Abstract
To evaluate the real-world treatment outcomes in patients with neovascular age-related macular degeneration (nAMD) in Korea, focusing on retinal fluid resolution. This multi-institutional retrospective chart review study, analyzed medical records of patients with nAMD (age ≥ 50 years) who received their first anti-vascular endothelial growth factor (VEGF) treatment in ophthalmology clinics across South Korea between January 2017 and March 2019. The primary endpoint was the proportion of patients with retinal fluid after 12 months of anti-VEGF treatment. The association between fluid-free period and VA gains was also evaluated. A total of 600 patients were enrolled. At baseline, 97.16% of patients had retinal fluid; after 12 months of anti-VEGF treatment, 58.10% of patients had persistent retinal fluid. VA improvements were relatively better in patients with absence of retinal fluid compared with presence of retinal fluid (+ 12.29 letters vs. + 6.45 letters at month 12; P < .0001). Longer duration of absence of retinal fluid over first 12 months correlated with better VA gains at month 12 (P < .01). More than half of the study patients with nAMD had retinal fluid even after 12 months of treatment with their current anti-VEGF. Presence of retinal fluid was associated with relatively worse VA outcomes.
Similar content being viewed by others
Introduction
Age-related macular degeneration (AMD) is the most common cause of irreversible vision loss in the elderly1. A nationwide population-based study using data from the Korean National Health Claims database found that neovascular AMD (nAMD) had a prevalence of 36.43 per 10,000 people and an incidence of 3.02 per 10,000 person-years in people over the age of 40 years2. nAMD is associated with limitations in both mobility and activity as a result of vision loss, leading to a negative impact on the quality of life of patients3. The use of anti-vascular endothelial growth factor (anti-VEGF) agents as a standard of care for nAMD has resulted in a significant decrease in the incidence of blindness and visual impairment4.
In Korea, ranibizumab (Lucentis; Novartis Pharma, Basel, Switzerland; Genentech, South San Francisco, CA, USA) and aflibercept (Eylea; Regeneron Pharma, Tarrytown, NY, USA; Bayer, Basel, Switzerland) are approved anti-VEGFs to treat visual impairment due to nAMD5. Off-label use of bevacizumab (Avastin; Genentech) for nAMD is also common in Korea due to cost issue6. Despite remarkable success in clinical trials, the 2 major challenges in the application of anti-VEGF therapy in real-world practice are the high treatment burden, requiring frequent hospital visits and injections, and the associated high costs7. As a result, patients with nAMD usually have suboptimal long-term outcomes with anti-VEGF therapy in real-world settings; this might be due to delays in diagnosis and/or treatment approval and initiation, differences in individual patient response to different anti-VEGF therapies, lapses in physician regimentation of anti-VEGF injection and monitoring, and inadequate patient adherence to treatment and monitoring7,8,9,10,11.
Morphological signs of disease activity on optical coherence tomography (OCT) are crucial because they correspond to early signs of recurrence, which are usually observed prior to visual acuity (VA) loss12.Central subfield thickness (CST) and fluid status, measured by OCT, are frequently used by clinicians as the key indicators of disease activity and for driving treatment decisions13,14. Retinal fluid is an important parameter in nAMD disease activity, and one of the objectives of nAMD treatment is to achieve complete fluid resolution. The presence of fluid is a key retreatment criterion across the landmark clinical trials for nAMD, as well as in current major clinical practice guidelines globally13,14,15,16.
The aim of this study was to better understand the real-world treatment patterns and outcomes of patients with nAMD in Korea, with a focus on retinal fluid resolution with current anti-VEGF therapies.
Results
Patient disposition
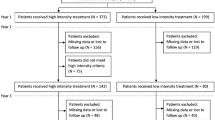
A total of 600 patients were enrolled; cohort 1 included all 600 patients who had a minimum follow-up period of 12 months, and cohort 2 included 453 patients who had a minimum follow-up period of 24 months (Fig. 1).
Patient demographic and baseline clinical characteristics
The mean (SD) age of the study patients was 73.33 (8.88) years, and a higher proportion of the patients were male (57.83%). At baseline, a majority of the patients (67.83%) had subfoveal lesions; 28.33% and 9.5% of patients had polypoidal choroidal vasculopathy and retinal angiomatous proliferation, respectively (Table 1).
Treatment pattern
The mean (95% CI) number of injections and visits during the first year of treatment was 5.54 (5.36, 5.72) and 9.51 (9.32, 9.71), respectively. A similar proportion of patients were treated as per the treat and extend (T&E; 50.5%) and pro re nata (PRN; 43.8%) regimens. During the second year, the mean (95% CI) number of injections and visits was 3.48 (3.26, 3.73) and 7.10 (6.86, 7.36), respectively, and 49.0% and 45.0% of patients were treated as per the T&E and PRN treatment regimens, respectively. Approximately 6% of patients were treated as per “Other” treatment regimens, which included a hybrid of T&E and PRN. During the first year, 417 patients (69.5%) were treated using single anti-VEGF agent, while the remaining 183 patients (30.5%) underwent treatment switch. During the second year, 213 patients of the 453 (47.02%) in the cohort having a follow-up of 24 months remained on single anti-VEGF, while 150 (33.11%) underwent treatment switch (Table 2).
Retinal fluid status over time
Figure 2 depicts the change in the proportion of patients with retinal fluid from baseline to 24 months, and by the type of retinal fluid (IRF, SRF, and sub-RPE fluid). At baseline, 97.16% of patients had retinal fluid (IRF: 39.97%, SRF: 90.13%, and sub-RPE fluid: 32.44%). There was a decline in the proportion of patients with retinal fluid, IRF, SRF, and sub-RPE fluid up to month 24 after anti-VEGF treatment compared to baseline. The greatest decline was observed at 3 months, but thereafter a slight increase in the proportion of patients with retinal fluid between months 6 and 24 was seen. At month 12, the proportion of patients with retinal fluid were 58.10% (IRF: 24.66%, SRF: 37.59%, and sub-RPE fluid: 21.21%). At month 24, 66.02% of patients had presence of retinal fluid (IRF: 25.89%, SRF: 43.69%, and sub-RPE fluid: 23.62%) (Fig. 2).
Visual acuity and central subfield thickness changes over time
Following anti-VEGF treatment, the maximum gain in VA (Early Treatment Diabetic Retinopathy Study [ETDRS] letters) from baseline (mean VA: 54.14 letters) was observed at 3 months (mean: 62.03 letters, mean change: + 8.10 letters). The VA gains were maintained through 12 months (mean: 63.18 letters, mean change: + 8.91 letters) but showed a trend of gradual decline by 24 months of treatment (mean: 61.81 letters, mean change: + 6.98 letters) (Fig. 3a).
Similarly, a significant decrease in CST from baseline was observed after 3 months of anti-VEGF treatment (mean: 256.72 μm, mean change: − 124.17 μm), which remained stable over 12 months (mean: 273.00 μm, mean change: − 106.90 μm). There was a small increase in CST between months 12 and 24 (mean: 282.50 μm, mean change: − 94.80 μm) (Fig. 3b).
Visual acuity changes by presence of retinal fluid at each time point
VA gains from baseline were significantly greater in patients with absence of retinal fluid versus those with presence of retinal fluid, with a P value of < 0.05 at months 3, 6, 9, 12, 15, and 21. At months 18 and 24, though VA gains were higher in the absence of retinal fluid compared to presence of fluid, the difference in between the two groups was not significant (P value > 0.05). At month 12, the mean difference in VA gain between patients with absence of retinal fluid versus those with presence of retinal fluid was 5.84 letters. Similarly, at any given time point, VA gains were relatively better in patients with absence of IRF, SRF, and sub-RPE fluid. (Fig. 4) The mean VA at each time point is shown in Fig. S1.
Visual acuity change by absence/presence of retinal fluid at each time point (cohort 1). ETDRS, Early Treatment Diabetic Retinopathy Study; IRF, intraretinal fluid; RPE, retinal pigment epithelium; SE, standard error; SRF, subretinal fluid; VA, visual acuity. P value analyzed for difference between presence and absence of retinal fluid groups (Wilcoxon signed-rank test). *P < .05, **P < .01, ***P < .001.
Visual acuity changes by presence of retinal fluid at month 3
We assessed the VA outcome at all time points based on the presence/absence of retinal fluid and the types of retinal fluid only at month 3 (after the loading dose for anti-VEGF therapy) (Fig. 5). VA gains were relatively better in patients who achieved absence of retinal fluid at month 3 versus those with presence of retinal fluid, with a P value of < 0.05 at months 3 and 6. VA gains were numerically greater in patients with absence of SRF and sub-RPE fluid at month 3, from month 3 to approximately month 24. However, the difference was not enough to attain statistical significance (P value > 0.05). VA gains were numerically lower in patients with absence of IRF at month 3, but baseline VA was lower in patients with presence of IRF at month 3 (mean VA: 55.66 letters for absence of IRF vs 45.32 letters for presence of IRF). The mean VA at each time point is shown in Fig. S2.
Visual acuity change by absence/presence of retinal fluid at month 3 (cohort 1). ETDRS, Early Treatment Diabetic Retinopathy Study; IRF, intraretinal fluid; RPE, retinal pigment epithelium; SE, standard error; SRF, subretinal fluid; VA, visual acuity. P value analyzed for difference between presence and absence of retinal fluid groups (Wilcoxon signed-rank test). *P < .05,**P < .01,***P < .001. The patient eyes were divided into the presence or absence of fluid at Month 3 and change in VA was stratified of those groups at all subsequent time points.
Visual acuity change by quartile time with absence of retinal fluid
After categorizing patients into quartiles based on time of absence with retinal fluid, patients with a longer duration of absence of retinal fluid from 1 to 12 months showed higher VA gains. There were substantial differences in VA gains at month 12 for the Q1–Q2 subgroup compared with Q3 (P = 0.0038) and Q4 subgroups (P = 0.0004) (Fig. 6a).
Visual acuity from baseline to month 12 and 24 stratified by quartile of time with absence of retinal fluid over (a) months 1 to 12 (cohort 1) and (b) months 1 to 24 (cohort 2). ETDRS, Early Treatment Diabetic Retinopathy Study; N, number of patients; Q, quartile; SE, standard error; VA, visual acuity. aPatients' eyes were divided into quartiles groups based on time with absence of fluid over months 1 to 12, and change in VA was stratified for those quartile groups. Lower quartile = 0.0, median = 1.0, upper quartile = 139.5 days. Q1-Q2: less than median, (<1 day); Q3: median to less than upper quartile (≥ 1 day to < 139.5 days); Q4: more than or equal to upper quartile (≥ 139.5 days). P value test for difference between Q1-Q2 and each quartile group (Wilcoxon signed-rank test). bPatients' eyes were divided into quartiles based on time with absence of fluid over months 1 to 24, and change in VA was stratified for those quartile groups. Lower quartile = 1.0, median = 86.0, upper quartile = 288.0 days. Q1: less than lower quartile (<1 day); Q2: lower quartile to less than median (≥ 1 to < 86 days); Q3: median to less than upper quartile (≥86 to <288 days); Q4: more than or equal to upper quartile (≥ 288 days). P value test for difference between Q1 and each quartile group (Wilcoxon signed-rank test). cNumber of patients with both baseline and month 12 or 24 VA; changes in VA were calculated in these patients.
Similarly, when patients were divided into quartiles based on time of absence of retinal fluid from 1 to 24 months, VA gains at month 24 were also relatively higher in the group with a longer duration of absence of retinal fluid. However, the P values for the differences between the groups were > 0.05 (Fig. 6b).
Discussion
To the best of our knowledge, the PROOF study is the first multicenter study to assess the efficacy of anti-VEGFs in resolving retinal fluid in nAMD in the real-world setting in Korea. This study found that despite their current anti-VEGF treatment, a high proportion of patients with nAMD continued to have retinal fluid at year 1 as well as year 2 of treatment. Further, patients who achieved early fluid resolution showed a trend towards better VA gains.
Disease activity in nAMD has been defined based on 3 parameters: (1) a loss of ≥ 5 letters in VA, (2) evidence of new hemorrhage, and (3) the presence of retinal fluid, including IRF and SRF17. Clinical practice guidelines from the American Academy of Ophthalmology14 and the European Society of Retina Specialists13 recommend retreatment with anti-VEGFs if retinal fluid is detected on OCT as it is an indication of active disease. A nAMD study from United Kingdom including 1190 eyes showed that presence of IRF and SRF has greater influence on real-world anti-VEGF retreatment decisions compared to the presence of pigment epithelial detachment or vision loss18.
In the present study, the reduction in the proportion of patients with retinal fluid was most noticeable by 3 months (after the loading dose), although only 54.55% of patients achieved fluid-free status. However, > 50% of the patients treated with anti-VEGFs had retinal fluid at the end of both year 1 and year 2 of the treatment. One possible reason for the high proportion of patients without resolution of retinal fluid in this study was the use of PRN regimen in nearly half of the patients. However, a subset of patients may have been either undertreated, or they did not respond adequately to the current anti-VEGF treatments and/or could have required a more intensive treatment. In the current study, anti-VEGFs received by patients with nAMD in Korea included ranibizumab, aflibercept, and off-label use of bevacizumab. A treatment that could more effectively provide better disease control without increasing the treatment burden is an unmet need for nAMD management in Korea.
There are several emerging treatments for nAMD that are under investigation, including longer-acting anti-VEGF agents, sustained systems, topical treatments, gene therapy, and molecules targeting novel angiogenesis checkpoints19,20. Notably, there are novel agents that can extend treatment intervals up to 12 to 16 weeks21,22,23. The most recent anti-VEGF therapy for nAMD includes brolucizumab, a single-chain antibody fragment inhibitor of all isoforms of VEGF-A21. In phase 3 studies, brolucizumab 6 mg demonstrated a better disease control including superior fluid resolution, with > 50% patients treated on a q12w interval after loading up to week 48 versus aflibercept 2 mg dosed q8w21,22. However, higher incidence of intraocular inflammation (IOI) was reported in patients treated with brolucizumab (4.7%) compared to aflibercept (< 1%)21,22. Following post-marketing reports, a rare safety signal of adverse event of retinal vasculitis (RV) and/ or retinal vascular occlusion (RO) which may result in severe vision loss was identified with brolucizumab.These events typically occured in the presence of IOI24,25,26. Brolucizumab was approved by the Food and Drug Administration in 2019 and European Medicines Agency in 2020 for the treatment of nAMD27, and it recently received healthcare reimbursement approval in South Korea.
The VA outcome in this study mirrored the trend observed for retinal fluid status, with the maximum improvement by 3 months after anti-VEGF treatment. In this study, the mean VA gains at month 3 were maintained through 12 months and showed a trend of decline towards 24 months. The VA gain reported in the present study at 12 months (mean + 8.91 letters with 5.54 injections) was relatively higher than in other real-world studies. A systematic review and meta-analysis that assessed the real-world effectiveness of ranibizumab and aflibercept in treatment-naive nAMD patients showed that the VA gain (ETDRS) and injection numbers at 12 months were + 4.24 letters and 5.88 injections with ranibizumab treatment, and + 5.30 letters and 7.10 injections with aflibercept treatment28. In the global LUMINOUS study, treatment-naive nAMD patients treated with ranibizumab for 1 year showed a mean VA gain of 3.1 letters with 5.0 injections29. A real-world study from US that included 2213 nAMD patients showed that patients treated with anti-VEGFs (ranibizumab, aflibercept, or bevacizumab) for 2 years had a mean change in VA of + 3.1 letters from baseline with 12.1 injections30.
This study demonstrates that the retinal fluid status at each time point influenced the VA outcome, whereby patients with absence of retinal fluid had better VA gains than patients with presence of retinal fluid. The same trend was observed for the individual retinal fluid components, including IRF, SRF, and sub-RPE fluid. In some previous studies the presence of SRF was not found to be associated with poor visual outcome31,32. Similarly, Core et al., reported that the long-term persisting SRF did not have an impact on VA outcomes through 2 years of follow-up in nAMD patients33. However, several recent studies demonstrated contrary results18,34. Post hoc analysis of the FLUID study suggested that IRF in the central 1 mm and SRF in the 1 to 6 mm of macular area were negatively associated with best-corrected VA34.
Another study with 321 eyes with nAMD showed that absence of IRF or SRF at ≥ 2 clinic visits resulted in a gain of 5 ETDRS letters from baseline, compared with 2 letters gained in eyes with < 2 clinic visits with absence of IRF or SRF18. The question of whether SRF can be tolerated is still being debated. In the present study, patients with absence of SRF had overall better VA gain compared with those who had SRF, especially at 12 months after treatment, with 4.52 letters difference in VA gain between the 2 groups. This result may indicate potential negative impact of SRF on visual outcome, implying the need for appropriate SRF control.
We further assessed if the fluid status at month 3 affects the VA outcome at subsequent time points. Compared to patients with presence of retinal fluid, patients with absence of retinal fluid at month 3 achieved slightly better VA gains (~ 1–2 letters) from 3 to 21 months; analyses of SRF and sub-RPE fluid followed a similar pattern. However, in our study, visual outcomes related to the IRF status at month 3 were somewhat different. Closer examination revealed that patients with presence of IRF at month 3 had a relatively lower baseline VA compared with patients with absence of IRF at month 3 (45.32 vs. 55.66 letters) (Fig. S1 and S2). The ceiling effect, whereby patients with higher baseline VA have limited potential to gain more letters, while those with lower baseline VA have little possibility for further loss of vision, may have caused a relatively lower VA gain in the patient subgroup with absence of IRF and vice versa. This finding is in agreement with previous studies, which have reported that the presence of IRF at baseline and during treatment is detrimental to vision35,36. It is also in line with the findings from a retrospective 1-year follow-up study of anti-VEGF–treated nAMD eyes showing that presence of IRF was associated with a lower VA at baseline37.Patients with presence of IRF at baseline represent a population with more advanced progression of nAMD, highlighting the need to treat IRF aggressively until maximum resolution is achieved38. Recently, Core et al. reported the presence of IRF in ≥ 80% of visits through year 2 was closely associated with worse long-term VA and scar development in nAMD patients, confirming the negative impact of persisting IRF on visual prognosis39. Our results confirmed that the presence of IRF, SRF, and sub-RPE fluid is associated with a worse VA outcome and suggest that all retinal fluids are pathological. In general, the presence of sub-RPE fluid is not considered to be closely associated with visual prognosis40. But few studies have suggested that the sub-RPE fluid can be associated with the prognosis in some circumstances41,42. In addition, the incidence of sub-RPE fluid has not been studied in a large Korean cohort. For these reasons, the incidence and clinical significance of sub-RPE fluid were analyzed in this study.
We further assessed the effect of time of absence of retinal fluid on VA outcomes. Patients who were fluid-free for a longer time had relatively better VA gains at both 12 and 24 months, as compared to patients with the shortest time with absence of retinal fluid. This result highlights the importance of long-term fluid control.
In the current study, a similar proportion of patients were treated using the PRN or T&E treatment regimen, with a small proportion of patients adopting an approach of a combination of both regimens. The number of injections and visits for treatment-naive patients with nAMD was 5.54 and 9.51, respectively, in year 1, and 3.48 and 7.10, respectively, in year 2. This was consistent with the real-world ranibizumab treatment pattern for the Korean patient population with nAMD in the LUMINOUS study, in which treatment-naive patients received 5.2 injections and 9.2 monitoring visits by year 143. The number of injections and visits was relatively higher than that in real-world studies conducted in other regions44,45,46 but was relatively lower than that in a study conducted in the US47. By 12 months, the patients achieved a VA improvement of + 8.1 letters and CST reduction of 106.90 µm. The results were comparatively better than those from other real-world studies for ranibizumab and aflibercept28,30,48.
Overall, the findings of this study are clinically meaningful as they reflect the actual clinical settings in South Korea and provide insights to fluid management strategies. The demographics and clinical characteristics of the study population were consistent with that reported in previous real-world studies from Korea6,43. Further, the study included a large sample population of 600 nAMD patients, adding strength to the findings.
Limitations include the retrospective nature of the study. The study cohort was heterogeneous compared to clinical trials. In addition, patients who received ranibizumab, aflibercept, or bevacizumab were pooled in the same cohort, which also included patients who had received two or more different anti-VEGF agents during treatment. Since efficacy might differ amidst the different anti-VEGF agents used, this serves as a major limitation of the study. Decisions regarding treatment regimens and schedule were made based on the physicians’ discretion (PRN or T&E and continuation/discontinuation/switch of treatment). Assessment variables, such as collection and interpretation of retinal images, may also have differed based on the institutions’ practice. Furthermore, the present study evaluated the influence of each fluid compartment on visual outcome separately. For this reason, we could not evaluate the influence of co-existing two or more fluid compartments. In addition, the difference in the impact of different fluid locations (foveal vs extrafoveal) was not evaluated. Lastly, since the current study focused on real-world anti-VEGF treatment patterns, we did not include safety data, which are well-known and reported in the prior literature.
In conclusion, our study found that a high proportion of Korean patients with nAMD on currently available anti-VEGF therapies had reported presence of retinal fluid at year 1 and year 2. Patients who achieved early fluid-free status following anti-VEGF treatment and those who had a longer time of absence of retinal fluid show a trend toward better visual outcomes. New and emerging anti-VEGF therapies that can provide sustained disease control with longer duration of action could lead to improved outcomes and address the high treatment burden for patients requiring more aggressive treatment, thus potentially fulfilling the unmet needs of patients with nAMD in Korea.
Methods
Study design and treatment
The PROOF study was a retrospective chart review of patients with nAMD who received their first anti-VEGF treatment between January 2017 and March 2019 in 10 ophthalmology clinics across South Korea. The primary objective of the study was to evaluate the presence of retinal fluid in patients with nAMD after 1 year of anti-VEGF treatment. The study was approved by institutional review board/independent ethics committee for each participating center.
The study period was between July 1, 2016 and March 31, 2020, which allowed for a 6-month pre-index period and an at least 12-month follow-up period. The index/baseline date was defined as the date of the first anti-VEGF injection in patients’ eyes. Patients were administered anti-VEGF treatment, which included ranibizumab, bevacizumab, or aflibercept; the choice of treatment and the decision to discontinue treatment were at the discretion of the prescribing investigator and the patient.
Eligibility criteria
Treatment-naive patients with nAMD who (1) had received their first anti-VEGF treatment between January 1, 2017 and March 31, 2019; (2) had at least 1 record of nAMD for diagnosis, treatment indication, or clinical findings on or within 6 months prior to the index date; (3) were ≥ 50 years of age at baseline; (4) had retinal fluid status on OCT captured at baseline and at 1 year of follow-up; and (5) had undergone standard examination procedures, including retinal imaging (eg, fundus photography and OCT) at baseline and follow-up, were included. Patients were excluded if they had received any other anti-VEGF and/or photodynamic therapy in the study eye prior to baseline, had concurrent progressive retinal diseases requiring treatment to prevent vision loss (eg, diabetic macular edema, retinal vein occlusion), were receiving any other treatments such as intravitreal steroids or cataract surgery, or participating in any other clinical trial during the study period.
Based on fluorescein angiography (FA) results, the location of choroidal neovascularization (CNV) was categorized into three: subfoveal (involving the foveal center), juxtafoveal (1–199 µm from the foveal center), and extrafoveal (≥ 200 µm from the foveal center). The CNV lesion composition was also classified based on FA results, according to the previously suggested criteria: predominantly classic (the area of classic CNV was at least 50% of the area of the entire lesion), minimally classic (the area of classic CNV was < 50% of the area of the entire lesion, and occult (lesions without classic CNV component)49.
Endpoints
The primary endpoint was the proportion of patients who had retinal fluid (retinal fluid was defined as either presence of intraretinal fluid [IRF], subretinal fluid [SRF], and/or sub-retinal pigment epithelium [RPE] fluid) following 1 year of anti-VEGF treatment. The secondary endpoints included in this report were the proportion of patients with retinal fluid (IRF, SRF, sub-RPE fluid) following anti-VEGF treatment from baseline to month 24, change in VA and CST from baseline to month 24, change in VA stratified by presence/absence of retinal fluid and time with absence of retinal fluid, number of injections and visits, and treatment regimen during the first and second year of treatment.
Secondary data captured in the medical records were extracted retrospectively as the source of the data. An electronic data capture (EDC) method, provided by LSK Global Pharma Services Co. Ltd., Korea, was used in this study; anonymized patient data from the study site were entered directly into the database by the investigator or investigator’s designee via a web browser to establish the patients’ electronic case report form (eCRF). The EDC system verified the integrity of the project progression by conducting system validation.
Statistical analyses
The primary analysis was performed in patients who met the inclusion criteria of the study and had a minimum follow-up for a period of 12 months (cohort 1, n = 600). The longer-term outcomes up to 24 months were additionally evaluated in a subset of patients from cohort 1 who had a minimum follow-up of 24 months (cohort 2, n = 453).
For the primary endpoint of the proportion of patients with retinal fluid during the first year of treatment, precision was calculated based on the following assumptions that at least 70% of the patients have retinal fluid and patients would have at least 1 year of follow-up (N > 600). With a sample size of 600, the 2-sided 95% confidence interval (CI) for \(\pi\) = 0.7 was estimated to range between 66 and 74%. Under the worst case scenario of \(\pi\) = 0.5, the 2-sided 95% CI would range between 46 and 54%.
This study was exploratory in nature, and thus, no comparative analysis was planned. All patients who received any anti-VEGFs for nAMD were pooled in the same cohort, and there was no censoring at the time of switching to another anti-VEGF. In patients who received bilateral treatment, only the first treated eye was analyzed. Descriptive statistics (number of observations, proportions, means and standard deviations [SDs], median and minimum and maximum values, and 2-sided 95% CIs of the mean) were tabulated for the demographic and clinical characteristics and all outcome variables unless otherwise stated. Two-sided 95% CIs were derived for all proportions. No data imputation was performed.
For VA analysis, either the naked eye vision or corrected vision was used for each patient. VA was analyzed in subgroups stratified by (1) absence/presence of retinal fluids at each time point: VA at month N was stratified by absence/presence of fluids at month N; (2) absence/presence of retinal fluids at month 3: VA at all time points was stratified by absence/presence of fluids at month 3; (3) time with absence of fluid over 1 to 12 months (cohort 1) and 1 to 24 months (cohort 2): time was calculated as the sum of fluid-absence duration (time from the absence of fluid to a day before subsequent presence from day 1 to 360 and day 1 to 720 in days). Patients were divided into quartiles groups based on their time with absence of fluid. Quartile values for cohort 1: lower quartile = 0.0 day, median = 1.0 day, upper quartile = 139.5 days; quartile values for cohort 2: lower quartile = 1.0 day, median = 86.0 days, upper quartile = 288.0 days.
The Wilcoxon signed-rank test was used to assess the difference in VA change between patients with presence and absence of retinal fluid, and in patients with different quartiles of time with absence of retinal fluid. Two-sided P values were reported. All analyses were performed by LSK Global Pharma Services Co. Ltd., Korea, using Statistical Analysis System (SAS) version 9.4 software.
Ethical approval
This study was conducted in accordance with the Guidelines for Good Pharmacoepidemiology Practices issued by the International Society for Pharmacoepidemiology and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines, and adhered to the ethical principles derived from the Declaration of Helsinki. Each center’s institutional review board/independent ethics committee (IRB/IEC) reviewed and approved the study protocol.
Informed consent
Patients from Samsung Medical Center provided written informed consent, and the IRB/IEC of the other study sites approved the waiver of patient consent. All research adhered to the tenets of the Declaration of Helsinki. No animal subjects were included in this study.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
World Health Organization ROftWP. The Asia-Pacific perspective: redefining obesity and its treatment (2010).
Park, S. J., Kwon, K.-E., Choi, N.-K., Park, K. H. & Woo, S. J. Prevalence and incidence of exudative age-related macular degeneration in South Korea: A nationwide population-based study. Ophthalmology 122, 2063-2070.e2061. https://doi.org/10.1016/j.ophtha.2015.06.018 (2015).
Spooner, K. L., Mhlanga, C. T., Hong, T. H., Broadhead, G. K. & Chang, A. A. The burden of neovascular age-related macular degeneration: A patient’s perspective. Clin. Ophthal. 12, 2483–2491. https://doi.org/10.2147/OPTH.S185052 (2018).
Finger, R. P. et al. Anti-vascular endothelial growth factor in neovascular age-related macular degeneration – a systematic review of the impact of anti-VEGF on patient outcomes and healthcare systems. BMC Ophthalmol. 20, 294. https://doi.org/10.1186/s12886-020-01554-2 (2020).
Woo, S. J., Cho, G. E. & Cho, J. H. Short-term efficacy and safety of ranibizumab for neovascular age-related macular degeneration in the real world: A post-marketing surveillance study. Korean J. Ophthalmol. KJO 33, 150–166. https://doi.org/10.3341/kjo.2018.0081 (2019).
Baek, S. K., Kim, J. H., Kim, J. W. & Kim, C. G. Increase in the population of patients with neovascular age-related macular degeneration who underwent long-term active treatment. Sci. Rep. https://doi.org/10.1038/s41598-019-49749-y (2019).
Wykoff, C. C. et al. Optimizing anti-VEGF treatment outcomes for patients with neovascular age-related macular degeneration. J. Manag. Care Spec. Pharm. 24, S3–S15. https://doi.org/10.18553/jmcp.2018.24.2-a.s3 (2018).
Hsu, J. & Regillo, C. D. Poorer outcomes in real-world studies of anti–vascular endothelial growth factor therapy for neovascular age-related macular degeneration. Ophthalmology 127, 1189–1190. https://doi.org/10.1016/j.ophtha.2020.03.034 (2020).
Monés, J. et al. Undertreatment of neovascular age-related macular degeneration after 10 years of anti-vascular endothelial growth factor therapy in the real world: The need for a change of mindset. Ophthalmologica 243, 1–8. https://doi.org/10.1159/000502747 (2020).
Mehta, H. et al. Trends in real-world neovascular amd treatment outcomes in the UK. Clin. Ophthal. 14, 3331–3342. https://doi.org/10.2147/OPTH.S275977 (2020).
Khanani, A. M. et al. SIERRA-AMD: A Retrospective, real-world evidence study of patients with neovascular age-related macular degeneration in the United States. Ophthalmol. Retina 4, 122–133. https://doi.org/10.1016/j.oret.2019.09.009 (2020).
Kodjikian, L. et al. Fluid as a critical biomarker in neovascular age-related macular degeneration management: Literature review and consensus recommendations. Eye 35, 2119–2135. https://doi.org/10.1038/s41433-021-01487-0 (2021).
Schmidt-Erfurth, U. et al. Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA). Br. J. Ophthalmol. 98, 1144–1167. https://doi.org/10.1136/bjophthalmol-2014-305702 (2014).
Flaxel, C. J. et al. Age-related macular degeneration preferred practice pattern®. Ophthalmology 127, P1–P65. https://doi.org/10.1016/j.ophtha.2019.09.024 (2020).
Gillies, M. C. et al. Effect of ranibizumab and aflibercept on best-corrected visual acuity in treat-and-extend for neovascular age-related macular degeneration: A randomized clinical trial. JAMA Ophthalmol. 137, 372–379. https://doi.org/10.1001/jamaophthalmol.2018.6776 (2019).
Fung, A. E. et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am. J. Ophthalmol. 143, 566-583.e562. https://doi.org/10.1016/j.ajo.2007.01.028 (2007).
Arnold, J. J., Markey, C. M., Kurstjens, N. P. & Guymer, R. H. The role of sub-retinal fluid in determining treatment outcomes in patients with neovascular age-related macular degeneration - a phase IV randomised clinical trial with ranibizumab: The FLUID study. BMC Ophthalmol. 16, 31. https://doi.org/10.1186/s12886-016-0207-3 (2016).
Chakravarthy, U. et al. Association between visual acuity, lesion activity markers and retreatment decisions in neovascular age-related macular degeneration. Eye 34, 2249–2256. https://doi.org/10.1038/s41433-020-0799-y (2020).
Hussain, R. M. Addressing the Anti-VEGF Treatment Burden, Review of Opthalmology (2018).
Klufas, M. A. & D’Amico, D. J. Targeting Unmet Needs in nAMD Treatment. Retina Specilalist (2018).
Dugel, P. U. et al. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology 127, 72–84. https://doi.org/10.1016/j.ophtha.2019.04.017 (2020).
Dugel, P. U. et al. HAWK and HARRIER: ninety-six-week outcomes from the phase 3 trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology 128, 89–99. https://doi.org/10.1016/j.ophtha.2020.06.028 (2021).
Heier, J. S. et al. Faricimabin Neovascular Age-Related Macular Degeneration TENAYA and LUCERNE Study Results: Phase 3, Multicenter, Randomized, Double-Masked, Active Comparator–Controlled Studies to Evaluate the Efficacy and Safety of Faricimabin Patients With Neovascular Age-Related Macular Degeneration. presented at Angiogenesis (Virtual, 2021).
Baumal, C. R. et al. Retinal Vasculitis and Intraocular Inflammation after Intravitreal Injection of Brolucizumab. Ophthalmology 127, 1345–1359. https://doi.org/10.1016/j.ophtha.2020.04.017 (2020).
Monés, J. et al. Risk of Inflammation, retinal vasculitis, and retinal occlusion-related events with brolucizumab: Post hoc review of HAWK and HARRIER. Ophthalmology 128, 1050–1059. https://doi.org/10.1016/j.ophtha.2020.11.011 (2021).
Singer, M. et al. Clinical Characteristics and outcomes of eyes with intraocular inflammation after brolucizumab: Post hoc analysis of HAWK and HARRIER. Ophthalmol Retina 6, 97–108. https://doi.org/10.1016/j.oret.2021.05.003 (2022).
Rahman, E. Z. & Singer, M. A. Brolucizumab as treatment of wet age-related maculopathy. Drugs Today 56, 699–704. https://doi.org/10.1358/dot.2020.56.11.3199812 (2020).
Carrasco, J. et al. Real-world effectiveness and real-world cost-effectiveness of intravitreal aflibercept and intravitreal ranibizumab in neovascular age-related macular degeneration: Systematic review and meta-analysis of real-world studies. Adv. Ther. 37, 300–315. https://doi.org/10.1007/s12325-019-01147-6 (2020).
Holz, F. G. et al. Ranibizumab treatment in treatment-naive neovascular age-related macular degeneration: Results from Luminous, a global real-world study. Retina 40, 1673–1685. https://doi.org/10.1097/IAE.0000000000002670 (2020) (Philadelphia, Pa).
Ciulla, T. A. et al. Real-world outcomes of anti-vascular endothelial growth factor therapy in neovascular age-related macular degeneration in the United States. Ophthalmology. Retina 2, 645–653. https://doi.org/10.1016/j.oret.2018.01.006 (2018).
Jaffe, G. J. et al. Macular morphology and visual acuity in the comparison of age-related macular degeneration treatments trials. Ophthalmology 120, 1860–1870. https://doi.org/10.1016/j.ophtha.2013.01.073 (2013).
Guymer, R. H. et al. Tolerating subretinal fluid in neovascular age-related macular degeneration treated with ranibizumab using a treat-and-extend regimen: Fluid study 24-month results. Ophthalmology 126, 723–734. https://doi.org/10.1016/j.ophtha.2018.11.025 (2019).
Core, J. Q. et al. Predominantly persistent subretinal fluid in the comparison of age-related macular degeneration treatments trials. Ophthalmol. Retina 5, 962–974. https://doi.org/10.1016/j.oret.2021.06.003 (2021).
Reiter, G. S. et al. Analysis of fluid volume and its impact on visual acuity in the fluid study as quantified with deep learning. Retina 41, 1318–1328. https://doi.org/10.1097/IAE.0000000000003023 (2021).
Lai, T.-T., Hsieh, Y.-T., Yang, C.-M., Ho, T.-C. & Yang, C.-H. Biomarkers of optical coherence tomography in evaluating the treatment outcomes of neovascular age-related macular degeneration: A real-world study. Sci. Rep. https://doi.org/10.1038/s41598-018-36704-6 (2019).
Wickremasinghe, S. S. et al. Implication of recurrent or retained fluid on optical coherence tomography for visual acuity during active treatment of neovascular age-related macular degeneration with a treat and extend protocol. Retina 36, 1331–1339. https://doi.org/10.1097/IAE.0000000000000902 (2016) (Philadelphia, Pa).
Leung, K. F. C., Downes, S. M. & Chong, V. A retrospective analysis of the effect of subretinal hyper-reflective material and other morphological features of neovascular age-related macular degeneration on visual acuity outcomes in eyes treated with intravitreal aflibercept over one year. Vision (Basel, Switzerland) https://doi.org/10.3390/vision2010005 (2018).
Zinkernagel, M. S. All retinal fluid should be eliminated to maximise outcomes for wet AMD patients. presented at European Society of Retina Specialist (2019).
Core, J. Q. et al. Predominantly persistent intraretinal fluid in the comparison of age-related macular degeneration treatments trials. Ophthalmol. Retina https://doi.org/10.1016/j.oret.2022.03.024 (2022).
Khanani, A. M., Eichenbaum, D., Schlottmann, P. G., Tuomi, L. & Sarraf, D. Optimal management of pigment epithelial detachments in eyes with neovascular age-related macular degeneration. Retina 38, 2103–2117. https://doi.org/10.1097/iae.0000000000002195 (2018).
Schmidt-Erfurth, U., Waldstein, S. M., Deak, G. G., Kundi, M. & Simader, C. Pigment epithelial detachment followed by retinal cystoid degeneration leads to vision loss in treatment of neovascular age-related macular degeneration. Ophthalmology 122, 822–832. https://doi.org/10.1016/j.ophtha.2014.11.017 (2015).
Fang, M. et al. Morphological characteristics of eyes with neovascular age-related macular degeneration and good long-term visual outcomes after anti-VEGF therapy. Br. J. Ophthalmol. https://doi.org/10.1136/bjophthalmol-2021-319602 (2021).
Sagong, M., Woo, S. & Lee, Y. Real-world effectiveness, treatment pattern, and safety of ranibizumab in Korean patients with neovascular age-related macular degeneration: Subgroup analyses from the luminous study. Clin. Ophthalmol. 15, 1995–2011. https://doi.org/10.2147/OPTH.S303884 (2021).
Chen, S.-N. et al. One-year real-world outcomes of ranibizumab 0.5 mg treatment in Taiwanese patients with polypoidal choroidal vasculopathy: a subgroup analysis of the REAL study. Int. J. Ophthalmol. 11, 1802–1808 (2018).
Cohen, J. A. et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N. Engl. J. Med. 362, 402–415. https://doi.org/10.1056/NEJMoa0907839 (2010).
Eldem, B. et al. An analysis of ranibizumab treatment and visual outcomes in real-world settings: The UNCOVER study. Graefes. Arch. Clin. Exp. Ophthalmol. 256, 963–973. https://doi.org/10.1007/s00417-017-3890-8 (2018).
Lotery, A., Griner, R., Ferreira, A., Milnes, F. & Dugel, P. Real-world visual acuity outcomes between ranibizumab and aflibercept in treatment of neovascular AMD in a large US data set. Eye 31, 1697–1706. https://doi.org/10.1038/eye.2017.143 (2017).
Gillies, M. C. et al. Twelve-month outcomes of ranibizumab vs. aflibercept for neovascular age-related macular degeneration: Data from an observational study. Ophthalmology 123, 2545–2553. https://doi.org/10.1016/j.ophtha.2016.08.016 (2016).
Barbazetto, I. et al. Photodynamic therapy of subfoveal choroidal neovascularization with verteporfin: Fluorescein angiographic guidelines for evaluation and treatment–TAP and VIP report No. 2. Arch. Ophthalmol. 121, 1253–1268. https://doi.org/10.1001/archopht.121.9.1253 (2003).
Acknowledgements
Medical writing support was provided by Khi Khi Choo (Novartis Corporation, Malaysia) in accordance with Good Publication Practice (GPP3) guidelines.
Funding
Financial support was provided by Novartis Korea Ltd. The sponsor or funding organization participated in the design of the study; management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript.
Author information
Authors and Affiliations
Contributions
J.H.K., M.S., S.J.W., Y.C.K., H.C., Y.H.L., I.B., Y.J.J., H.S.C., S.W.K.: Conceptualization, methodology, investigation, writing—original draft, writing—review & editing, visualization, and supervision; Y.L.: Conceptualization, methodology, writing—original draft, writing—review & editing, visualization, project administration, and funding acquisition. J.E.C.: Methodology, software, formal analysis, data curation, writing—original draft, writing –review & editing, and visualization.
Corresponding author
Ethics declarations
Competing interests
Jae Hui Kim reports grants from Novartis and Bayer. Min Sagong reports honoraria from Allergan, Bayer, Novartis, and Roche for lecture fees, consultancy, and board membership and reports research grants from Allergan, Bayer, and Novartis. Se Joon Woo is a consultant of Samsung Bioepis, Curacle, Novelty Nobility, Sometech, Philophos, Janssen, and Allergan; reports grants from Samsung Bioepis, Curacle, and Alteogen; reports lecture fees from Novartis, Bayer, Allergan/AbbVie, and Santen; and owns equity of RetiMark and Panolos Bioscience. Yu Cheol Kim is a consultant for Novartis and Bayer; received honoraria from Allergan, Bayer, and Novartis; and research grants from Chong Kun Dang Pharm., SCD Pharm., Bayer, and Novartis. Heeyoon Cho reports lecture fees and consultancy from Allergan, Bayer, and Novartis. Young Hoon Lee has no financial disclosures. Iksoo Byon is a consultant/advisor for Novartis, Bayer, and AIinsight Inc and reports grants from Novartis and Busan Technopark. Young Joon Jo reports grants from Bayer and Novartis. Hee Seung Chin has no financial disclosures.Youkyung Lee is a full-time employee of Novartis Korea Ltd. Jae Eun Chae is a full-time employee of LSK Global Pharma Services, Korea. Se Woong Kang has served on advisory boards for Novartis, Bayer, Allergan, Alcon, and Samchundang and has received consultancy fees and payments for lectures from these companies.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Kim, J.H., Sagong, M., Woo, S.J. et al. A real-world study assessing the impact of retinal fluid on visual acuity outcomes in patients with neovascular age-related macular degeneration in Korea. Sci Rep 12, 14166 (2022). https://doi.org/10.1038/s41598-022-18158-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-18158-z
- Springer Nature Limited