Abstract
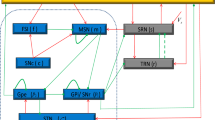
The cerebral cortex, thalamus and basal ganglia together form an important network in the brain, which is closely related to several nerve diseases, such as parkinson disease, epilepsy seizure and so on. Absence seizure can be characterized by 2–4 Hz oscillatory activity, and it can be induced by abnormal interactions between the cerebral cortex and thalamus. Many experimental results have also shown that basal ganglia are a key neural structure, which closely links the corticothalamic system in the brain. Presently, we use a corticothalamic-basal ganglia model to study which pathways in corticothalamic system can induce absence seizures and how these oscillatory activities can be controlled by projections from the substantia nigra pars reticulata (SNr) to the thalamic reticular nucleus (TRN) or the specific relay nuclei (SRN) of the thalamus. By tuning the projection strength of the pathway “Excitatory pyramidal cortex-SRN”, ”SRN-Excitatory pyramidal cortex” and “SRN–TRN” respectively, different firing states including absence seizures can appear. This indicates that absence seizures can be induced by tuning the connection strength of the considered pathway. In addition, typical absence epilepsy seizure state “spike-and-slow wave discharges” can be controlled by adjusting the activation level of the SNr as the pathways SNr–SRN and SNr–TRN open independently or together. Our results emphasize the importance of basal ganglia in controlling absence seizures in the corticothalamic system, and can provide a potential idea for the clinical treatment.










Similar content being viewed by others
References
Biraben A, Semah F et al (2004) PET evidence for a role of the basal ganglia in patients with ring chromosome 20 epilepsy. Neurology 63:73–77
Breakspear M, Roberts JA et al (2006) A unifying explanation of primary generalized seizures through nonlinear brain modeling and bifurcation analysis. Cereb Cortex 16:1296–1313
Chen MM, Guo DQ et al (2014) Bidirectional control of absence seizures by the Basal Ganglia: a computational evidence. PLoS Comput Biol 10(3):e1003495
Coenen AM, van Luijtelaar EL (2003) Genetic animal models for absence epilepsy: a review of the WAG/Rij strain of rats. Behav Genet 33:635–655
Crunelli V, Leresche N (2002) Childhood absence epilepsy: genes, channels, neurons and networks. Nat Rev Neurosci 3:371–382
Deransart C, Depaulis A (2002) The control of seizures by the basal ganglia? A review of experimental data. Epileptic Disord Suppl 3:S61–72
Deransart C, Vercueil L et al (1998) The role of basal ganglia in the control of generalized absence seizures. Epilepsy Res 32:213–223
Gatev P, Wichmann T (2008) Interactions between cortical rhythms and spiking activity of single basal ganglia neurons in the normal and parkinsonian state. Cereb Cortex 19(6):1330–1344
Groenewegen HJ (2003) The basal ganglia and motor control. Neural Plast 10:107–120
Gulcebi MI, Ketenci S et al (2012) Topographical connections of the substantia nigra pars reticulata to higher-order thalamic nuclei in the rat. Brain Res Bull 87:312–318
Humphries MD, Gurney K (2012) Network effects of subthalamic deep brain stimulation drive a unique mixture of responses in basal ganglia output. Eur J Neurosci 36:2240–2251
Jasper HH, Kershman J (1941) Electroencephalographic classification of the epilepsies. Arch Neurol Psychiatry 45:903–943
Kase D, Inoue T et al (2012) Roles of the subthalamic nucleus and subthalamic HCN channels in absence seizures. J Neurophysiol 107:393–406
Marten F, Rodrigues S et al (2009) Onset of polyspike complexes in a mean-field model of human electroencephalography and its application to absence epilepsy. Phil Trans R Soc A 367:1145–1161
Massimo A (2012) A brief history on the oscillating roles of thalamus and cortex in absence seizures. Epilepsia 53(5):779–789
Park C, Rubchinsky LL (2012) Potential Mechanisms for Imperfect Synchronization in Parkinsonian basal Ggnglia. PLOS ONE 7(12):e51530
Paz JT, Bryant AS et al (2011) A new mode of corticothalamic transmission revealed in the \(Gria4^{-/-}\) model of absence epilepsy. Nat Neurosci 14(9):1167–1173
Paz JT, Chavez M et al (2007) Activity of ventral medial thalamic neurons during absence seizures and modulation of cortical paroxysms by the nigrothalamic pathway. J Neurosci 27:929–941
Paz JT, Deniau JM et al (2005) Rhythmic bursting in the cortico-subthalamo-pallidal network during spontaneous genetically determined spike and wave discharges. J Neurosci 25(8):2092–2101
Roberts JA, Robinson PA (2008) Modeling absence seizure dynamics: implications for basic mechanisms and measurement of thalamocortical and corticothalamic latencies. J Theor Biol 253:189–201
Robinson PA, Rennie CJ et al (1998) Steady states and global dynamics of electrical activity in the cerebral cortex. Phys Rev E 58:3557–3571
Robinson PA, Rennie CJ et al (2001) Prediction of electroencephalographic spectra from neurophysiology. Phys Rev E 63:021903
Robinson PA, Rennie CJ et al (2002) Dynamics of large-scale brain activity in normal arousal states and epileptic seizures. Phys Rev E 65:041924
Robinson PA, Rennie CJ et al (2003) Estimation of multiscale neurophysiologic parameters by electroencephalographic means. Hum Brain Mapp 23:53–72
Rodrigues S, Barton D et al (2009) Transitions to spike-wave oscillations and epileptic dynamics in a human cortico-thalamic mean-field model. J Comput Neurosci 27(3):507–526
Timofeev I, Steriade M (2004) Neocortical seizures: initiation, development and cessation. Neuroscience 123:299–336
van Albada SJ, Gray RT et al (2009) Mean-field modeling of the basal ganglia-thalamocortical system. II: dynamics of parkinsonian oscillations. J Theor Biol 257:664–688
van Albada SJ, Robinson PA (2009) Mean-field modeling of the basal ganglia-thalamocortical system. I: firing rates in healthy and parkinsonian states. J Theor Biol 257:642–663
van Luijtelaar G, Sitnikova E (2006) Global and focal aspects of absence epilepsy: the contribution of genetic models. Neurosci Biobehav Rev 30:983–1003
Volman V, Perc M, Bazhenov M (2011) Gap junctions and epileptic seizures: two sides of the same coin? PLoS One 6(5):e20572
Acknowledgments
This research was supported by the National Science Foundation of China (Grant Nos. 11325208, 11172017 and 61201278).
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Unless otherwise noted, we use these parameter values for simulations as follows (Chen et al. 2014; Marten et al. 2009; Massimo 2012; Paz et al. 2007; Roberts and Robinson 2008; Rodrigues et al. 2009; van Albada et al. 2009; van Albada and Robinson 2009).
Parameter | Mean | Value |
|---|---|---|
\(Q_{e}^{max},Q_{i}^{max}\) | Cortical maximum firing rate | 250 Hz |
\(Q_{d_{1}}^{max},Q_{d_{2}}^{max}\) | Striatum maximum firing rate | 65 Hz |
\(Q_{p_{1}}^{max}\) | SNr maximum firing rate | 250 Hz |
\(Q_{p_{2}}^{max}\) | GPe maximum firing rate | 300 Hz |
\(Q_{\zeta }^{max}\) | STN maximum firing rate | 500 Hz |
\(Q_{s}^{max}\) | SRN maximum firing rate | 250 Hz |
\(Q_{r}^{max}\) | TRN maximum firing rate | 250 Hz |
\(\theta _{e},\theta _{i}\) | Mean firing threshold of cortical populations | 15 mV |
\(\theta _{d_{1}},\theta _{d_{2}}\) | Mean firing threshold of striatum | 19 mV |
\(\theta _{p_{1}}\) | Mean firing threshold of SNr | 10 mV |
\(\theta _{p_{2}}\) | Mean firing threshold of GPe | 9 mV |
\(\theta _{\zeta }\) | Mean firing threshold of STN | 10 mV |
\(\theta _{s}\) | Mean firing threshold of SRN | 15 mV |
\(\theta _{r}\) | Mean firing threshold of TRN | 15 mV |
\(\gamma _{e}\) | Cortical dam** rate | 100 Hz |
\(\tau\) | Time delay due to slow synaptic kinetics of \(GABA_{B}\) | 50 ms |
\(\alpha\) | Synaptodendritic decay time constant | 50 \({\rm s}^{-1}\) |
\(\beta\) | Synaptodendritic rise time constant | 200 \({\rm s}^{-1}\) |
\(\sigma\) | Threshold variability of firing rate | 6 mV |
\(\phi _{n}\) | Nonspecific subthalamic input onto SRN | 2 mV s |
Coupling strength | Source | Target | Value (mV s) |
|---|---|---|---|
\(\nu _{ee}\) | Excitatory pyramidal neurons | Excitatory pyramidal neurons | 1 |
\(\nu _{ei}\) | Inhibitory interneurons | Excitatory pyramidal neurons | −1.8 |
\(\nu _{re}\) | Excitatory pyramidal neurons | TRN | 0.05 |
\(\nu _{rs}\) | SRN | TRN | 0.5 |
\(\nu _{sr}^{A,B}\) | TRN | SRN | −0.48 |
\(\nu _{d_{1}e}\) | Excitatory pyramidal neurons | Striatal D1 neurons | 1 |
\(\nu _{d_{1}d_{1}}\) | Striatal D1 neurons | Striatal D1 neurons | −0.2 |
\(\nu _{d_{1}s}\) | SRN | Striatal D1 neurons | 0.1 |
\(\nu _{d_{2}e}\) | Excitatory pyramidal neurons | Striatal D2 neurons | 0.7 |
\(\nu _{d_{2}d_{2}}\) | Striatal D2 neurons | Striatal D2 neurons | −0.3 |
\(\nu _{d_{2}s}\) | SRN | Striatal D2 neurons | 0.05 |
\(\nu _{p_{1}d_{1}}\) | Striatal D1 neurons | SNr | −0.1 |
\(\nu _{p_{1}p_{2}}\) | GPe | SNr | −0.03 |
\(\nu _{p_{1}\zeta }\) | STN | SNr | 0–0.6 |
\(\nu _{p_{2}d_{2}}\) | Striatal D2 neurons | GPe | −0.3 |
\(\nu _{p_{2}p_{2}}\) | GPe | GPe | −0.075 |
\(\nu _{p_{2}\zeta }\) | STN | GPe | 0.45 |
\(\nu _{\zeta p_{2}}\) | GPe | STN | −0.04 |
\(\nu _{es}\) | STN | Excitatory pyramidal neurons | 1.8 |
\(\nu _{se}\) | Excitatory pyramidal neurons | SRN | 2.2 |
\(\nu _{\zeta e}\) | Excitatory pyramidal neurons | STN | 0.1 |
\(\nu _{sp_{1}}\) | SNr | SRN | −0.035 |
\(\nu _{rp_{1}}\) | SNr | TRN | −0.035 |
Rights and permissions
About this article
Cite this article
Hu, B., Guo, D. & Wang, Q. Control of absence seizures induced by the pathways connected to SRN in corticothalamic system. Cogn Neurodyn 9, 279–289 (2015). https://doi.org/10.1007/s11571-014-9321-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11571-014-9321-1