Abstract
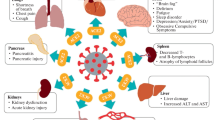
Persistent post-COVID syndrome, also referred to as long COVID, is a pathologic entity, which involves persistent physical, medical, and cognitive sequelae following COVID-19, including persistent immunosuppression as well as pulmonary, cardiac, and vascular fibrosis. Pathologic fibrosis of organs and vasculature leads to increased mortality and severely worsened quality of life. Inhibiting transforming growth factor beta (TGF-β), an immuno- and a fibrosis modulator, may attenuate these post-COVID sequelae. Current preclinical and clinical efforts are centered on the mechanisms and manifestations of COVID-19 and its presymptomatic and prodromal periods; by comparison, the postdrome, which occurs in the aftermath of COVID-19, which we refer to as persistent post-COVID-syndrome, has received little attention. Potential long-term effects from post-COVID syndrome will assume increasing importance as a surge of treated patients are discharged from the hospital, placing a burden on healthcare systems, patients’ families, and society in general to care for these medically devastated COVID-19 survivors. This review explores underlying mechanisms and possible manifestations of persistent post-COVID syndrome, and presents a framework of strategies for the diagnosis and management of patients with suspected or confirmed persistent post-COVID syndrome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Winston Churchill, the eminently aphoristic and epigrammatic Prime Minister of Great Britain from 1941 to 1945, once wrote: “Now this is not the end. It is not even the beginning of the end. But it is, perhaps, the end of the beginning.” The SARS-CoV-2 pandemic is a story in three parts: prologue or onset, middle chapters, and epilogue or resolution, of which only the prologue and some of the middle chapters have been written, marking the end of the beginning but also hopefully the beginning of the end as the containment and mitigation measures to limit the spread of the disease finally begin to take effect [28]. In the exudative phase, release of proinflammatory cytokines such as IL-1β, TNF, and IL-6, influx of neutrophils, and endothelial-epithelial barrier disruption occur, which leads to alveolar flooding and respiratory distress [29]. The exudative phase is followed by a fibroproliferative phase, in which fibrocytes, fibroblasts, and myofibroblasts accumulate in the alveolar compartment, leading to excessive deposition of matrix components including fibronectin, collagen I, and collagen III [30].
One of the mechanisms that contribute to the development of a fibroproliferative response in ARDS is mechanical ventilation since shear forces not only induce secretion of transforming growth factor β1 but also activate collagen synthesis and inhibit collagenase production [31].
A subset of ARDS survivors and, hence, also, by extension, COVID-19 patients progress to pulmonary fibrosis, of which exercise-induced breathlessness and chronic dry cough are the prominent symptoms and for which management is largely supportive consisting of supplemental oxygen, pulmonary rehabilitation, and vaccination against Streptococcus pneumoniae and influenza [32]. Two FDA-approved medications, nintedanib and pirfenidone, are non-curative but have been demonstrated to slow the progression of pulmonary fibrosis [33]. These patients, whose mortality risk is elevated, may continue to present with exercise limitations and reduced quality of life for up to 5 years post-ARDS [34].
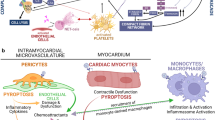
Cardiac Fibrosis and Dysfunction
COVID-19 patients commonly present with signs of myocardial injury including heart failure and myocarditis and/or exacerbation of existing cardiovascular disease as determined by elevated levels of troponin T (TnT) and brain natriuretic peptide (BNP) [35]. Potential mechanisms of injury include the following:
-
increased pulmonary vascular resistance with subsequent pulmonary hypertension and right heart failure
-
overstimulation of the renin-angiotensin system (RAS), which mediates deleterious effects on the cardiovascular system including secondary hyperaldosteronism, leading to hypokalemia and cardiac arrhythmias [36]
-
atherosclerotic plaque rupture via the action of pro-inflammatory cytokines, precipitating infarction, especially in the context of pre-existing coronary artery diseases [37]
-
ACE-2-mediated viral invasion of cardiomyocytes, resulting in myocarditis
-
myocardial oxygen supply/demand mismatch from the combination of decreased venous return and severe hypoxemia due to ARDS, leading to myocardial ischemia/necrosis
-
possible cardiotoxicity of potential anti-COVID agents including the macrolide antibiotic, azithromycin, associated with a prolonged QT interval [38], chloroquine/hydroxychloroquine, which may produce conduction defects in the heart, tocilizumab, which increases cholesterol levels [39], and lopinavir/ritonavir, the protease inhibitors that may prolong PR and QT intervals and also inhibit CYP3A4 activity, which influences the metabolism of other cardiac medications including statins [40].
The common denominator of myocardial injury is a remodeling process that includes hypertrophy and fibrosis of the left ventricular wall, leading to reduced contractility and impaired global function [41], of which TGF-β, as the main profibrotic cytokine, is a major player. While it is perhaps too early to predict long-term cardiac consequences of COVID-19, extrapolation is possible with SARS-CoV-1 patients, given the genetic similarities between SARS-CoV-1 and SARS-CoV-2, that at 12-years of follow-up demonstrated cardiovascular abnormalities in 40% [42].
Neurological Fibrosis and Dysfunction
Infection with SARS-CoV-2 commonly leads to respiratory symptoms typical of a viral pneumonia, including fever, cough, dyspnea, and sore throat but also, interestingly, anosmia and dysgeusia [43], which suggests that the virus is neurotropic. In a retrospective case series of 214 patients in Wuhan, China, a high incidence of neurologic symptoms was seen. Seventy-eight (36.4%) patients had central nervous system (CNS) (24.8%), peripheral nervous system (PNS) (8.9%), or skeletal muscle symptoms (10.7%). The two most common CNS symptoms were dizziness (16.8%) and headache (13.1%). Acute cerebrovascular disease, ataxia, epilepsy, and impaired consciousness were also reported [44].
Tissue fibrosis is a common response to damage in most organs of the body except the brain because fibrogenic cells are restricted to particular niches [45]. However, with disruption of the blood brain barrier due to cytokine storm, for example, or direct viral injury to nervous tissue, scar formation is induced.
Neurologic and psychiatric sequelae are commonly seen in sepsis survivors [46, 47]. Likewise, neuropsychiatric symptoms have also been reported with after SARS-CoV-2 infection [48]. These symptoms include depression, anxiety, and psychosis [49].
Since multiple neurological disorders including AIDS dementia complex, Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis (ALS), multiple sclerosis, anxiety, depression, and schizophrenia [50] are linked to the deregulation of the TGF-β signaling pathway, this cytokine is a potential therapeutic target for COVID-19-induced neuropsychiatric symptoms.
COVID-19-Associated Coagulopathy
Some patients with severe COVID-19 infection develop a DIC-like coagulopathy with fulminant activation of coagulation and consumption of coagulation factors. This is characterized by delayed clotting times (PT and aPTT), low platelets, and decreased fibrinogen (< 1.0 g/L) due to their consumption. Thrombotic complications include pulmonary embolism and strokes, which suggest the need for pharmacological thrombosis prophylaxis especially in ICU patients [51].
Thrombotic “after-effects” include the potential for recurrence, long-term anticoagulation with Coumadin or enoxaparin, which increases the risk of hemorrhage, physical impairments from cerebral vascular accident (CVA) [52], myocardial infarction (MI) or pulmonary embolism, and alterations of behavior and emotion.
Management
Management strategies for the treatment of post-COVID sequelae will vary greatly depending on the symptomatic profile and needs of each individual patient. Management strategies should account for prior pre-existing medical conditions and care teams should provide regular follow-up for each patient until symptoms subside and for some time thereafter. A framework of general recommendations for the management of patients with suspected or confirmed PPCS is presented in Table 2. In the following, we explore potential treatment strategies for specific post-COVID manifestations.
By analogy with sepsis, which COVID-19-induced ARDS parallels, hyperinflammation during SARS-CoV-2 infection is followed by a prolonged immunoparalytic and profibrotic state that drives heightened vulnerability to secondary infections and organ dysfunction even after so-called recovery from the disease (Fig. 2). On this basis, immunodulatory therapies are seemingly warranted, to prevent or reverse the anti-inflammatory phenotype, although many of these immunodulatory therapies (e.g., GM-CSF, pooled intravenous immunoglobulins (IVIG), IFNγ, interleukin-7, PD-L1 inhibitors, and IL-3) have been tried during sepsis, with mostly mixed results, possibly due to the complex temporal fluctuations of pro- and anti-inflammatory cytokines in sepsis [53].
However, in post-sepsis syndrome and, by extension, PPCS, persistent inflammation, immunosuppression, and catabolism or PICS predominates, which resembles the malignant phenotype, hence the rationale to investigate immunomodulatory therapies such as checkpoint inhibitors, TGF-β inhibitors, hematopoietic growth factors, cytokines, and chemokines specifically in this post-infectious setting, rather than during ongoing sepsis per se, when inflammation fluctuates dynamically. In particular, for PPCS and for post-sepsis/post-ICU syndromes, TGF-β inhibitors may hold promise as agents that neutralize or reverse immune suppression as well as fibrosis.
Several potentially repurposable TGF-β inhibitors are under evaluation in the clinic for the treatment of cancer. These include Trabedersen (AP12009, Antisense Pharma), an antisense oligonucleotide, Belagenpneumatucel-L (Lucanix, NovaRx), a TGF-β2, antisense allogenic tumor cell vaccine, galunisertib monohydrate (LY2157299, Eli Lilly), a small-molecule inhibitor of TβRI, vactosertib (EW-7197 or TEW-7197), a novel small-molecule inhibitor of ALK5 that inhibits TGF-β1-induced Smad/TGFβ signaling, fresolimumab (GC1008, Genzyme/Sanofi), a fully human monoclonal antibody blocking pan-TGF-β (TGF-β1, TGF-β2, and TGF-β3), tasisulam (LY573636), a small-molecule inhibitor of TGF-β, and BETA PRIME (AdAPT-001, EpicentRx), a modified replicating oncolytic adenovirus that encodes the TGF-β type II receptor to trap or neutralize TGF-β [54]. Table 3 summarizes a number of these clinical TGF-β inhibitors.
Standard of care for many acute events includes not only acute management but also mitigates the risk of subsequent complications in scenarios where the risks and appropriate interventions are widely recognized. Examples include treating with antiplatelet therapy (such as Aggrenox) after stroke, anticoagulation which is increasingly in the form of direct oral anticoagulants (such as rivaroxaban or apixaban) after orthopedic surgery, and post-myocardial infarction regimens including statins, anti-platelet agents, ACE inhibitors, and beta blockers. While post-ARDS complications have begun to be recognized, the surge of COVID-19 is poised to bring PPCS and post-ARDS syndrome to the forefront and open the question of how it should be handled when there is no current standard of care.
Conclusion
At the forefront of clinical care for acute COVID-19 are multiple guidelines, recommendations, and best practices that have been promulgated and prioritized for prevention and management; however, presumably because the focus is on the immediate, day-to-day “anti-COVID fight” rather than on a potential future one, no guidelines are currently available for postinfectious care or recovery and there is a notable dearth of information on and strategies about how to assess and manage post-COVID patients.
The purpose of this review is to make the case and to raise awareness (and the alarm) for persistent post-COVID syndrome (PPCS), a newly coined umbrella term, by analogy to post-sepsis/post-ICU syndrome that covers a loose confederation of heterogeneous symptoms for which no pathognomonic laboratory test exists, making it easy to overlook or ignore. However, to overlook or ignore the hidden “iceberg” of PPCS, which may be unique or a version of post-sepsis/post-ICU is to possibly replace or supplant one epidemic with another, as evidenced by the rising tide of physical and psychological disabilities that have been described in post-COVID patients [55,56,57] and which have the potential to re-inundate an already overburdened health care system.
Nevertheless, evidence of a causal association between COVID-19 diagnosis and subsequent morbidity is difficult to establish, especially since chronic illness and persistent post-COVID syndrome (PPCS) may share common risk factors and antecedents, such as older age, diabetes, smoking, malnutrition or obesity, immunosuppression, and hypertension, which reflect a broad vulnerability to these pathologies. Other factors, which may increase diagnostic and management difficulties include.
-
the temporal separation between the acute and chronic symptoms
-
a lack of awareness of post-COVID and post-ICU pathology, potentially resulting in a failure to “connect the dots” with regard to the multi-system signs and symptoms
-
the chicken-and-egg question about whether and to what extent critical illness per se is responsible for and causally related to prolonged post-COVID illness, or whether and to what extent pre-existing comorbidities and pre-COVID clinical trajectories influence the post-COVID burden and are responsible for pushing frail patients with low resiliency past a tip** point.
Finally, it is probably unrealistic to expect a “magic bullet” treatment for PPCS that will completely “roll back” the symptoms; however, since the central issues for PPCS are potentially immune paresis and, hence, susceptibility to secondary infections as well as fibrotic remodeling in the lungs, heart, and brain that develops as the end result of a chronic inflammatory process, then immunomodulatory therapy, and particularly inhibition of TGF-β, stands at the intersection between inflammation, immunosuppression, and fibrosis and may serve as a mechanistic lynchpin linking post-infectious immunoparalysis with fibrosis to facilitate the design of new targeted strategies for the prevention of these devastating sequelae of COVID-19.
One of the first American battle cries was, “The redcoats are coming!”. The most recent may be “re-COVID is coming” due to seasonal recurrence of the SARS-CoV-2 virus and along with it, according to the premise of this review, persistent post-COVID syndrome.
References
Milne GJ, **e S (2020) The effectiveness of social distancing in mitigating COVID-19 spread: a modelling analysis. medRxiv. 03.20.20040055
Misra DP, Agarwal V, Gasparyan AY et al (2020) Rheumatologists’ perspective on coronavirus disease 19 (COVID-19) and potential therapeutic targets. Clin Rheumatol
https://nypost.com/2020/03/13/coronavirus-survivors-may-suffer-from-reduced-lung-function/
Baud D, Qi X, Nielsen-Saines K, Musso D, Pomar L, Favre G (2020) Real estimates of mortality following COVID-19 infection [published online ahead of print, 2020 Mar 12]. Lancet Infect Dis S1473–3099(20)30195-X
Mostel Z, Perl A, Marck M et al (2019) Post-sepsis syndrome - an evolving entity that afflicts survivors of sepsis. Mol Med 26(1):6. Published 31 Dec 2019
Stam HJ, Stucki G, Bickenbach J (2020) Covid-19 and post intensive care syndrome: a call for action. J Rehabil Med Apr 15;52(4):jrm00044
Angus DC (2010) The lingering consequences of sepsis: a hidden public health disaster? JAMA Oct 27;304(16):1833-4
Hotchkiss RS, Monneret G, Payen D (2013) Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol 13:862–874
Bone RC (1996) Sir Isaac Newton, sepsis, SIRS, and CARS. Crit Care Med 24:1125–1128
Sugimoto MA, Sousa LP, Pinho V, Perretti M, Teixeira MM (2016) Resolution of inflammation: what controls its onset?. Front Immunol 7:160. Published 2016 Apr 26
Bozza FA, Salluh JI, Japiassu AM, Soares M, Assis EF, Gomes RN, Bozza MT, Castro-Faria-Neto HC, Bozza PT (2007) Cytokine profiles as markers of disease severity in sepsis: a multiplex analysis. Crit Care 11(2):R49
Delano MJ, Ward PA (2016) The immune system's role in sepsis progression, resolution, and long-term outcome. Immunol Rev 274(1):330–353
Kell DB, Pretorius E (2018) To what extent are the terminal stages of sepsis, septic shock, systemic inflammatory response syndrome, and multiple organ dysfunction syndrome actually driven by a prion/amyloid form of fibrin?. Semin Thromb Hemost 44(3):224–238
Biradar V, Moran JL (2011) SIRS, Sepsis and Multiorgan Failure. In: Fitridge R, Thompson M, editors. Mechanisms of vascular disease: a reference book for vascular specialists [Internet]. Adelaide (AU): University of Adelaide Press 17. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534275/
Hamers L, Kox M, Pickkers P (2015) Sepsis-induced immunoparalysis: mechanisms, markers, and treatment options. Minerva Anestesiol Apr;81(4):426-39. Epub 2014 May 30
Walton AH, Muenzer JT, Rasche D et al (2014) Reactivation of multiple viruses in patients with sepsis. PLoS One 9(2):e98819. Published 11 Jun 2014
Xu K1, Cai H, Shen Y, Ni Q, Chen Y, Hu S, Li J et al (2020) [Management of corona virus disease-19 (COVID-19): the Zhejiang experience].[Article in Chinese] Zhejiang Da Xue Xue Bao Yi Xue Ban Feb 21;49(1):0
Shi H, Han X, Jiang N et al (2020) Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis 20(4):425–434
Hosseiny M, Kooraki S, Gholamrezanezhad A, Reddy S, Myers L (2020)Radiology Perspective of Coronavirus Disease 2019 (COVID-19): Lessons From Severe Acute Respiratory Syndrome and Middle East Respiratory Syndrome. AJR Am J Roentgenol Feb 28:1-5
Russell B, Moss C, George G et al (2020) Associations between immune-suppressive and stimulating drugs and novel COVID-19-a systematic review of current evidence. Ecancer Med Sci 14:1022. Published 27 Mar 2020
Tian S, Hu W, Niu L, Liu H, Xu H, **ao SY (2020) Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer [published online ahead of print, 2020 Feb 28]. J Thorac Oncol S1556–0864(20)30132–5
Russell B, Moss C, George G et al (2020) Associations between immune-suppressive and stimulating drugs and novel COVID-19-a systematic review of current evidence. Ecancer Med Sci 14:1022. Published 27 Mar 2020
NIHR (2020) Living with covid-19. A dynamic review of the evidence around ongoingcovid-19 symptoms (often called long covid). https://evidence.nihr.ac.uk/themedreview/living-with-covid19
https://www.uptodate.com/contents/coronavirus-disease-2019-covid-19-critical-care-issues
Matuschak GM, Lechner AJ (2010) Acute lung injury and the acute respiratory distress syndrome: pathophysiology and treatment. Mo Med 107(4):252–258
Walkey AJ, Summer R, Ho V, Alkana P (2012) Acute respiratory distress syndrome: epidemiology and management approaches. Clin Epidemiol 4:159–169
Williams AE, Chambers RC (2014) The mercurial nature of neutrophils: still an enigma in ARDS?. Am J Physiol Lung Cell Mol Physiol 306(3):L217–L230
Burnham EL, Janssen WJ, Riches DW, Moss M, Downey GP (2014) The fibroproliferative response in acute respiratory distress syndrome: mechanisms and clinical significance. Eur Respir J 43(1):276–285
Kouzbari K, Hossan MR, Arrizabalaga JH et al (2019) Oscillatory shear potentiates latent TGF-β1 activation more than steady shear as demonstrated by a novel force generator. Sci Rep 9, 6065
Johannson K, Collard HR (2013) Acute exacerbation of idiopathic pulmonary fibrosis: a proposal. Curr Respir Care Rep2(4):https://doi.org/10.1007/s13665-013-0065-x
Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE Jr, Kondoh Y, Myers J, Müller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schünemann HJ (2011) ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183(6):788
Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A et al (2011) Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 364(14):1293–1304
Bansal M (2020) Cardiovascular disease and COVID-19. Diabetes Metab Syndr 14(3):247–250
Vaidya A, Dluhy R (2000) Hyperaldosteronism. [Updated 2016 Oct 19]. In: Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279065/
Young BE, Ong SWX, Kalimuddin S et al (2020) Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA ([Internet]. Available from:): 1–7
Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, Liu X, Zhao L, Dong E, Song C, Zhan S, Lu R, Li H, Tan W, Liu D (2020) In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin Infect Dis
Soubrier M, Pei J, Durand F, Gullestad L, John A (2017) Concomitant Use of Statins in Tocilizumab-Treated Patients with Rheumatoid Arthritis: A Post Hoc Analysis. Rheumatol Ther Jun;4(1):133-149
Tan W, Aboulhosn J (2020) The cardiovascular burden of coronavirus disease 2019 (COVID-19) with a focus on congenital heart disease [published online ahead of print, 2020 Mar 28]. Int J Cardiol S0167–5273(20)31593-X
Biernacka A, Frangogiannis NG (2011) Aging and Cardiac Fibrosis. Aging Dis 2(2):158–173
Wu Q, Zhou L, Sun X (2017) Altered lipid metabolism in recovered SARS patients twelve years after infection. Sci Rep 7:9110
Giacomelli A, Pezzati L, Conti F et al (2020) Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study. Clin Infect Dis. [epub ahead of print]
Mao L, ** H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B (2020) Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol
Fernández-Klett F, Priller J (2014) The fibrotic scar in neurological disorders. Brain Pathol 24(4):404-13
Gawlytta R, Niemeyer H, Böttche M, Scherag A, Knaevelsrud C, Rosendahl J (2017) Internet-based cognitive-behavioural writing therapy for reducing post-traumatic stress after intensive care for sepsis in patients and their spouses (REPAIR): study protocol for a randomised-controlled trial. BMJ Open 7(2):e014363
Annane D, Sharshar T (2015) Cognitive decline after sepsis. Lancet Respir Med 3(1):61–9
Wu, Yeshun, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, Liu C, Yang C (2020) Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain. Behav. Immun. https://doi.org/10.1016/j.bbi.2020.03.031
Troyer EA, Kohn JN, Hong S (2020) Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms [published online ahead of print, 2020 Apr 13]. Brain Behav Immun S0889–1591(20)30489-X
Kashima R, Akiko Hata A (2018) The role of TGF-β superfamily signaling in neurological disorders. Acta Biochimica et Biophysica Sinica 50(1):106–120
Klok FA, Kruip MJHA, van der Meer NJM et al (2020) Incidence of thrombotic complications in critically ill ICU patients with COVID-19 [published online ahead of print, 2020 Apr 10]. Thromb Res S0049–3848(20)30120–1
Mao L, Wang M, Chen S, He O, Chang J et al (2020)Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series study. medRxiv 02.22.20026500
Horiguchi H, Loftus TJ, Hawkins RB et al (2018) Innate immunity in the persistent inflammation, immunosuppression, and catabolism syndrome and its implications for therapy. Front Immunol 9:595. Published 4 Apr 2018
Parichatikanond W, Luangmonkong T, Mangmool S, Kurose H (2020) Therapeutic targets for the treatment of cardiac fibrosis and cancer: focusing on TGF-β signaling. Front Cardiovasc Med 7:34. Published 10 Mar 2020
Allahwala UK, Denniss AR, Zaman S, Bhindi R (2020) Cardiovascular disease in the post-COVID-19 era – the impending tsunami? [published online ahead of print, 2020 Apr 16]. Heart Lung Circ
Das N (2020) Psychiatrist in post-COVID-19 era - Are we prepared? [published online ahead of print, 2020 Apr 7]. Asian J Psychiatr 51:102082
McInnes IB (2020) COVID-19 and rheumatology: first steps towards a different future? Annals Rheumatic Diseases 79:551-552
Acknowledgements
The authors wish to acknowledge Dr. Arnold Oronsky, great man, father, role model, mentor and best friend, for his guidance and sage insights and for always lending a hel** hand
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
EpicentRx, Inc., funds research of the AdAPT-001 product. Authors B.O., C.L. and T.R. are employed by EpicentRx, Inc.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Oronsky, B., Larson, C., Hammond, T.C. et al. A Review of Persistent Post-COVID Syndrome (PPCS). Clinic Rev Allerg Immunol 64, 66–74 (2023). https://doi.org/10.1007/s12016-021-08848-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-021-08848-3