Abstract
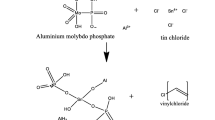
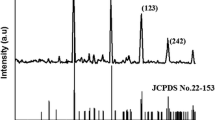
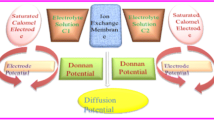
Polyvinyl chloride (PVC)-derived cobalt molybdophosphate (CMP) nanocomposite material was synthesized by sol-gel technique. The physico-chemical properties such as composition, functional groups, thermal stability, phase transition, and surface structure were characterized using TGA, FT-IR, SEM, and TEM analyses. Membranes from the nanocomposite material were prepared to elucidate the electrochemical properties. The ionic potential was measured using strong electrolytes such as KCl, NaCl, and LiCl. Electrolytes have the following cation-sequenced with observed membrane potentials Li+ > Na+ > K+. The estimated membrane charge density was calculated for membrane performance. Membrane characteristics, such as transport number, mobility ratio, and charge efficiency, were determined using the Teorell–Meyer–Sievers (TMS) theory framework. LiCl has the highest value of t+ and \(\overline{\omega}\). The nanocomposite membrane is lithium cation-selective, which can, therefore, be effectively utilized in various electro-membrane processes such as lithium-ion extraction and removal.














Similar content being viewed by others
Data availability
The data supporting this study’s findings are available from the corresponding author upon reasonable request.
References
Zhang R, Liu Y, He M et al (2016) Antifouling membranes for sustainable water purification: strategies and mechanisms. Chem Soc Rev 45:5888–5924. https://doi.org/10.1039/C5CS00579E
Shannon MA, Bohn PW, Elimelech M et al (2008) Science and technology for water purification in the coming decades. Nature 452(7185):301–310. https://doi.org/10.1038/nature06599
Kim S, Nam S-N, Jang A et al (2022) Review of adsorption–membrane hybrid systems for water and wastewater treatment. Chemosphere 286:131916. https://doi.org/10.1016/j.chemosphere.2021.131916
Shahzaib A, Shaily AI et al (2023) Ultrarapid and highly efficient reduction of nitroaromatic compounds using cyclodextrin MOF. Catal Commun 174:106569. https://doi.org/10.1016/j.catcom.2022.106569
Shahzaib A, Shaily AI et al (2023) One pot synthesis of cyclodextrin MOF as a promising heterogeneous catalyst for the reduction of nitroaromatic compounds and azo dyes. Res Chem Intermed. https://doi.org/10.1007/s11164-023-04986-9
Rivera-Utrilla J, Sánchez-Polo M, Gómez-Serrano V et al (2011) Activated carbon modifications to enhance its water treatment applications. . An overview. J Hazard Mater 187:1–23. https://doi.org/10.1016/j.jhazmat.2011.01.033
Ju X-J, Zhang S-B, Zhou M-Y et al (2009) Novel heavy-metal adsorption material: ion-recognition P(NIPAM-co-BCAm) hydrogels for removal of lead(II) ions. J Hazard Mater 167:114–118. https://doi.org/10.1016/j.jhazmat.2008.12.089
Meena AK, Mishra GK, Rai PK et al (2005) Removal of heavy metal ions from aqueous solutions using carbon aerogel as an adsorbent. J Hazard Mater 122:161–170. https://doi.org/10.1016/j.jhazmat.2005.03.024
Boivin MJ, Giordani B (1995) A risk evaluation of the neuropsychological effects of childhood lead toxicity. Dev Neuropsychol 11:157–180. https://doi.org/10.1080/87565649509540611
Blöcher C, Dorda J, Mavrov V et al (2003) Hybrid flotation—membrane filtration process for the removal of heavy metal ions from wastewater. Water Res 37:4018–4026. https://doi.org/10.1016/S0043-1354(03)00314-2
Werber JR, Osuji CO, Elimelech M (2016) Materials for next-generation desalination and water purification membranes. Nat Rev Mater 1:16018. https://doi.org/10.1038/natrevmats.2016.18
Elimelech M, Phillip WA (2011) The future of seawater desalination: energy, technology, and the environment. Science 333:712–717. https://doi.org/10.1126/science.1200488
Tang Y, Cai Z, Sun X et al (2022) Electrospun nanofiber-based membranes for water treatment. Polymers (Basel) 14:2004. https://doi.org/10.3390/polym14102004
Fernandez-Gonzalez C, Dominguez-Ramos A, Ibañez R, Irabien A (2019) Desalination by renewable energy-powered electrodialysis processes. In: Current Trends and Future Developments on (Bio-) Membranes. Elsevier, pp 111–131
Semiat R (2008) Energy issues in desalination processes. Environ Sci Technol 42:8193–8201. https://doi.org/10.1021/es801330u
Baker RW (2012) Membrane technology and applications. Wiley
Gin DL, Noble RD (2011) Designing the next generation of chemical separation membranes. Science 332:674–676. https://doi.org/10.1126/science.1203771
Larbot A, Fabre JP, Guizard C, Cot L (1988) Inorganic membranes obtained by sol-gel techniques. J Memb Sci 39:203–212. https://doi.org/10.1016/S0376-7388(00)80929-1
Rodriguez JA, Hanson JC, Chaturvedi S et al (2000) Studies on the behavior of mixed-metal oxides: structural, electronic, and chemical properties of β-FeMoO 4. J Phys Chem B 104:8145–8152. https://doi.org/10.1021/jp001652c
Cao B, Veith GM, Diaz RE et al (2013) Cobalt molybdenum oxynitrides: synthesis, structural characterization, and catalytic activity for the oxygen reduction reaction. Angew Chemie Int Ed 52:10753–10757. https://doi.org/10.1002/anie.201303197
Rodriguez JA, Chaturvedi S, Hanson JC, Brito JL (1999) Reaction of H 2 and H 2 S with CoMoO 4 and NiMoO 4 : TPR, XANES, time-resolved XRD, and molecular-orbital studies. J Phys Chem B 103:770–781. https://doi.org/10.1021/jp983115m
Ertl G, Knözinger H, Weitkamp J (1997) Handbook of heterogeneous catalysis. Wiley
Haber J (1974) Cobalt and other transition metal molybdate catalysts. J Less Common Met 36:277–287. https://doi.org/10.1016/0022-5088(74)90112-X
Allulli S, Ferragina C, La GA et al (1977) Ion-exchange mechanism of some bivalent transition-metal ions on half-converted sodium and lithium forms of crystalline zirconium phosphate. J Chem Soc Dalt Trans 1879. https://doi.org/10.1039/dt9770001879
Imteyaz S, Rafiuddin R (2015) Effects of monovalent ions on membrane potential and permselectivity: evaluation of fixed charge density of polymer based zirconium aluminophosphate nanocomposite membrane. RSC Adv 5:96008–96018. https://doi.org/10.1039/C5RA17193H
Nabi SA, Akhtar A, Khan MDA, Khan MA (2014) Synthesis, characterization and electrical conductivity of Polyaniline-Sn(IV)tungstophosphate hybrid cation exchanger: analytical application for removal of heavy metal ions from wastewater. Desalination 340:73–83. https://doi.org/10.1016/j.desal.2014.02.020
Shim J, Ha HY, Hong S-A, Oh I-H (2002) Characteristics of the Nafion ionomer-impregnated nanocomposite membrane for polymer electrolyte fuel cells. J Power Sources 109:412–417. https://doi.org/10.1016/S0378-7753(02)00106-4
Nabi SA, Naushad M (2008) Synthesis, characterization and analytical applications of a new nanocomposite cation exchanger cellulose acetate-Zr(IV) molybdophosphate. Colloids Surf Physicochem Eng Asp 316:217–225. https://doi.org/10.1016/j.colsurfa.2007.09.005
Kim YS, Wang F, Hickner M et al (2003) Fabrication and characterization of heteropolyacid (H3PW12O40)/directly polymerized sulfonated poly(arylene ether sulfone) copolymer nanocomposite membranes for higher temperature fuel cell applications. J Memb Sci 212:263–282. https://doi.org/10.1016/S0376-7388(02)00507-0
Tchicaya-Bouckary L, Jones DJ, Rozière J (2002) Hybrid polyaryletherketone membranes for fuel cell applications. Fuel Cells 2:40–45. https://doi.org/10.1002/1615-6854(20020815)2:1<40::AID-FUCE40>3.0.CO;2-U
Blöcher C, Noronha M, Fünfrocken L et al (2002) Recycling of spent process water in the food industry by an integrated process of biological treatment and membrane separation. Desalination 144:143–150. https://doi.org/10.1016/S0011-9164(02)00303-X
Côté P, Mourato D, Güngerich C et al (1998) Immersed membrane filtration for the production of drinking water: case studies. Desalination 117:181–188. https://doi.org/10.1016/S0011-9164(98)00090-3
Bowen WR, Mohammad AW, Hilal N (1997) Characterisation of nanofiltration membranes for predictive purposes — use of salts, uncharged solutes and atomic force microscopy. J Memb Sci 126:91–105. https://doi.org/10.1016/S0376-7388(96)00276-1
Bowen WR, Welfoot JS (2002) Predictive modelling of nanofiltration: membrane specification and process optimisation. Desalination 147:197–203. https://doi.org/10.1016/S0011-9164(02)00534-9
Navarro R, González MP, Saucedo I et al (2008) Effect of an acidic treatment on the chemical and charge properties of a nanofiltration membrane. J Memb Sci 307:136–148. https://doi.org/10.1016/j.memsci.2007.09.015
Szymczyk A, Fievet P, Mullet M et al (1998) Comparison of two electrokinetic methods – electroosmosis and streaming potential – to determine the zeta-potential of plane ceramic membranes. J Memb Sci 143:189–195. https://doi.org/10.1016/S0376-7388(97)00340-2
Takagi R, Nakagaki M (1990) Theoretical study of the effect of ion adsorption on membrane potential and its application to collodion membranes. J Memb Sci 53:19–35. https://doi.org/10.1016/0376-7388(90)80003-5
Levenstein R (1996) Utilization of the Donnan effect for improving electrolyte separation with nanofiltration membranes. J Memb Sci 116:77–92. https://doi.org/10.1016/0376-7388(96)00029-4
Bowen WR, Welfoot JS (2002) Modelling of membrane nanofiltration—pore size distribution effects. Chem Eng Sci 57:1393–1407. https://doi.org/10.1016/S0009-2509(01)00412-2
Mohammad AW, Hilal N, Al-Zoubib H et al (2007) Modelling the effects of nanofiltration membrane properties on system cost assessment for desalination applications. Desalination 206:215–225. https://doi.org/10.1016/j.desal.2006.02.068
Barragán VM, Pérez-Haro MJ (2011) Correlations between water uptake and effective fixed charge concentration at high univalent electrolyte concentrations in sulfonated polymer cation-exchange membranes with different morphology. Electrochim Acta 56:8630–8637. https://doi.org/10.1016/j.electacta.2011.07.060
Westermann-Clark GB, Christoforou CC (1986) The exclusion-diffusion potential in charged porous membranes. J Electroanal Chem Interfacial Electrochem 198:213–231. https://doi.org/10.1016/0022-0728(86)90001-X
Hickner MA, Ghassemi H, Kim YS et al (2004) Alternative polymer systems for proton exchange membranes (PEMs). Chem Rev 104:4587–4612. https://doi.org/10.1021/cr020711a
Chung T-S, Jiang LY, Li Y, Kulprathipanja S (2007) Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation. Prog Polym Sci 32:483–507. https://doi.org/10.1016/j.progpolymsci.2007.01.008
Tasaka M, Aoki N, Kondo Y, Nagasawa M (1975) Membrane potentials and electrolyte permeation velocities in charged membranes. J Phys Chem 79:1307–1314. https://doi.org/10.1021/j100580a017
Kamo N, Oikawa M, Kobatake Y (1973) Effective fixed charge density governing membrane phenomena. V. Reduced expression of permselectivity. J Phys Chem 77:92–95. https://doi.org/10.1021/j100620a020
Nagasawa M, Kobatake Y (1952) The theory of membrane potential. J Phys Chem 56:1017–1024. https://doi.org/10.1021/j150500a024
Kobatake Y (1958) Irreversible electrochemical processes of membranes. J Chem Phys 28:146–153. https://doi.org/10.1063/1.1744057
Hou J-F, Gao J-F, Kong L-B (2020) Boosting the performance of cobalt molybdate nanorods by introducing nanoflake-like cobalt boride to form a heterostructure for aqueous hybrid supercapacitors. J Colloid Interface Sci 565:388–399. https://doi.org/10.1016/j.jcis.2020.01.040
** X, Li J, Xue P, Jia M (2014) Preparation and characterization of PVC-based form-stable phase change materials. Sol Energy Mater Sol Cells 130:435–441. https://doi.org/10.1016/j.solmat.2014.07.013
Janardhan E, Reddy MM, Reddy PV, Reddy MJ (2018) Synthesis of SnO nanoparticles—a hydrothermal approach. World J Nano Sci Eng 08:33–37. https://doi.org/10.4236/wjnse.2018.82002
Sharma S, Kothiyal NC, Rathore BS, Sharma S (2015) Use of cellulose acetate–tin (lV) phosphate nanocomposite (CA/TPC) in highly effective removal and recovery of heavy metal ions. Int J Ind Chem 6:43–58. https://doi.org/10.1007/s40090-015-0031-2
Siddiqui WA, Khan SA (2007) Synthesis, characterization and ion exchange properties of zirconium(IV) tungstoiodophosphate, a new cation exchanger. Bull Mater Sci 30:43–49. https://doi.org/10.1007/s12034-007-0008-7
Semagne B, Diaz I, Kebede T, Taddesse AM (2016) Synthesis, characterization and analytical application of polyaniline tin(IV) molybdophosphate nanocomposite with nanocrystalline domains. React Funct Polym 98:17–23. https://doi.org/10.1016/j.reactfunctpolym.2015.11.004
Wan Y, Zhao (2007) On the controllable soft-templating approach to mesoporous silicates. Chem Rev 107:2821–2860. https://doi.org/10.1021/cr068020s
Li J, Zhang L, Yang P, Cheng X (2018) Morphological evolution of Co phosphate and its electrochemical and photocatalytic performance. CrystEngComm 20:6982–6988. https://doi.org/10.1039/C8CE01535J
Wang Y, Wang Y, Jiang R, Xu R (2012) Cobalt phosphate–ZnO nanocomposite photocatalysts for oxygen evolution from photocatalytic water oxidation. Ind Eng Chem Res 51:9945–9951. https://doi.org/10.1021/ie2027469
Liu R, Shi Y, Wan Y et al (2006) Triconstituent co-assembly to ordered mesostructured polymer−silica and carbon−silica nanonanocomposites and large-pore mesoporous carbons with high surface areas. J Am Chem Soc 128:11652–11662. https://doi.org/10.1021/ja0633518
Sen T, Tiddy GJT, Casci JL, Anderson MW (2004) Synthesis and characterization of hierarchically ordered porous silica materials. Chem Mater 16:2044–2054. https://doi.org/10.1021/cm034946u
Khan A, Khan A (2007) Preparation and characterization of a new organic–inorganic nano-nanocomposite poly-o-toluidine Th(IV) phosphate: its analytical applications as cation-exchanger and in making ion-selective electrode. Talanta 72:699–710. https://doi.org/10.1016/j.talanta.2006.11.044
Titiloye JO, Hussain I (2008) Synthesis and characterization of silicalite-1/carbon-graphite membranes. J Colloid Interface Sci 318:50–58. https://doi.org/10.1016/j.jcis.2007.10.025
Resina M, Macanás J, de Gyves J, Muñoz M (2007) Development and characterization of hybrid membranes based on an organic matrix modified with silanes for metal separation. J Memb Sci 289:150–158. https://doi.org/10.1016/j.memsci.2006.11.049
Imteyaz S (2016) Transport studies of ions across polystyrene based nanocomposite membrane: evaluation of fixed charge density using theoretical models. J Mol Struct 1123:116–123. https://doi.org/10.1016/j.molstruc.2016.06.020
Teorell T (1935) Studies on the “diffusion effect” upon ionic distribution. Some theoretical considerations. Proc Natl Acad Sci 21:152–161. https://doi.org/10.1073/pnas.21.3.152
Hall MS, Starov VM, Lloyd DR (1997) Reverse osmosis of multicomponent electrolyte solutions Part I. Theoretical development. J Memb Sci 128:23–37. https://doi.org/10.1016/S0376-7388(96)00300-6
Beg M, Matin M (2002) Studies with nickel phosphate membranes: evaluation of charge density and test of recently developed theory of membrane potential. J Memb Sci 196:95–102. https://doi.org/10.1016/S0376-7388(01)00582-8
Arfin T (2009) Transport studies of nickel arsenate membrane. J Electroanal Chem 636:113–122. https://doi.org/10.1016/j.jelechem.2009.09.019
Imteyaz S (2016) Synthesis of phosphonated poly(vinyl alcohol)-based nanocomposite membrane: effects of counter and co-ions on its electrochemical properties for separation applications. Ind Eng Chem Res 55:12655–12666. https://doi.org/10.1021/acs.iecr.6b03387
Teixeira M, Rosa M, Nystrom M (2005) The role of membrane charge on nanofiltration performance. J Memb Sci 265:160–166. https://doi.org/10.1016/j.memsci.2005.04.046
Afonso MD, de Pinho MN (2000) Transport of MgSO4, MgCl2, and Na2SO4 across an amphoteric nanofiltration membrane. J Memb Sci 179:137–154. https://doi.org/10.1016/S0376-7388(00)00495-6
Peeters JMM, Mulder MHV, Strathmann H (1999) Streaming potential measurements as a characterization method for nanofiltration membranes. Colloids Surfaces A Physicochem Eng Asp 150:247–259. https://doi.org/10.1016/S0927-7757(98)00828-0
Klaysom C, Moon S-H, Ladewig BP et al (2011) The influence of inorganic filler nanoparticle size on nanocomposite ion-exchange membranes for desalination. J Phys Chem C 115:15124–15132. https://doi.org/10.1021/jp112157z
Shahi VK, Thampy SK, Rangarajan R (1999) Studies on transport properties of surfactant immobilized anion-exchange membrane. J Memb Sci 158:77–83. https://doi.org/10.1016/S0376-7388(99)00029-0
Benavente J, Cañas A (1999) Transport of NaNO3 solutions across an activated nanocomposite membrane: electrochemical and chemical surface characterizations. J Memb Sci 156:241–250. https://doi.org/10.1016/S0376-7388(98)00348-2
Mauro A (1962) Space charge regions in fixed charge membranes and the associated property of capacitance. Biophys J 2:179–198. https://doi.org/10.1016/S0006-3495(62)86848-9
Sasidhar V, Ruckenstein E (1982) Anomalous effects during electrolyte osmosis across charged porous membranes. J Colloid Interface Sci 85:332–362. https://doi.org/10.1016/0021-9797(82)90003-0
Shang W-J, Wang X-L, Yu Y-X (2006) Theoretical calculation on the membrane potential of charged porous membranes in 1-1, 1-2, 2-1 and 2-2 electrolyte solutions. J Memb Sci 285:362–375. https://doi.org/10.1016/j.memsci.2006.09.005
Chou T-J, Tanioka A (1998) Membrane potential across charged membranes in organic solutions. J Phys Chem B 102:7198–7202. https://doi.org/10.1021/jp981673v
Tanioka A, Matsumoto H, Yamamoto R (2004) Charge effectiveness of sulfonated polymer membranes under low-water-content condition. Sci Technol Adv Mater 5:461–468. https://doi.org/10.1016/j.stam.2004.01.014
Geise GM, Cassady HJ, Paul DR et al (2014) Specific ion effects on membrane potential and the permselectivity of ion exchange membranes. Phys Chem Chem Phys 16:21673–21681. https://doi.org/10.1039/C4CP03076A
Acknowledgements
We are very grateful to the Chairman, Department of Chemistry of Aligarh Muslim University, Aligarh, for providing research facilities. We appreciate the Departmental Instrumentation facilities to support XRD and FT-IR. We also appreciate the SEM and TEM facilities at the University Sophisticated Instrumentations Facility (USIF), AMU. In addition, we acknowledge the University Grants Commission (UGC), New Delhi, for financial support.
Author information
Authors and Affiliations
Contributions
Nisar Ahmad: study design, conceptualization, experimental design, data analysis, writing – review and manuscript editing. Rafiuddin: supervision, visualization, formal analysis.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahmad, N., Rafiuddin Polyvinyl chloride grounded cobalt molybdophosphate nanocomposite membrane: comparative study of fixed charge density. J Nanopart Res 25, 220 (2023). https://doi.org/10.1007/s11051-023-05869-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-023-05869-1