing a real-time assessment of excision completeness. 1 year later, Serrano et al. employed Thallium-201chloride (202Tl) as a guide for biopsy sample and tumour resection in 6 patients with malignant astrocytoma, previously assessed by 201Tl SPECT [9]. In all cases, residual uptake was detected by the γ probe in the surgical bed, confirmed as tumour tissue by the pathological examination. In 3 out of 6 patients, residual areas of pathological uptake were completely removed. More recently, Seddighi et al. evaluated 22 glioma patients, equally randomized into two groups [10]. The use of RGS and 99mTc-MIBI (first group) increased the extent of tumour resection in comparison with the conventional tumour removal (control group), as assessed by contrast MRI. In particular, a radical excision was achieved in 8 patients (72.7%) in the radioguided group, whereas a complete removal was performed only in three patients (27.2%) in the control group. Moreover, the tumour detection time in the radioguided group was significantly less than the control group. These few results, obtained with 99mTc or 201Tl, suggested the feasibility and usefulness of RGS and γ probes in guiding and maximizing the brain tumour resection. However, some important technical aspects should be taken into account. Regarding γ-photon emission, the low probability of low-energy (< 150 keV) photons interacting in the traversed media has a direct impact on the characteristics and limitations of the so-called γ-RGS. Indeed, to detect such weakly interacting particles, γ probes are typically based on high-density materials, leading to a somehow difficult use of such devices in minimally invasive surgery. Moreover, since these types of photons penetrate tens of cm in human tissue, the emission of a small tumour remnant could be shadowed by the abundant photon flux due to the physiological uptake of the radiopharmaceutical by the surrounding healthy tissue. This non-negligible background in the nearby healthy tissue can decrease the tumour-to-background ratio and limit γ-RGS efficacy for the accurate delineation of resection margins and/or tumour remnants [11, 12].
β -based RGS
Although positron emission tomography (PET)/CT imaging with several radiotracers has an important role in the management of brain tumours [13, 14], there are no studies assessing the potential application of RGS using a β+ probe. This technique would benefit from the better signal-to-background ratio of PET tracers compared to γ-emitting radiopharmaceuticals. In fact, β particles tend to interact much more with the crossed tissue; as a result, the most common beta emitters (i.e., 18F, 68 Ga, 90Y) produce electrons that, despite their different energies (600, 1900 and 2100 keV, respectively) have a penetration of few mm compared to several cm for γ radiation. Detecting β particles selectively, would make it possible to apply RGS also in those cases with high tracer uptake in healthy tissues surrounding the tumour, since βs originating outside the tumour have a very small probability of reaching the β probe. On the other hand, the short penetration of beta particles might give false-negative results, particularly in cases of tumour necrosis or heterogeneity where layers of healthy tissue can hide high uptake areas.
Nevertheless, this theoretical precision sparked interest in the possible use of β emission to intraoperatively detect tumours in high background scenarios, such as cerebral gliomas. The first attempt dates back to 1949, when Selverstone et al. used Phosphorus-32 (P32), a pure β− emitter tracer, to intraoperatively identify tumour residuals in 14 patients diagnosed with glioblastoma, astroblastoma, astrocytoma or atypical glioma [15]. Each patient received 0.95–4.2 mCi of P32 intravenously and the probe (an ad hoc designed, needle-shaped miniaturized Geiger–Muller counter) was employed to accurately identify the location of deep tumours, which was possible within 12–24 s of sampling time. Despite the limitations of using a probe not particularly suitable for surgery and a radiotracer with a half-life of several days, this study is still significantly important, being the initial true appearance of β-RGS technique itself. Already in 1951, Sweet WH reported the successful results of RGS using P32 in 114 patients with brain tumours: a correct localisation was obtained in 105/114 (92%) cases [16]. The first modern attempt is the development of an intraoperative β probe for glioma surgery by Bonzom et al., in 2007 [17]. The detector, specifically aimed at localising the residual tumour labelled with positron emitters, consists of two series of scintillating fibres, arranged in concentric annular distributions. However, these plastic filaments are also sensitive to the abundant γ radiation when using PET tracers. As a result, scintillating fibres of the outermost layer of the detector are covered by a stainless steel thin sheet, shielding them against β radiation. The presence of this shielding leads to what is called a “dual-detector”, i.e., a device based on the simultaneous detection of two different components of the signal (β plus γ and just γ), relying on the real-time subtraction of these two contributions to obtain the pursued result. This type of detector has been characterized in terms of sensitivity and spatial resolution by both Monte Carlo simulations and experimental measurements, showing a very high γ rejection efficiency (99.4%). A brain phantom modelling of the surgical cavity has demonstrated the probe ability to detect tumour discs as small as 5 mm in diameter for tumour-to-background ratios higher than 3:1 and with an acquisition time of around 4 s. Despite this initial validation, no further steps towards the clinical implementation of this detector have been reported in literature. A possible explanation can be found in the final form factor of the probe, due to its “dual nature”, hardly compatible with a minimally invasive setting such as brain surgery.
Probably, the most relevant novelty in this field lies in the introduction of innovative, high efficiency organic scintillating materials. P-terphenyl, for example, is characterized by a very high light yield (i.e., the amount of scintillating light produced per unit of deposited energy), thus enabling the detection of even very small energy depositions, such as those from low-energy electrons. Likewise, this material also has a low density, as it is common in organic scintillators that, on the other hand, have typically reduced high light yield. Given its low density, p-terphenyl has an intrinsic substantial transparency to γ radiation: therefore, it can be exploited to design a probe capable of selectively detecting β particles, even in the presence of a γ background. The first p-terphenyl-based probe was proposed by Camillocci et al. in 2014 [18]. In particular, a 5 mm thick, 6 mm diameter cylindrical scintillator is coupled with a solid-state light detector that allows an effective signal reading produced without any electrical hazard for the patient, being operated at 5 V. Due to the very small penetration of β particles, a 3 mm thick lateral shielding is sufficient to ensure detection directionality and the probe can be easily encapsulated in a “pen-like” form factor (Fig. 1). The first use of this innovative β probe was in cerebral tumours, using 90Y-DOTATOC as radiopharmaceutical. In the study by Collamati et al., 68 Ga-DOTATOC PET images of patients affected by meningioma and glioma were used to quantify the uptake of 90Y-DOTATOC in these tumours [12]. Uptakes were then used as inputs for a Monte Carlo simulation of the detector, allowing to obtain the expected performances of the probe in a realistic applicative scenario. As expected, meningiomas showed a higher uptake of 68 Ga-DOTATOC than gliomas. Moreover, tumour non-tumour ratios were 10–20 for patients with meningioma and 5–10 for patients with glioma. As regards the final feasibility of the RGS technique, under the assumption of injecting 3 MBq/kg of 90Y-DOTATOC and aiming to detect a 0.1 mL tumour residual, the required probing time was less than 1 s and less than 6 s for meningioma and gliomas, respectively. Such positive results have led to a first experimental validation of this innovative β-RGS technique, obtained on ex vivo samples of meningioma, in patients preoperatively injected with 90Y-DOTATOC [19]. In this study, 26 samples from 4 patients have been analysed with the β probe and the authors found that even injecting as low as 1.4 MBq/kg of radiotracer, tumour residual of 0.1 mL could be detected. It is worth noting that, given the nature of meningioma, usually benign and with clear surgical margins, this is not an actual case of interest for a possible RGS approach. It was indeed considered as a case study to test the possibility of predicting the real performance of the technique from the preoperative PET scan. Conversely, a much more interesting case would be the glioma, whose infiltrating nature poses a massive obstacle to complete the tumour resection and an ex vivo study on such tumours was indeed expected to follow the meningioma one by this group. However, this innovative β-RGS technique has been experienced outside the brain, in particular in neuroendocrine tumours [20, 21] as well as in prostate cancer [22]. Considering radioprotection issues, the use of β− radiation provides a reduction in the exposure for patients and surgery staff, as compared to γ-RGS [20].
Future developments
Regarding low-grade gliomas, 5-ALA uptake has been found in 10% to 20% of the cases [3]. In this context, a possible scenario could be to investigate the uptake of 68 Ga-DOTATOC in patients with low-grade glioma and using these uptakes as input for Monte Carlo simulations to obtain the expected performance of a β− probe, for example the p-terphenyl base one. According to these results, the β− probe could then be tested in vivo to distinguish residual tumour from normal brain tissue in low-glioma patients using 90Y-DOTATOC as β− emitting tracer. The rationale is that this tracer has high affinity with somatostatin receptor subtype 2 (SSTR2), whose expression has been described in glioma [23]. Another possible relevant study could be a comparison of 5-ALA with β− RGS to differentiate tumour recurrence from radionecrosis in glioma patients.
Conclusion
This mini-review has taken into account the possible role of RGS in hel** the neurosurgeon to achieve the most complete excision of the tumour. Although brain tumours were the first proposed application of RGS as early as 1949, no real progress has been made in this field for several decades. Indeed, despite the growing success of γ-based RGS in other clinical applications, the long penetration of photons and the radiopharmaceutical uptake by healthy tissue hinder the use of γ emission in brain surgery.
In this context, together with the emerging use of 68 Ga-DOTATOC PET/CT or PET/MRI for stereotactic image-guided treatment plan including robotic radiosurgery by Cyberknife [24], the recent introduction of new scintillating materials, characterized by high sensitivity to β particles and intrinsic transparency to γ contamination, has allowed to reconsider the possibility of performing RGS as an alternative treatment of brain tumours using β-emitting tracers. In particular, after an initial Monte Carlo study suggesting the possibility of performing RGS in meningioma and, with reduced efficacy, in glioma, a first ex vivo experimental study has been successfully performed on meningioma samples. However, the transition to the much more interesting case of glioma has not been performed yet. Accordingly, it is not possible at present to assess whether and how this novel RGS technique could prove effective in the surgical resection.
As regards future perspectives, it is worth considering further improvements in detector technologies. The development of radiation probes based on semiconductors [25], for example, would allow to perform RGS even with lower uptake values, as in gliomas. Simultaneously, miniaturization now allows the production of probes compatible with a minimally invasive surgery context.
References
Hervey-Jumper SL, Berger MS (2016) Maximizing safe resection of low- and high-grade glioma. J Neurooncol 130:269–282. https://doi.org/10.1007/s11060-016-2110-4
Eatz TA, Eichberg DG, Lu VM et al (2022) Intraoperative 5-ALA fluorescence-guided resection of high-grade glioma leads to greater extent of resection with better outcomes: a systematic review. J Neurooncol 156:233–256. https://doi.org/10.1007/s11060-021-03901-9
Stummer W, Pichlmeier U, Meinel T et al (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7:392–401. https://doi.org/10.1016/S1470-2045(06)70665-9
Valdés Olmos RA, Rietbergen DDD, Rubello D et al (2020) Sentinel node imaging and radioguided surgery in the era of SPECT/CT and PET/CT: toward new interventional nuclear medicine strategies. Clin Nucl Med 45:771–777. https://doi.org/10.1097/RLU.0000000000003206
Tsuchimochi M, Hayama K (2013) Intraoperative gamma cameras for radioguided surgery: technical characteristics, performance parameters, and clinical applications. Phys Med 29:126–138. https://doi.org/10.1016/j.ejmp.2012.05.002
Schneebaum S, Even-Sapir E, Cohen M et al (1999) Clinical applications of gamma-detection probes—radioguided surgery. Eur J Nucl Med 26:S26-35. https://doi.org/10.1007/pl00014792
Kojima T, Kumita S-I, Yamaguchi F et al (2004) Radio-guided brain tumorectomy using a gamma detecting probe and a mobile solid-state gamma camera. Surg Neurol 61:229–238. https://doi.org/10.1016/j.surneu.2003.07.015
Bhanot Y, Rao S, Parmeshwaran RV (2007) Radio-guided neurosurgery (RGNS): early experience with its use in brain tumour surgery. Br J Neurosurg 21:382–388. https://doi.org/10.1080/02688690701491204
Serrano J, Rayo JI, Infante JR et al (2008) Radioguided surgery in brain tumors with thallium-201. Clin Nucl Med 33:838–840. https://doi.org/10.1097/RLU.0b013e31818bf26a
Seddighi A, Akbari ME, Seddighi AS et al (2015) Radioguided surgery using gamma detection probe technology for resection of cerebral glioma. Hell J Nucl Med 18(Suppl 1):68–75
Cuccurullo V, Di Stasio GD, Mansi L (2017) Radioguided surgery with radiolabeled somatostatin analogs: not only in GEP-NETs. Nucl Med Rev Cent East Eur 20:49–56. https://doi.org/10.5603/NMR.2017.0003
Collamati F, Pepe A, Bellini F et al (2015) Toward radioguided surgery with β- Decays: Uptake of a somatostatin analogue, DOTATOC, in meningioma and high-grade glioma. J Nucl Med 56:3–8. https://doi.org/10.2967/jnumed.114.145995
Law I, Albert NL, Arbizu J et al (2019) Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [18F]FDG: version 1.0. Eur J Nucl Med Mol Imaging 46:540–557. https://doi.org/10.1007/s00259-018-4207-9
Albert NL, Weller M, Suchorska B et al (2016) Response assessment in neuro-oncology working group and european association for neuro-oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol 18:1199–1208. https://doi.org/10.1093/neuonc/now058
Selverstone B, Solomon AK, Sweet WH (1949) Location of brain tumors by means of radioactive phosphorus. J Am Med Assoc 140:277. https://doi.org/10.1001/jama.1949.02900380017004
Sweet WH (1951) The uses of nuclear disintegration in the diagnosis and treatment of brain tumor. N Engl J Med 245:875–878. https://doi.org/10.1056/NEJM195112062452301
Bonzom S, Menard L, Pitre S et al (2007) An Intraoperative beta probe dedicated to glioma surgery: design and feasibility study. IEEE T Nucl Sci 54:30–41. https://doi.org/10.1109/TNS.2006.885574
Camillocci ES, Baroni G, Bellini F et al (2014) A novel radioguided surgery technique exploiting β(-) decays. Sci Rep 4:4401. https://doi.org/10.1038/srep04401
Solfaroli Camillocci E, Schiariti M, Bocci V et al (2016) First ex vivo validation of a radioguided surgery technique with β-radiation. Phys Med 32:1139–1144. https://doi.org/10.1016/j.ejmp.2016.08.018
Collamati F, Bocci V, Castellucci P et al (2018) Radioguided surgery with β radiation: a novel application with Ga68. Sci Rep 8:16171. https://doi.org/10.1038/s41598-018-34626-x
Collamati F, Maccora D, Alfieri S et al (2020) Radioguided surgery with β- radiation in pancreatic neuroendocrine tumors: a feasibility study. Sci Rep 10:4015. https://doi.org/10.1038/s41598-020-61075-2
Collamati F, van Oosterom MN, De Simoni M et al (2020) A DROP-IN beta probe for robot-assisted 68Ga-PSMA radioguided surgery: first ex vivo technology evaluation using prostate cancer specimens. EJNMMI Res 10:92. https://doi.org/10.1186/s13550-020-00682-6
Kiviniemi A, Gardberg M, Frantzén J et al (2015) Somatostatin receptor subtype 2 in high-grade gliomas: PET/CT with (68)Ga-DOTA-peptides, correlation to prognostic markers, and implications for targeted radiotherapy. EJNMMI Res 5:25. https://doi.org/10.1186/s13550-015-0106-2
Acker G, Kluge A, Lukas M et al (2019) Impact of 68Ga-DOTATOC PET/MRI on robotic radiosurgery treatment planning in meningioma patients: first experiences in a single institution. Neurosurg Focus 46:E9. https://doi.org/10.3171/2019.3.FOCUS1925
Collamati F, Amoruso R, Servoli L et al (2020) Stability and efficiency of a CMOS sensor as detector of low energy β and γ particles. J Inst 15:P11003–P11003. https://doi.org/10.1088/1748-0221/15/11/P11003
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Francesco Collamati is listed among the inventors on an Italian patent application (RM2013A000053) entitled “Utilizzo di radiazione beta- per la identificazione intraoperatoria di residui tumorali e la corrispondente sonda di rivelazione” and on the PCT patent application (PCT/IT2014/000025) entitled “Intraoperative detection of tumor residues using beta- radiation and corresponding probes,” covering the method and instruments described in this paper. No other potential conflict of interest relevant to this article was reported. Renato Valdés Olmos declares that he has no conflict of interest. Alessio Albanese declares that he has no conflict of interest. Fabrizio Cocciolillo declares that he has no conflict of interest. Daniela Di Giuda declares that she has no conflict of interest. Angela Collarino declares that she has no conflict of interest.
Ethical approval
This article does not report any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Collamati, F., Valdés Olmos, R., Albanese, A. et al. Current use and potential role of radioguided surgery in brain tumours. Clin Transl Imaging 10, 451–456 (2022). https://doi.org/10.1007/s40336-022-00503-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-022-00503-x