Abstract
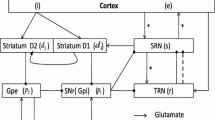
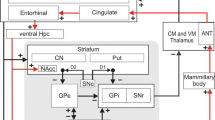
Absence epileptiform activities are traditionally considered to be primarily induced by abnormal interactions between the cortical and thalamic neurons, which form the thalamocortical circuit in the brain. The basal ganglia, as an organizational unit in the brain, has close input and output relationships with the thalamocortical circuit. Although several studies report that the basal ganglia may participate in controlling and regulating absence epileptiform activities, to date, there have been no studies regarding whether the basal ganglia directly cause absence epileptiform activities. In this paper, we built a basal ganglia-corticothalamic network model to determine the role of basal ganglia in this disease. We determined that absence epileptiform activities might be directly induced by abnormal coupling strengths on certain pivotal pathways in the basal ganglia. These epileptiform activities can be well controlled by the coupling strengths of three major pathways that project from the thalamocortical network to the basal ganglia. The results implied that the substantia nigra pars compacta (SNc) can be considered to be the effective treatment target area for inhibiting epileptiform activities, which supports the observations of previous studies. Particularly, as a major contribution of this paper, we determined that the final presentation position of the epileptic slow spike waves is not limited to the cerebral cortex; these waves may additionally appear in the thalamus, striatal medium spiny neurons, striatal fast spiking interneuron, the SNc, subthalamic nucleus, substantia nigra pars reticulata and globus pallidus pars externa. In addition, consistent with several previous studies, the delay in the network was observed to be a critical factor for inducing transitions between different types of absence epileptiform activities. Our new model not only explains the onset and control mechanism but also provides a unified framework to study similar problems in neuron systems.








Similar content being viewed by others
References
Arakaki T, Mahon S, Charpier S, Leblois A, Hansel D (2016) The role of striatal feedforward inhibition in the maintenance of absence seizures. J Neurosci 36(37):9618–9632
Biraben A, Semah F, Ribeiro MJ, Douaud G, Remy P, Depaulis A (2004) PET evidence for a role of the basal ganglia in patients with ring chromosome 20 epilepsy. Neurology 63(1):73–77
Bogacz R (2015) Basal Ganglia: beta oscillations. In: Jaeger D, Jung R (eds) Encyclopedia of Computational Neuroscience. Springer, New York, NY, pp 327–330
Bonhaus DW, Walters JR, McNamara JO (1986) Activation of substantia nigra neurons: role in the propagation of seizures in kindled rats. J Neurosci 6(10):3024–3030
Breakspear M, Roberts JA, Terry JR, Rodrigues S, Mahant N, Robinson PA (2005) A unifying explanation of primary generalized seizures through nonlinear brain modeling and bifurcation analysis. Cereb Cortex 16(9):1296–1313
Case M, Soltesz I (2011) Computational modeling of epilepsy. Epilepsia 52(s8):12–15
Chang AD, Berges VA, Chung SJ, Fridman GY, Baraban JM, Reti IM (2016) High-frequency stimulation at the subthalamic nucleus suppresses excessive self-grooming in autism-like mouse models. Neuropsychopharmacology 41(7):1813–1821
Chen MM, Guo D, Wang T et al (2014) Bidirectional control of absence seizures by the basal ganglia: a computational evidence. PLoS Comput Biol 10(3):e1003495
Chen MC, Ferrari L, Sacchet MD et al (2015a) Identification of a direct GABA ergic pallidocortical pathway in rodents. Eur J Neurosci 41(6):748–759
Chen MM, Guo DQ, Li M et al (2015b) Critical roles of the direct GABAergic pallido-cortical pathway in controlling absence seizures. PLoS Comput Biol 11(10):e1004539
Cheng H, Kuang Y, Liu Y et al (2015) Low-frequency stimulation of the external globus palladium produces anti-epileptogenic and anti-ictogenic actions in rats. Acta Pharmacol Sin 36(8):957–965
Cheong E, Shin HS (1828) T-type Ca2+ channels in absence epilepsy. BBA Biomembr 7:1560–1571
CoCaTotILA E (1989) Proposal for revised classification of epilepsies and epileptic syndromes. Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia 30(4): 389–399
Crunelli V, Leresche N (2002) Childhood absence epilepsy: genes, channels, neurons and networks. Nat Rev Neurosci 3(5):371–382
Dadok VM, Szeri AJ, Kirsch H, Sleigh J, Lopour B (2012) Interpretation of seizure evolution pathways via a mean-field cortical model. BMC Neurosci 13(1):P95
Dinner DS, Neme S, Nair D, Montgomery EB Jr, Baker KB, Rezai A, Lüders HO (2002) EEG and evoked potential recording from the subthalamic nucleus for deep brain stimulation of intractable epilepsy. Clin Neurophysiol 113(9):1391–1402
Dong L, Wang P, Peng R, Jiang S, Klugah-Brown B, Luo C, Yao D (2016) Altered basal ganglia-cortical functional connections in frontal lobe epilepsy: a resting-state fMRI study. Epilepsy Res 128:12–20
Feng L, Liu TT, Ye DW, Qiu Q, **ang HB, Cheung CW (2014) Stimulation of the dorsal portion of subthalamic nucleus may be a viable therapeutic approach in pharmacoresistant epilepsy: a virally mediated transsynaptic tracing study in transgenic mouse model. Epilepsy Behav 31:114–116
Gey L, Gernert M, Löscher W (2016) Continuous bilateral infusion of vigabatrin into the subthalamic nucleus: effects on seizure threshold and GABA metabolism in two rat models. Neurobiol Dis 91:194–208
Gibbs EL, Gibbs FA (1951) Electroencephalographic evidence of thalamic and hypothalamic epilepsy. Neurology 1:136–144
Graybiel AM (2000) The basal ganglia. Curr Biol 10(14):R509–R511
Guo H, Zhang H, Kuang Y, Wang C, **g X, Gu J, Gao G (2014) Electrical stimulation of the substantia nigra pars reticulata (SNr) suppresses chemically induced neocortical seizures in rats. J Mol Neurosci 53(4):546–552
Holgado AJN, Terry JR, Bogacz R (2010) Conditions for the generation of beta oscillations in the subthalamic nucleus-globus pallidus network. J Neurosci 30(37):12340–12352
Hu B, Wang Q (2015) Controlling absence seizures by deep brain stimulus applied on substantia nigra pars reticulata and cortex. Chaos Solitons Fractals 80:13–23
Hu B, Guo DQ, Wang QY (2015) Control of absence seizures induced by the pathways connected to SRN in corticothalamic system. Cogn Neurodyn 9(3):279–289
Hu B, Chen S, Chi HM, Chen J, Yuan P, Lai H, Dong W (2017) Controlling absence seizures by tuning activation level of the thalamus and striatum. Chaos Solitons Fractals 95:65–76
Kase D, Inoue T, Imoto K (2012) Roles of the subthalamic nucleus and subthalamic HCN channels in absence seizures. J Neurophysiol 107(1):393–406
Knowlton B (2015) Basal ganglia: habit formation. In: Jaeger D, Jung R (eds) Encyclopedia of Computational Neuroscience. Springer, New York, NY, pp 336–351
Kubu CS, Malone DA, Chelune G et al (2013) Neuropsychological outcome after deep brain stimulation in the ventral capsule/ventral striatum for highly refractory obsessive-compulsive disorder or major depression. Stereotact Funct Neurosurg 91(6):374–378
Kwon CS, Katnani H, Patel S, Abdel-Aziz S, Gale J, Eskandar E (2015) 199 temporally coordinated deep brain stimulation in the dorsal and ventral striatum synergistically enhances associative learning. Neurosurgery 62:232–233
Lee CY, Lim SN, Wu T, Lee ST (2017) Successful treatment of refractory status epilepticus using anterior thalamic nuclei deep brain stimulation. World Neurosurg 99:14–18
Lehtimäki K, Möttönen T, Järventausta K et al (2016) Outcome based definition of the anterior thalamic deep brain stimulation target in refractory epilepsy. Brain Stimul 9(2):268–275
Luo C, Li Q, **a Y et al (2012) Resting state basal ganglia network in idiopathic generalized epilepsy. Hum Brain Mapp 33(6):1279–1294
Lytton WW (2008) Computer modelling of epilepsy. Nat Rev Neurosci 9(8):626–637
Marten F, Rodrigues S, Benjamin O, Richardson MP, Terry JR (2009a) Onset of polyspike complexes in a mean-field model of human electroencephalography and its application to absence epilepsy. Philos Trans R Soc A 367(1891):1145–1161
Marten F, Rodrigues S, Suffczynski P, Richardson MP, Terry JR (2009b) Derivation and analysis of an ordinary differential equation mean-field model for studying clinically recorded epilepsy dynamics. Phys Rev E 79(2):021911
McNamara JO, Galloway MT, Rigsbee LC, Shin CHEOLSU (1984) Evidence implicating substantia nigra in regulation of kindled seizure threshold. J Neurosci 4(9):2410–2417
Morimoto K, Goddard GV (1987) The substantia nigra is an important site for the containment of seizure generalization in the kindling model of epilepsy. Epilepsia 28(1):1–10
Morrell MJ (2011) Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology 77(13):1295–1304
Möttönen T, Katisko J, Haapasalo J et al (2015) Defining the anterior nucleus of the thalamus (ANT) as a deep brain stimulation target in refractory epilepsy: delineation using 3 T MRI and intraoperative microelectrode recording. NeuroImage Clin 7:823–829
Moussawi K, Kalivas PW, Lee JW (2016) Abstinence from drug dependence after bilateral globus pallidus hypoxic-ischemic injury. Biol Psychiatry 80(9):e79–e80
Pavlides A, John Hogan S, Bogacz R (2012) Improved conditions for the generation of beta oscillations in the subthalamic nucleus-globus pallidus network. Eur J Neurosci 36(2):2229–2239
Pavlides A, Hogan SJ, Bogacz R (2015) Computational models describing possible mechanisms for generation of excessive beta oscillations in Parkinson’s disease. PLoS Comput Biol 11(12):e1004609
Paz JT, Chavez M, Saillet S, Deniau JM, Charpier S (2007) Activity of ventral medial thalamic neurons during absence seizures and modulation of cortical paroxysms by the nigrothalamic pathway. J Neurosci 27(4):929–941
Pinault D, Leresche N, Charpier S, Deniau JM, Marescaux C, Vergnes M, Crunelli V (1998) Intracellular recordings in thalamic neurones during spontaneous spike and wave discharges in rats with absence epilepsy. J Physiol 509(2):449–456
Polosan M, Chabardes S, Bougerol T, Ardouin C, Pollak P, Benabid AL, Krack P (2016) Long-term improvement in obsessions and compulsions with subthalamic stimulation. Neurology 87(17):1843–1844
Qiu MH, Vetrivelan R, Fuller PM, Lu J (2010) Basal ganglia control of sleep–wake behavior and cortical activation. Eur J Neurosci 31(3):499–507
Rajab A, Schuelke M, Gill E, Zwirner A, Seifert F, Gonzalez SM, Knierim E (2015) Recessive DEAF1 mutation associates with autism, intellectual disability, basal ganglia dysfunction and epilepsy. J Med Genet 52(9):607–611
Rektor I, Tomčk J, Mikl M et al (2013) Association between the basal ganglia and large-scale brain networks in epilepsy. Brain Topogr 26(2):355–362
Robinson PA, Rennie CJ, Rowe DL (2002) Dynamics of large-scale brain activity in normal arousal states and epileptic seizures. Phys Rev E 65(4):041924
Robinson PA, Rennie CJ, Rowe DL, O’connor SC (2004) Estimation of multiscale neurophysiologic parameters by electroencephalographic means. Hum Brain Mapp 23(1):53–72
Rodrigues S, Terry JR, Breakspear M (2006) On the genesis of spike-wave oscillations in a mean-field model of human thalamic and corticothalamic dynamics. Phys Lett A 355(4):352–357
Rodrigues S, Barton D, Szalai R, Benjamin O, Richardson MP, Terry JR (2009) Transitions to spike-wave oscillations and epileptic dynamics in a human cortico-thalamic mean-field model. J Comput Neurosci 27(3):507–526
Sabatino M, Gravante G, Ferraro G, Savatteri V, La Grutta V (1988) Inhibitory control by substantia nigra of generalized epilepsy in the cat. Epilepsy Res 2(6):380–386
Saunders A, Oldenburg IA, Berezovskii VK et al (2015) A direct GABAergic output from the basal ganglia to frontal cortex. Nature 521(7550):85
Shi LH, Luo F, Woodward D, Chang JY (2006) Deep brain stimulation of the substantia nigra pars reticulata exerts long lasting suppression of amygdala-kindled seizures. Brain Res 1090(1):202–207
Sprengers M, Vonck K, Carrette E, Marson AG, Boon P (2017) Deep brain and cortical stimulation for epilepsy. Cochrane Database Syst Rev 7:CD008497
Takeshita D, Sato YD, Bahar S (2007) Transitions between multistable states as a model of epileptic seizure dynamics. Phys Rev E 75(5):051925
Tepper JM, Abercrombie ED, Bolam JP (2007) Basal ganglia macrocircuits. Prog Brain Res 160:3–7
Timofeev I, Steriade M (2004) Neocortical seizures: initiation, development and cessation. Neurosci 123(2):299–336
van Albada SJ, Robinson PA (2009) Mean-field modeling of the basal ganglia-thalamocortical system. I: firing rates in healthy and Parkinsonian states. J Theor Biol 257(4):642–663
van Albada SJ, Gray RT, Drysdale PM, Robinson PA (2009) Mean-field modeling of the basal ganglia-thalamocortical system. II: dynamics of parkinsonian oscillations. J Theor Biol 257(4):664–688
Velazquez JLP, Erra RG, Rosenblum M (2015) The epileptic thalamocortical network is a macroscopic self-sustained oscillator: evidence from frequency-locking experiments in rat brains. Sci Rep 5:8423
Výtvarová E, Mareček R, Fousek J et al (2017) Large-scale cortico-subcortical functional networks in focal epilepsies: the role of the basal ganglia. NeuroImage Clin 14:28–36
Wilson MT, Sleigh JW, Steyn-Ross DA, Steyn-Ross ML (2006) General anesthetic-induced seizures can be explained by a mean-field model of cortical dynamics. J ASA 104(3):588–593
Yelnik J, Francis C, Percheron G, Tandéa D (1991) Morphological taxonomy of the neurons of the primate striatum. J Comp Neurol 313(2):273–294
Acknowledgements
This research was supported by the National key research and development program of China (No. 2017YFA0505500); the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB13040700); the National Science Foundation of China (Nos. 11602092, 31771476, 61602460); the Natural Science Foundation of Hubei Province (No. 2018CFB628); the China Postdoctoral Science Foundation (Nos. 2018M632184, 2016M600338); the National Undergraduate Training Program for Innovation and Entrepreneurship of Huazhong Agricultural University (Nos. 201710504092, 201810504104) and the Scientific and technological innovation fund for college students (SRF) of Huazhong Agricultural University (No. 2018323).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Unless otherwise noted, we used the following values for numerical simulations:
Parameter | Meaning of parameter | Unit | Values | References |
|---|---|---|---|---|
\(Q_{e}^{max},Q_{i}^{max}\) | The MFR of the cortex | Hz | 250 | Robinson et al. (2002), Marten et al. (2009a), Chen et al. (2014, 2015b), Breakspear et al. (2005) |
\(Q_{m}^{max}\) | The MFR of the MSN | Hz | 65 | van Albada and Robinson (2009), van Albada et al. (2009), Chen et al. (2014, 2015b) |
\(Q_{f}^{max}\) | The MFR of the FSI | Hz | 70 | van Albada and Robinson (2009); van Albada et al. (2009), Chen et al. (2014, 2015b) |
\(Q_{p_{1}}^{max}\) | The MFR of the SNr | Hz | 250 | van Albada and Robinson (2009); van Albada et al. (2009), Chen et al. (2014, 2015b) |
\(Q_{p_{2}}^{max}\) | The MFR of the GPe | Hz | 300 | van Albada and Robinson (2009); van Albada et al. (2009), Chen et al. (2014, 2015b) |
\(Q_{c}^{max}\) | The MFR of the SNc | Hz | 300 | Estimated |
\(Q_{s}^{max}\) | The MFR of the SRN | Hz | 250 | Robinson et al. (2002) , Marten et al. (2009a), Chen et al. (2014, 2015b), Breakspear et al. (2005) |
\(Q_{\zeta }^{max}\) | The MFR of the STN | Hz | 500 | van Albada and Robinson (2009); van Albada et al. (2009), Chen et al. (2014, 2015b) |
\(Q_{r}^{max}\) | The MFR of the TRN | Hz | 250 | Robinson et al. (2002), Marten et al. (2009a), Chen et al. (2014, 2015b), Breakspear et al. (2005) |
\(\theta _{e},\theta _{i}\) | The MTP of the cortex | mV | 15 | Robinson et al. (2002), Marten et al. (2009a), Chen et al. (2014, 2015b), Breakspear et al. (2005), Robinson et al. (2004) |
\(\theta _{m}\) | The MTP of the MSN | mV | 19 | van Albada and Robinson (2009); van Albada et al. (2009), Chen et al. (2014, 2015b) |
\(\theta _{f}\) | The MTP of the FSI | mV | 10 | van Albada and Robinson (2009); van Albada et al. (2009), Chen et al. (2014, 2015b) |
\(\theta _{p_{2}}\) | The MTP of the GPe | mV | 9 | van Albada and Robinson (2009); van Albada et al. (2009), Chen et al. (2014, 2015b) |
\(\theta _{p_{1}}\) | The MTP of the SNr | mV | 10 | van Albada and Robinson (2009); van Albada et al. (2009), Chen et al. (2014, 2015b) |
\(\theta _{\zeta }\) | The MTP of the STN | mV | 10 | van Albada and Robinson 2009; van Albada et al. 2009, Chen et al. (2014, 2015b) |
\(\theta _{r}\) | The MTP of the TRN | mV | 15 | Robinson et al. (2002), Marten et al. (2009a), Chen et al. (2014, 2015b), Breakspear et al. (2005), Robinson et al. (2004) |
\(\theta _{s}\) | The MTP of the SRN | mV | 15 | Robinson et al. (2002), Marten et al. (2009a), Chen et al. (2014, 2015b), Breakspear et al. (2005), Robinson et al. (2004) |
\(\theta _{c}\) | The MTP of the SNc | mV | 10 | Estimated |
\(\gamma _{e}\) | Cortical dam** rate | Hz | 100 | Robinson et al. (2002), Marten et al. (2009a), Chen et al. (2014, 2015b), Breakspear et al. (2005) |
\(\tau\) | The delay | ms | 55 | |
\(\alpha\) | Synaptodendritic decay time | \(s^{-1}\) | 50 | Robinson et al. (2002), Marten et al. (2009a), Chen et al. (2014, 2015b), Breakspear et al. (2005), Robinson et al. (2004) |
\(\sigma\) | Standard deviation of firing thresholds | mV | 6 | Robinson et al. (2002), Chen et al. (2014, 2015b), Breakspear et al. (2005), Robinson et al. (2004) |
\(\beta\) | Synaptodendritic rise time | \(s^{-1}\) | 200 | Robinson et al. (2002), Marten et al. (2009a), Chen et al. (2014, 2015b), Breakspear et al. (2005), Robinson et al. (2004) |
\(V_{s}\) | The sensory stimuli constant | mV s | 2.1 | Estimated |
Coupling strength | The output nuclei | The receiving nuclei | Values (mV s) | References |
|---|---|---|---|---|
\(\nu _{ei}\) | IIN | EPN | − 1.6 | Marten et al. (2009a), Chen et al. (2014, 2015b), Breakspear et al. (2005) |
\(\nu _{ee}\) | EPN | EPN | 1.1 | Marten et al. (2009a), Chen et al. (2014, 2015b), Breakspear et al. (2005) |
\(\nu _{rs}\) | SRN | TRN | 0.51 | Robinson et al. (2002), Marten et al. (2009a), Chen et al. (2014, 2015b) |
\(\nu _{re}\) | EPN | TRN | 0.052 | Robinson et al. (2002), Marten et al. (2009a), Chen et al. (2014, 2015b) |
\(\nu _{sr}^{A,B}\) | TRN | SRN | − 2.3 | Robinson et al. (2002), Chen et al. (2014, 2015b), Robinson et al. (2004) |
\(\nu _{me}\) | EPN | MSN | 1.1 | van Albada and Robinson (2009), van Albada et al. (2009), Chen et al. (2014, 2015b) |
\(\nu _{mm}\) | MSN | MSN | − 0.22 | van Albada and Robinson (2009), van Albada et al. (2009), Chen et al. (2014, 2015b) |
\(\nu _{ms}\) | SRN | MSN | 0.12 | van Albada and Robinson (2009), van Albada et al. (2009), Chen et al. (2014, 2015b) |
\(\nu _{fe}\) | EPN | FSI | 0.72 | van Albada and Robinson (2009), van Albada et al. (2009), Chen et al. (2014, 2015b) |
\(\nu _{fc}\) | SNc | FSI | 0.35 | Estimated |
\(\nu _{mc}\) | SNc | MSN | 0.35 | Estimated |
\(\nu _{p_{1}m}\) | MSN | SNr | − 0.08 | van Albada and Robinson (2009), van Albada et al. (2009), Chen et al. (2014, 2015b) |
\(\nu _{p_{1}p_{2}}\) | GPe | SNr | − 0.031 | van Albada and Robinson (2009), van Albada et al. (2009), Chen et al. (2014, 2015b) |
\(\nu _{p_{1}\zeta }\) | STN | SNr | 0.32 | van Albada and Robinson (2009), van Albada et al. (2009), Chen et al. (2014, 2015b) |
\(\nu _{p_{2}m}\) | MSN | GPe | − 0.31 | van Albada and Robinson (2009), van Albada et al. (2009), Chen et al. (2014, 2015b) |
\(\nu _{p_{2}p_{2}}\) | GPe | GPe | − 0.07 | van Albada and Robinson (2009), van Albada et al. (2009), Chen et al. (2014, 2015b) |
\(\nu _{p_{1}p_{1}}\) | SNr | SNr | − 0.006 | van Albada and Robinson (2009), van Albada et al. (2009), Chen et al. (2014, 2015b) |
\(\nu _{p_{2}\zeta }\) | STN | GPe | 0.47 | van Albada and Robinson (2009), van Albada et al. (2009), Chen et al. (2014, 2015b) |
\(\nu _{\zeta p_{2}}\) | GPe | STN | − 0.042 | van Albada and Robinson (2009), van Albada et al. (2009), Chen et al. (2014, 2015b) |
\(\nu _{es}\) | SRN | EPN | 1.9 | Robinson et al. (2002), Marten et al. (2009a), Chen et al. (2014, 2015b) |
\(\nu _{se}\) | EPN | SRN | 2.1 | |
\(\nu _{sp_{1}}\) | SNr | SRN | − 0.03 | van Albada and Robinson (2009), van Albada et al. (2009), Chen et al. (2014, 2015b) |
\(\nu _{\zeta e}\) | EPN | STN | 0.15 | van Albada and Robinson (2009) van Albada et al. (2009), Chen et al. (2014, 2015b) |
\(\nu _{rp_{1}}\) | SNr | TRN | − 0.03 | |
\(\nu _{ep_{2}}\) | GPe | EPN | − 0.04 | Chen et al. (2015b) |
\(\nu _{ep_{1}}\) | SNr | EPN | − 0.04 | Estimated |
\(\nu _{mf}\) | FSI | MSN | − 0.3 | van Albada and Robinson (2009), van Albada et al. (2009), Chen et al. (2014, 2015b) |
Rights and permissions
About this article
Cite this article
Hu, B., Wang, D., **a, Z. et al. Regulation and control roles of the basal ganglia in the development of absence epileptiform activities. Cogn Neurodyn 14, 137–154 (2020). https://doi.org/10.1007/s11571-019-09559-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11571-019-09559-4