Abstract
Introduction
The aim of this study was to describe the management of neovascular age-related macular degeneration (nAMD) in French patients between 2008 and 2018.
Methods
This was a retrospective longitudinal cohort study using exhaustive nationwide health records from the French National Health Information database. Enrollment criteria were adults aged ≥ 50 years, nAMD diagnosis, or reimbursement for nAMD treatments (anti-vascular epithelial growth factor [VEGF] injection or dynamic phototherapy with verteporfin). Exclusion criteria were high myopia, diagnosis of other retinal diseases, and treatments for other macular diseases (dexamethasone implant, laser). Main outcome measures were consumption of medical care and nAMD treatments per calendar year and number of years of follow-up.
Results
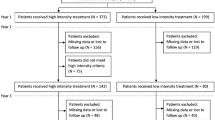
Between 2008 and 2018, we identified 342,961 patients who have been treated for nAMD. Median duration of ophthalmological follow-up exceeded 7 years (90 months). The median annual number of ophthalmology consultations decreased from nine visits in year 1 after treatment initiation to four visits from year 7 onwards. The median duration of nAMD treatment was 10.1 months for all patients, with 48.5% of patients undergoing treatment for < 1 year. Only 24.4% of patients had maintained treatment at year 11. Patients remaining under treatment had a median of four anti-VEGF treatments per year throughout the 10-year study period. Ranibizumab was the more common first-line treatment (67.5% of patients) compared to aflibercept (32.4%). About 20% of patients who initiated treatment switched treatment at least once.
Conclusions
LANDSCAPE provides exhaustive nationwide data on the real-world management of nAMD in France over a 10-year period. Further investigation into short treatment duration is required, especially in terms of understanding its relation to visual outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Little is known of the management of neovascular age-related macular degeneration (nAMD) in the real world. |
We extracted exhaustive nationwide data on the real-world management of nAMD in the entire French population over a 10-year period from the French National Health Information database. |
What was learned from the study? |
The results showed that nAMD treatment duration in the real world was short (median 10 months) despite a median ophthalmological follow-up of 7 years and that the percentage of patients maintaining treatment decreased by one-half in follow-up year 2 to 49.6%. |
The short treatment duration was a surprising result and needs further investigation, especially in terms of how short treatment duration is linked to visual outcomes. |
Introduction
Neovascular age-related macular degeneration (nAMD) is a leading cause of visual impairment and blindness in people aged > 50 years [1]. In a previous LANDSCAPE study, we estimated the incidence and prevalence of nAMD to be 0.15% and 1.06%, respectively, in the French population aged ≥ 50 years in 2018 [2].
Vascular endothelial growth factor inhibitor/antagonist (anti-VEGF) medications have transformed the care of nAMD, significantly reducing visual impairment [3, 4]. In France, common anti-VEGF medications used for treating nAM include ranibizumab, available since 2007, aflibercept, available since 2012, and bevacizumab, recommended for off-label use in 2015. Real-world evidence shows that patients are often treated less frequently with anti-VEGFs than recommended in the respective Summaries of Product Characteristics [5] and in the randomized clinical trials (RCTs) leading to marketing authorization. These real-world studies also suggest that visual acuity improvements in the real-world setting are inferior to those achieved in the RCTs. It is therefore important to better understand the management of nAMD and anti-VEGF use in real-world settings.
The aim of the present study was to describe nAMD management before and after the initiation of treatment for nAMD, using exhaustive nationwide population data from health insurance claims for the French population between 2008 and 2018.
Methods
Study Design and Data Sources
This was a retrospective longitudinal population study based on data extracted from the French National Health Insurance Database (Système National des Données de Santé; SNDS), which contains comprehensive patient-level data for all individuals in France covered by national health insurance. The SNDS covers 99% of the French population from the time of birth or immigration to France, up to the time of to death or emigration from France [6].
Data in the SNDS are pseudonymized and contain individual-level data on demographics (age, sex, area of residence, date of birth), reimbursed drugs (dispensation date and number of units), long-term chronic disease diagnosis, dates and descriptions of paramedical interventions, procedures and laboratory tests, private and public hospitalizations (admission date, duration, main and associated diagnoses, medical consultations etc.), and date and cause of death. Coding, data dictionaries, and data quality control have been described in detail elsewhere [7, 8]. The SNDS only includes data by patient and thus does not distinguish between eyes of a given patient.
The LANDSCAPE study was approved by the relevant French agencies for data protection and health data (the Commission Nationale de l’Informatique et des Libertés [CNIL] and the INDS [Institut National des Données de Santé]). Ethical approval was not required for this observational study, in line with French regulations. Studies using anonymous SNDS data do not require individual informed consent from patients in the database. This study was conducted according to the Declaration of Helsinki and adhered to all relevant regulations in France.
Identification of nAMD Patients
The nAMD patients in the SNDS were identified as described previously [2]. Briefly, we used the following criteria to identify nAMD patients: age > 50 years of age, covered by French national health insurance, and at least one anti-VEGF treatment reimbursed for nAMD (bevacizumab, pegaptanib, ranibizumab, aflibercept) administrated by intravitreal (IVT) injection between 2008 and 2018. Exclusion criteria were: other ocular diseases (including those treated with anti-VEGFs for other diseases, such as diabetic macular edema and retinal vein occlusion), severe myopia (reimbursement for high corrective refractive glasses in previous years), retinal disease other than AMD (International Classification of Diseases, Tenth Revision [ICD-10] codes H30-H36: e.g., diabetic retinopathy) or treatments for other macular diseases (dexamethasone implant, macular laser, or panretinal photocoagulation), or resident in the French overseas region Mayotte (because of incomplete SNDS data).
However, even if exclusion criteria were present, nAMD was confirmed if a patient had at least one hospital stay or long-term disease with an ICD-10 diagnosis of AMD (ICD code H35.3) or if they were treated by phototherapy with verteporfin.
The treatment initiation date was defined as the first IVT anti-VEGF dispensing date or the date of laser photocoagulation. Patients were followed until they finished consuming healthcare or having treatment, or the patient died or emigrated (thus exiting the SNDS database).
Variables
Comorbidities were described for newly treated patients in 2018 and were identified via hospitalization records (for cataract surgery) and dispensation of topical treatments (for dry eye, hypertension, glaucoma, and other ocular comorbidities). Non-ocular comorbidities and other long-term diseases were identified by treatments and ICD codes from hospitalization and included: high blood pressure (treated by antihypertensive drugs), diabetes (identified using a validated algorithm [9]), myocardial infarction, congestive heart failure, stroke, dementia, kidney disease, and cancer without metastasis.
Medical consumption and treatments were analyzed for the period prior to anti-VEGF treatment initiation (90 days before) and then for up to 10 years following treatment initiation (years 1 to 11). We analyzed data for each treatment year, and until the end of consumption of healthcare (regardless of treatment type) or the patient exited the SNDS database (due to death or emigration).
We analyzed consumption of medical care provided by ophthalmologists, general practitioners, orthoptists, and other specialists. Data from ophthalmological imaging examinations were analyzed in terms of diagnosis and monitoring, including optical coherence tomography (OCT), fluorescein angiography, and indocyanine green angiography.
Ophthalmological follow-up was defined by any reimbursed care or medical action that the patient received from an ophthalmologist, so as to account for the variety of reimbursement claims arising from a visit. These included consultations, ocular imaging or treatment (IVT injection, laser procedure, among others). Of note, the SNDS database does not distinguish “classic” OCT from OCT-angiography.
Statistical Analyses
Quantitative variables were described using the mean, standard deviation (SD), median, quartiles, and minimum and maximum. Categorical variables were described using counts and percentages. Patient characteristics (age, sex, comorbidities) were summarized descriptively.
Ophthalmological follow-up consultations (defined as a consultation, image, or medical action) are presented by number and frequency. We calculated the time from the last ophthalmologist consultation to the initiation of anti-VEGF treatment (excluding patients who had their first anti-VEGF treatment on the same day as their diagnosis) We also calculated the time between anti-VEGF intravitreal injections.
A Kaplan–Meier survival analysis was performed to assess the duration of ophthalmologic follow-up over the study period (this analysis was not affected even if there was a change in the treating ophthalmologist since the SNDS still captured information on ophthalmologist consultations). The duration of ophthalmologic follow-up was calculated as the time (date) from anti-VEGF treatment initiation to the stop date of ophthalmologic follow-up (defined as a gap of > 12 months without an ophthalmologist visit). Patients without ophthalmologic follow-up were removed from the study (censured) at the date of end of their follow-up in the study (date of death, date of end of study or gap of > 12 months without healthcare consumption).
A Kaplan–Meier survival analysis was also performed to assess treatment duration for patients initiating a treatment from 2008 to 2017. A similar analysis performed to assess duration of first-line anti-VEGF treatment was limited to patients initiating a treatment from 2014 to 2017 (since aflibercept was reimbursed for nAMD from October 2013 onwards). The treatment switch was analyzed for these patients and visualized with a sunburst diagram.
SAS Enterprise Guide® version 7.1 (SAS Institute, Cary, NC, USA) was used for all analyses.
Results
Patient Characteristics
A total of 342,961 patients with nAMD who were treated between 2008 and 2018 were identified in the SNDS database. The mean (SD) age of newly treated nAMD patients was 78.8 (8.1) years in 2008, increasing to 81.2 (7.9) years in 2018. About two thirds of newly treated nAMD patients were female (67.5% in 2008, 64.1% in 2018) (Table 1). Comorbid hypertension was reported for 69.5% of patients, and almost 10% had cardiovascular or neurovascular comorbidities (Table 1).
Care Prior to Treatment Initiation
In the 3 months prior to initiation of nAMD treatment, 90.4% of patients with nAMD consulted an ophthalmologist at least once, 82% consulted a general practitioner, and 4.5% consulted an orthoptist.
The percentage of patients with at least one consultation with an ophthalmologist in the pre-treatment period increased over time across the 10-year study period, from 87.0% in 2008 to 95.0% in 2018 (Fig. 1). The median (interquartile range [IQR]) number of days between initiation of anti-VEGF treatment and the previous ophthalmology consultation decreased over time across the study, from 10.0 (5.0–20.0) days in 2008 to 7.0 (4.0–14.0) days in 2018 (Fig. 1).
Ophthalmology consultations in the 3 months prior to initiation of treatment with anti-vascular epithelial growth factor. The left axis presents the percentage of patients who had at least one consultation with an ophthalmologist (defined as a consultation, ocular image or medical/treatment act) in the 30 days prior to initiation of treatment for neovascular age-related vascular degeneration. The right axis presents the median (IQR) number of ophthalmological consultations per patient in the 30 days prior to treatment initiation. IQR Interquartile range
Regarding imaging in the 3 months prior to treatment initiation, the percentage of patients who had OCT imaging during this period increased from 59% in 2008 to 87% in 2018. Conversely, the percentage of patients who had angiography decreased over the same period, being lower for both fluorescein angiography (decreasing from 54% in 2008 to 23% in 2018) and indocyanine green angiography (decreasing from 16% in 2008 to 9% in 2018).
Care Following Treatment Initiation
In the year following the initiation of treatment for nAMD, nearly all (98.1%) patients had ophthalmological follow-up (any consultation, ocular imagining, or medical action). However, this percentage declined steadily in the following years. In year 11 following treatment initiation, only 57% of patients still alive had at least one ophthalmological follow-up consultation. For those patients continuing to be followed-up by an ophthalmologist, the median annual number of ophthalmological follow-up consultations also decreased over the 10-year study period, from nine consultations in year 1 to four consultations from year 7 onwards (Fig. 2). The median duration of ophthalmological follow-up exceeded 7 years (90 months) (Fig. 3).
Ophthalmological follow-up consultations following initiation of treatment with anti-vascular epithelial growth factor. The left axis presents the percentage of patients who had at least one ophthalmological consultation (defined as a consultation, ocular image, or medical/treatment activity) in years 1 to 10 following initiation of treatment for neovascular age-related vascular degeneration. The right axis presents the median number of ophthalmological consultations per patient in the 10 years (Year 1 to Year 11) following treatment initiation. VEGF Vascular epithelial growth factor
Survival analysis of ophthalmological follow-up and anti-VEGF treatment over the 10-year study period. Ophthalmological follow-up was defined as a consultation, ocular image, or medical/treatment activity in order to account for the variety in reimbursement claims arising from a visit to the ophthalmologist. Treatment duration with any anti-VEGF is presented, along with treatment duration on the first-line anti-VEGF, before treatment switch
The percentage of patients who had an angiography examination declined with increasing number of years following treatment initiation for both fluorescein angiography (from 20% to 3%) and indocyanine green angiography (from 6% to < 1%). The mean number of OCT and angiography examinations remained generally stable from year to year during the study period (Table 2).
Anti Vascular Endothelial Growth Factor Treatment
In the year immediately followinmg anti-VEGF treatment initiation, nearly all patients were re-treated with anti-VEGFs (99.2%) (Fig. 4); 0.8% had photodynamic therapy. The percentage of patients receiving at least one anti-VEGF treatment decreased by 50% from year 1 to year 2, then declined gradually but steadily to 24.4% in year 11.
Treatment with anti-VEGF over the 10-year study period. The left axis presents the percentage of patients receiving at least one administration of an anti-VEGF in years 1 to 11 following treatment initiation for neovascular age-related macular degeneration. The right axis presents the median (IQR) number of anti-VEGF administrations to patients in years 1 to 11 following treatment initiation for patients still under treatment
Survival analysis showed the median treatment duration of anti-VEGF treatment to be 10.1 months (95% confidence interval 10–10.2), with 48.5% of patients having a treatment duration of < 1 year (Fig. 3).
The median (IQR) number of anti-VEGF injections in patients still under treatment was four (3–7) during the first year of treatment. In subsequent treatment years, the median remained at around four anti-VEGF injections per year across the 10-year study period.
The median (IQR) time between injections in year 1 after treatment initiation was 44.6 (32.5–63.0) days. In subsequent years, this lengthened to a median of approximately 2 months (between 56 and 58 days).
Over the 10-year study period, a few hundred patients were treated with bevacizumab via an early access program (0% when expressed as a percentage of the nAMD population in France during the 10-year study period). Similarly, pegaptanib sodium was administered for around 2300 patients (0.7%) between 2008 and 2013, when reimbursement was discontinued.
Treatment Switch
Treatment switch was investigated for the 141,305 patients with nAMD starting ranibizumab or aflibercept treatment between 2014 and 2017, the period when both anti-VEGFs became commercially available in France for nAMD treatment. Other treatments, such as bevacizumab, was not included in the analysis of treatment switch due to the vanishingly small percentage of nAMD patients who received those treatment options.
For these patients, first-line treatment was more commonly with ranibizumab (67.5% of patients) than with aflibercept (32.4%). The median (IQR) treatment duration of the first-line anti-VEGF therapy was longer for aflibercept (8.7 [8.5–9] months) than for ranibizumab (6.2 [6.2–6.3] months).
About 20% of patients switched anti-VEGF treatment at least once (Fig. 5). Of the patients on first-line ranibizumab, 22% switched to aflibercept, of whom 33% switched back to ranibizumab. Of the patients on first-line aflibercept, 21% switched to ranibizumab, of whom 43% switched back to aflibercept (Fig. 5).
Treatment switch between ranibizumab and aflibercept for patients initiating treatment between 2014 and 2017. The inner circle represents first-line therapy (ranibizumab in blue and aflibercept in orange). The middle circle presents the patients who switched treatment to a second-line therapy. The outer circle presents patients who switched back to their original first-line therapy
Other Treatments
Over the 10-year study period, 3.7% of nAMD patients were treated at least once by macular laser and 6.4% by photodynamic therapy. Use of these therapies decreased year on year between 2008 and 2018 (Electronic Supplementary Material Table 1).
Discussion
We used exhaustive nationwide data from the French National Health Insurance Database (SNDS) to describe nAMD management prior to and following initiation of nAMD treatment (anti-VEGF or dynamic phototherapy) for the 342,961 patients with nAMD who had been previously identified in the French population between 2008 and 2018 [2]. Our results revealed a short duration of treatment for nAMD (median 10 months) despite a median ophthalmological follow-up period of 7 years. The percentage of treated patients decreased by 50% in year 2 to 49.6%. The most common first-line treatment was ranibizumab (67.5% of patients), and about 20% of all patients switched treatment at least once.
In the 90 days prior to the initiation of nAMD treatment, most patients consulted an ophthalmologist (90.4%) or a general practitioner (82%). It is possible that some of the remaining 9.6% of patients may have initiated anti-VEGF treatment on the same day as receiving the nAMD diagnosis, which can happen in larger hospital-based clinics. The time from treatment initiation and the previous ophthalmology consultation (as a proxy of nAMD diagnosis) accelerated from a median of 10 days to a median of 7 days across the 10-year study period. This change could be due to increased confidence in the treatment and to treatment recommendations to start anti-VEGF therapy within 10 days of nAMD diagnosis [10]. We do not know why 25% of nAMD patients in LANDSCAPE started anti-VEGF treatment ≥ 15 days after the previous ophthalmology consultation. Efforts should be made to understand and address any issues related to this phenomenon.
OCT examinations became progressively more frequent as diagnostic modality up to 2018, while fluoresceine angiography became less frequent, reflecting changing practices, availability of new OCT-A technology, and changing preferences for noninvasive vascular examination. Indocyanine angiography was still performed for approximately 9% of the patients in the study, probably for patients needing confirmation of the presence of polyps or for differential diagnosis (vs. central serous chorioretinopathy) [11, 12].
After the initiation of nAMD-specific treatment, patients were initially well monitored. In the year immediately following the initiation of nAMD treatment, 98% of patients consulted an ophthalmologist (median of 9 consultations); this decreased over across the years of the study to 57% of patients consulting an ophthalmologist in year 11 (median of 4 consultations). Other real-world studies have also shown ophthalmological follow-up to decrease over time. The changing number of ophthalmological consultations could also be influenced by the evolution of treatment regimens from pro-re-nata (PRN) in 2012 to treat-and-extend (T&E) by 2018 [13]. Median ophthalmological follow-up was 7 years; the reasons for follow-up of < 7 years are unknown. A 2015 French study on adherence found that 57% of patients were lost to follow-up 5 years after the initiation of ranibizumab treatment, with the main reasons for discontinuing follow-up reported as distance from home to hospital (51.7% of patients), a feeling of doubtful benefits of treatment (34.5%), and the high burden of follow-up (24.1%) [14].
The percentage of patients receiving anti-VEGFs decreased by 50% over the study period, from 99.2% in year 1 to 49.6% in year 2, then declined further in years 3–11. Treated patients had a median of four injections each year, including year 1. Evidence from real-world and population-based studies also show the trend of declining number of treatments after the first year. A 2016 meta-analysis of real-world studies found that patients had a mean of 6.3 injections and 8.4 visits in year 1, declining to 4.4 injections and 7.7 visits in year 2 [15]. In the 5-year real-world multinational Luminous study, the approximately 6000 patients had a mean number of 5.4 ranibizumab injections and 8.3 visits in year 1 [5]. A recent French study also using SNDS data for all patients using anti-VEGFs (including those with AMD, diabetic macular edema, retinal vein occlusion, among others) reported a mean of 4.8 injections and 6.5 visits in year 1, then 2.2 injections and 4.6 visits in year 2 [16]. However, a recent South Korean study observed an increase in the number of patients receiving long-term (> 24 months) active treatment over the period 2014–2018, possibly suggesting that the number of patients without complete loss of vision is increasing thanks to the long-term, gradual changes in the method of anti-VEGF treatment [17].
We found the median duration of anti-VEGF treatment of 10.1 months to be short. Treatment duration was < 1 year for 48.5% of patients, despite a median ophthalmological follow-up of 7 years. Since the SNDS does not include clinical data or reasons for treatment stop, we were unable to identify the reasons for the treatment stop. Other real-world studies have previously reported short treatment durations, with 22–29% of patients stop** treatment within 1 year of initiation [18, 19], and 30.4% stop** treatment between 24 and 60 months after treatment initiation [20]. Treatment discontinuation could be due to the high treatment burden, treatment success or failure, or patient/ophthalmologist decision. Tolerability and side effects are also a barrier to anti-VEGF adherence, as reported by patients and family caregivers in a recent multinational qualitative study investigating adherence [21]. Given the universal and comprehensive healthcare system in France, it is unlikely that economic barriers or restricted access to healthcare underlies nAMD under-treatment. The shift to proactive T&E treatment in France over the study period could account for the low number of injections per year; however, the low median of four injections per year, especially in year 1, remains surprising. It is possible that the earlier years in our study period included some newly treated nAMD patients with advanced disease and therefore a limited potential for visual improvements. This factor could partly explain some under-treatment, despite the first anti-VEGFs becoming available in France in 2007.
Our results suggest that nAMD patients in the real-world setting were dosed less frequently than those in the RCTs whose results supported anti-VEGF approvals, and less frequently than recommended in treatment guidelines, similarly to the results of other real-world studies showing under-treatment [5, 15, 16, 22, 23]. A growing body of real-world evidence suggesting lower visual outcomes in real-world practice was recently confirmed by a large meta-analysis of 109,666 eyes, confirming that visual gains were statistically greater in RCTs for anti-VEGFs than in real-life clinical practice [23]. This meta-analysis also reported relatively better visual gains with proactive treatment regimens (monthly or T&E) and with more frequent injections [23]. Therefore, our findings of short treatment duration and a low median number of injections per year could mean that French nAMD patients have suboptimal visual outcomes.
In LANDSCAPE, ranibizumab was the more common first-line treatment (67.5% of patients) compared with aflibercept (32.4%) from 2014 to 2017 (this timeframe was chosen since aflibercept only became available in France in 2014). We found that around 20% of patients who initiated anti-VEGF treatment switched to another anti-VEGF treatment after a median of 8.7 months on first-line aflibercept, or after a median of 6.2 months on first-line ranibizumab. The longer first-line treatment duration with aflibercept may be due to the potentially less burdensome treatment regimen with aflibercept (every 2 months) [13]. The phenomenon of treatment switch has been well documented in nAMD patients. A French study using SNDS data found similar rates of anti-VEGF treatment switch, albeit after a longer mean (± SD) first-line therapy duration (13.8 ± 11.0 months) [16]. Other real-world studies have reported rates of nAMD treatment switch of 9.5% [5], 13.5% after a mean of around 4 months [24], up to rates of 59% by 36 months after treatment start [25]. A caveat to consider when using SNDS data to assess treatment switch is that it is not possible to know whether the second drug is a switched treatment for the first eye or a newly initiated treatment for the second eye, or to know the reasons for treatment switch. Results from other studies suggest that treatment switch arises due to a suboptimal response with the first-line therapy [26, 27].
Other treatments used for a minority (< 10%) of nAMD patients, such as macular laser, photodynamic therapy, bevacizumab, and pegaptanib sodium injection, declined throughout the study period as they were replaced by newer anti-VEGFs or as marketing of a drug ceased.
The strengths and weaknesses of the LANDSCAPE study were mainly associated with the SNDS data source. The SNDS health claims database covers around 99% of French residents due to the universal healthcare system in France and the comprehensive reimbursement of medical care. Therefore, the granular data in the SNDS allows large longitudinal population-level studies with high statistical power, no risk of selection bias, and a low rate of attrition. However, there are limitations to using SNDS data. The SNDS records healthcare consumption by patient, without distinguishing between eyes. Therefore, we do not know if nAMD patients received unilateral or bilateral anti-VEGF treatment. Neither do we know the treatment regimen used, be it PRN or T&E; knowledge of the treatment regimen is relevant since there was a move towards T&E during the study period. SNDS data are thought to be incomplete for fundus photography since ophthalmologists often include the examination in consultation fees. The SNDS does not contain clinical data on disease diagnosis, progression or management of disease, or clinical response to treatments as visual acuity or anatomic parameters. Such data would have given a more complete picture about why treatments were started, stopped, or switched. Lastly, our results may not be representative of other countries. This is especially relevant since the comprehensive reimbursement of healthcare in France removes economic barriers to treatment.
Conclusions
In conclusion, the LANDSCAPE study described real-world management of nAMD in the entire French population between 2008 and 2018 [2]. Our exhaustive study lends considerable weight to the growing consensus that patients with nAMD are not treated for a sufficiently long period, despite continued ophthalmological monitoring. Although our massive health claims-based study is limited by lack of clinical data, particularly on visual outcomes, it identifies an important evidence gap to be addressed by future studies with access to clinical data, namely, exploration of the reasons for short treatment durations and optimization of treatment strategies and visual outcomes. The present results emphasize the discrepancy between the need to avoid under-treatment of nAMD and the management of nAMD in the real-life setting.
References
GBD 2019 Blindness and Vision Impairment Collaborators, Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9(2):e144–60. https://doi.org/10.1016/S2214-109X(20)30489-7.
Creuzot-Garcher CP, Srour M, Baudin F, et al. Incidence and prevalence of neovascular age-related macular degeneration in France between 2008 and 2018: the LANDSCAPE study. Ophthalmol Sci. 2022;2(1):100114. https://doi.org/10.1016/j.xops.2022.100114.
Holz FG, Schmitz-Valckenberg S, Fleckenstein M. Recent developments in the treatment of age-related macular degeneration. J Clin Investig. 2014;124(4):1430–8. https://doi.org/10.1172/JCI71029.
Mitchell P, Liew G, Gopinath B, Wong TY. Age-related macular degeneration. Lancet). 2018;392(10153):1147–59. https://doi.org/10.1016/S0140-6736(18)31550-2.
Holz FG, Figueroa MS, Bandello F, et al. Ranibizumab treatment in treatment-naive neovascular age-related macular degeneration: results from LUMINOUS, a global real-world study. Retina (Philadelphia, Pa). 2020;40(9):1673–85. https://doi.org/10.1097/iae.0000000000002670.
Scailteux LM, Droitcourt C, Balusson F, et al. French administrative health care database (SNDS): the value of its enrichment. Therapies. 2019;74(2):215–23. https://doi.org/10.1016/j.therap.2018.09.072.
Tuppin P, Rudant J, Constantinou P, et al. Value of a national administrative database to guide public decisions: from the systeme national d’information interregimes de l’Assurance Maladie (SNIIRAM) to the systeme national des donnees de sante (SNDS) in France. Rev Epidemiol Sante Publique. 2017;65(Suppl 4):S149–67. https://doi.org/10.1016/j.respe.2017.05.004.
Daien V, Korobelnik JF, Delcourt C, et al. French medical-administrative database for epidemiology and safety in ophthalmology (EPISAFE): the EPISAFE collaboration program in cataract surgery. Ophthalmic Res. 2017;58(2):67–73. https://doi.org/10.1159/000456721.
Fuentes S, Cosson E, Mandereau-Bruno L, et al. Identifying diabetes cases in health administrative databases: a validation study based on a large French cohort. Int J Public Health. 2019;64(3):441–50. https://doi.org/10.1007/s00038-018-1186-3.
Schmidt-Erfurth U, Chong V, Loewenstein A, et al. Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA). Br J Ophthalmol. 2014;98(9):1144–67. https://doi.org/10.1136/bjophthalmol-2014-305702.
Wolff B, De Bats F, Tick S, Cornut PL, Souied E, Cohen SY. Actualisations de la Federation France Macula: diagnostic de la DMLA exsudative [Update from France Macula Federation: Diagnosis of wet AMD]. J Fr Ophtalmol. 2018;41(9):857–61. https://doi.org/10.1016/j.jfo.2018.05.004.
Cheung CMG, Lai TYY, Teo K, et al. Polypoidal choroidal vasculopathy: consensus nomenclature and non-indocyanine green angiograph diagnostic criteria from the Asia-Pacific ocular imaging society PCV workgroup. Ophthalmology. 2021;128(3):443–52. https://doi.org/10.1016/j.ophtha.2020.08.006.
Tick S, Cornut PL, De Bats F, Wolf B, Souied EH, Cohen SY. Actualisations de la Fédération France Macula: prise en charge thérapeutique de la DMLA exsudative [Update from France Macula Federation: Treatment of Wet AMD]. J Fr Ophtalmol. 2018;41(9):862–7. https://doi.org/10.1016/j.jfo.2018.06.002.
Boulanger-Scemama E, Querques G, About F, et al. Ranibizumab for exudative age-related macular degeneration: a five year study of adherence to follow-up in a real-life setting. J Fr Ophtalmol. 2015;38(7):620–7. https://doi.org/10.1016/j.jfo.2014.11.015.
Kim LN, Mehta H, Barthelmes D, Nguyen V, Gillies MC. Metaanalysis of real-world outcomes of intravitreal Ranibizumab for the treatment of neovascular age-related macular degeneration. Retina (Philadelphia, Pa). 2016;36(8):1418–31. https://doi.org/10.1097/IAE.0000000000001142.
de Gage SB, Bertrand M, Grimaldi S, Zureik M. Intravitreal anti-VEGF use in France: a cross-sectional and longitudinal nationwide observational study. Acta Ophthalmol. 2022;100(2):e502–11. https://doi.org/10.1111/aos.14929.
Baek SK, Kim JH, Kim JW, Kim CG. Increase in the population of patients with neovascular age-related macular degeneration who underwent long-term active treatment. Sci Rep. 2019;9(1):13264. https://doi.org/10.1038/s41598-019-49749-y.
MacCumber MW, Yu JS, Sagkriotis A, et al. Antivascular endothelial growth factor agents for wet age-related macular degeneration: an IRIS registry analysis. Can J Ophthalmol. 2023;58(3):252-61. https://doi.org/10.1016/j.jcjo.2021.10.008.
Barthelmes D, Nguyen V, Walton R, Gillies MC, Daien V. A pharmacoepidemiologic study of ranibizumab and aflibercept use 2013–2016. The Fight Retinal Blindness! Project. Graefes Arch Clin Exp Ophthalmol. 2018;256(10):1839–46. https://doi.org/10.1007/s00417-018-4061-2.
Bakri SJ, Karcher H, Andersen S, Souied EH. Anti-vascular endothelial growth factor treatment discontinuation and interval in neovascular age-related macular degeneration in the United States. Am J Ophthalmol. 2022;242:189–96. https://doi.org/10.1016/j.ajo.2022.06.005.
Giocanti-Aurégan A, García-Layana A, Peto T, et al. Drivers of and barriers to adherence to neovascular age-related macular degeneration and diabetic macular edema treatment management plans: a multi-national qualitative study. Patient Prefer Adherence. 2022;16:587–604. https://doi.org/10.2147/ppa.S347713.
Korobelnik JF, Delcourt C, Creuzot-Garcher C, et al. Real-life management of neovascular age-related macular degeneration (nAMD) in France: a nationwide observational study using retrospective claims data. J Med Econ. 2021;24(1):1087–97. https://doi.org/10.1080/13696998.2021.1971416.
Veritti D, Sarao V, Soppelsa V, Danese C, Chhablani J, Lanzetta P. Managing neovascular age-related macular degeneration in clinical practice: systematic review, meta-analysis, and meta-regression. J Clin Med. 2022. https://doi.org/10.3390/jcm11020325.
Kozak I, Gurbaxani A, Safar A, et al. Treatment patterns in patients with age-related macular degeneration and diabetic macular edema: a real-world claims analysis in Dubai. PLoS ONE. 2021;16(7):e0254569. https://doi.org/10.1371/journal.pone.0254569.
Verbraak FD, Ponsioen DL, Tigchelaar-Besling OAM, et al. Real-world treatment outcomes of neovascular age-related macular degeneration in the Netherlands. Acta Ophthalmol. 2021;99(6):e884–92. https://doi.org/10.1111/aos.14712.
Amoaku WM, Chakravarthy U, Gale R, et al. Defining response to anti-VEGF therapies in neovascular AMD. Eye (Lond). 2015;29(10):1397–8. https://doi.org/10.1038/eye.2015.159.
Salcedo-Villanueva G, Feria-Anzaldo E, Romo-Aguas JC, et al. Anti-VEGF treatment switch in neovascular age-related macular degeneration: a comparison of aflibercept versus ranibizumab after a single-dose switch. Int Ophthalmol. 2019;39(9):2023–31. https://doi.org/10.1007/s10792-018-1038-4.
Acknowledgements
Funding
This study and its publication (including editorial support and the journal’s Rapid Service fees) were funded by Novartis SAS, France. The sponsor or funding organization participated in design of the study, conducting the study, data collection, data management, data analysis, interpretation of the data, preparation, review, and approval of the manuscript.
Editorial Assistance
Editorial assistance was provided by Fiona Dunlevy PhD and Matrix Consultants. Support for this assistance was funded by Novartis SAS, France.
Author Contributions
Catherine P. Creuzot Garcher, Mayer Srour, Florian Baudin, Corinne Dot, Sylvia Nghiem-Buffet, Jean-Francois Girmens, Cedric Collin, Anne Ponthieux, and Cécile Delcourt all contributed to the study conception and design. Material preparation and data collection and analysis were performed by IQVIA France. The first draft of the manuscript was written by Anne Ponthieux, and all named authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosures
Anne Ponthieux is an employee of Novartis Pharma. Cedric Collin reports no conflict of interest. All other authors report receiving personal fees from Novartis when conducting the present study. Outside the submitted work: Catherine P. Creuzot Garcher reports personal fees from Thea Pharma, Allergan, Bayer, Novartis, Roche, Horus, and Alcon; Florian Baudin reports personal fees from Thea Pharma; Cécile Delcourt reports personal fees from Abbvie, Bayer, and Horus Pharma; Sylvia Nghiem-Buffet reports personal fees from Abbvie, Bayer, Novartis, and Roche; Jean-Francois Girmens reports personal fees from Abbvie/Allergan and Bayer, and personal fees and other reimbursements from Tilak; Corinne Dot reports personal fees from Allergan, Chauvin-Bausch + Lomb, Laboratoires Théa, and Apellis.
Prior Publication
This manuscript is based on work that has been previously presented at the 2023 ARVO Annual Meeting on 24 April 2023 (Posterboard Number: 2186—C0139) and at the 129th Congrès de la Société Française d’Ophtalmologie on 7 May 2023, Paris, France.
Compliance with Ethics Guidelines
LANDSCAPE was approved by the relevant French agencies for data protection and health data (the CNIL and the INDS). Ethical approval was not required for this observational study, in line with French regulations. Studies using anonymous SNDS data do not require individual informed consent from patients in the database. This study was conducted according to the Declaration of Helsinki and adhered to all relevant regulations in France.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available due to the French law on access to the French National Health Insurance Database (Système National des Données de Santé; SNDS).
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Creuzot Garcher, C.P., Srour, M., Baudin, F. et al. Management of Neovascular Age-Related Macular Degeneration Treatment in France from 2008–2018: The Nationwide LANDSCAPE Study. Ophthalmol Ther 12, 2687–2701 (2023). https://doi.org/10.1007/s40123-023-00772-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-023-00772-3