Abstract
Water treatment applications are in high demand recently. In this work, the titania nanotube (TNT) was successfully grown onto the outer surface of the carbon nanotubes (CNTs) via the hydrothermal method. The resultant prepared composite was doped with different ratios of nitrogen. The structural and morphological merits of the composites displayed the successful composition of the matrices, as well as the particle size of the composite within the nanoscale. The optical specifications of the composites demonstrate successful direct and indirect transitions with a high energy gap (> 3 eV). The testing of different oils in the water/oil separation exhibited a high rate of success to split oil and water (> 95%). In this regard, CNT-TNT 1.0 sample reflects the highest efficiency. Compared to other researchers that demonstrate the highest efficiency of their proposed structure, our membrane offers a decent separation efficiency. The proposed composite might provide a feasible and cost-effective method for water/oil separation application in the nanotechnological fields.
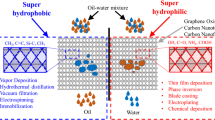
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The utilization of carbon nano-based materials in different applications acquired great attention in the last two decades, especially those related to environmental protection [1,2,3,4]. The water contaminated with oil not only affects all life being organisms, but also decelerates the industrial revolutions. The discharged water coming out from different factories contains multiple contaminants like dyes and oil [5]. The great challenges are not only separating oil from water but also removing other contaminants. The multi-function materials capable of performing multi-tasks are favored. The scientific society proposed different advanced techniques to clean up oil spills from water, such as coagulation and fluctuation, chemical oxidation, adsorption, biological treatment, photocatalytic treatment, and membrane technology [6,7,8]. Among all of the separation techniques, membrane technology is favored. The membranes are durable and cost-effective, consume less energy, are single-stage, have corrosive resistance, and are non-toxic, environmentally friendly, and efficient [9].
Due to their outstanding properties, sodium titanate nanotubes (TNTs) were subjected to massive research in past decades [10]. The application areas of TNT comprise energy storage [11,12,13], photocatalysis [14], sensors [14], dye removal [15,16,17], and fire protection [18]. Sodium titanate has a general Na2TinO2n+1 (n = 3–8) formula, with numerous crystalline phases with different physicochemical properties. Specifically, Na2Ti3O7 and Na2Ti6O13 have been comprehensively studied because of their exceptional stability and outstanding ionic conductivity [10]. Several approaches like sol–gel, solid-state, and hydrothermal have been reported for TNT preparation [19]. The hydrothermal method is a single-step process that produces many TNTs with different morphologies [20]. Carbon nanotubes (CNTs) are promising 1D materials with splendid functionalities used in different fields [21,22,23]. It was applied as a sensitive material to various physical, chemical, and biological stimuli; however, some limitations related to its intrinsic characteristics hinder its practical applications [24,25,26,27,28,29]. It was reported that the incorporation of CNTs with some functional materials (e.g., metal oxides) resulted in enhancing the functionality of the resultant composite [30, 31]. The incorporation of sodium titanate and CNTs was reported to have untraditional merits compared to their separate components. Payan et al., successfully synthesized TNT/SWCNT via a two-step hydrothermal route. The proposed composite was tested against 4-CP degradation under UV and solar irradiation [32]. Santos et al., have prepared TNT/MWCNTs through the hydrothermal method. They concluded that the modification of TNT with MWCNTs led to a remarkable enhancement in the oxidization of methyl yellow (MY) [33]. Mohamed et al., prepared MWCNTs/TNT nanocomposites loaded with platinum nanoparticles as methylene blue (MB) dye removal. Their research work demonstrated that the combination between TNT and MWCNT resulted in a superior photocatalytic activity in the degradation of MB [34]. Jiang et al., synthesized CNT/TNT composite to be applied as photocatalyst material for methyl orange (MO) dye. According to their results, the degradation rate reached 94% after irradiation for 40 min. Hongchao Li et al., successfully prepared carbon membranes by the mixing polyacrylonitrile and phenolic resin via the pyrolysis process. They approached an optimal rejection for oil with 94.2% [35]. Carbon membrane coated with polyethylene glutaraldehyde was prepared. The impacts of coating sorts and solutions used as well as the concentration for the structure and the separation efficiency for oily wastewater were explored while ~ 94% separation was affirmed in another report [36]. Herein, through this work, titanium dioxide nanoparticles (TiO2) were grown on the external surface of CNTs, and then transformed into TNT via a hydrothermal route. The earlier obtained composite was doped with nitrogen using a solid-state reaction followed by calcination. The main target of this study is to acquire a smart material having oil/water separation properties. The prepared composites were studied using different techniques including XRD, FTIR, UV–Vis, SEM, and HR-TEM, to assess their properties, and then tested for the application mentioned earlier. The microstructural-related parameters such as microstrain, dislocation density, and crystallinity degree were calculated from the XRD diffractograms. The conducted optical study reveals an interaction between the doped TNT and CNT. This interaction alters the energy level of the conduction band. The studied membrane was prepared using a simple vacuum filtration method. The efficiency of the synthesized membranes was investigated initially against n-hexane. The obtained results confirmed the sample CNTs-TNT1.0 has the highest separation efficiency. The latter was tested versus different oils.
Experimental
Materials
Petroleum ether (extra pure), acetic acid (99.7%), ethanol (99.5%), hexane (95%), sodium hydroxide (≥ 97%), sulfuric acid (95–98%), hydrochloric acid (36%), and urea (98%) were obtained from Fisher Scientific. Nitric acid was shipped from Sigma-Aldrich. The titanium tetra isopropoxide (TTIP) (98%) was supplied from Acros Organic. Multiwalled carbon nanotubes (MWCNTs) were synthesized by atmospheric chemical vapor deposition (CVD). More details regarding the synthesis method can be found in our previously published articles [30, 37, 38].
Synthesis of CNT-TiO2 composite
The first step of grafting TiO2 over CNTs is to modify their outer surface to get a functional group. Initially, 0.5 g of MWCNTs was treated with a mixture of HNO3 and H2SO4 (3:1, V:V) for 4 h to get a function group and eliminate the undesired impurities and catalyst particles. The MWCNTs were washed repeatedly with deionized water, followed by ethanol, and then deionized water, and finally dried at 60 °C until complete evaporation of the water.
The CNT-TiO2 composite was synthesized as per procedures described in the literature [39]. Briefly, 5 mL of TTIP was dissolved in 30 mL of absolute ethanol to form a clear solution, followed by the dispersion of 30 mg of MWCNTs in the solution using an ultrasonic homogenizer. Another solution comprising 2 mL acetic acid and 30 mL deionized water was dropped slowly into the above solution under continuous stirring for 3 h. The resultant solution was allowed to rest overnight. The precipitates were separated from the liquor solution by centrifugation, and then washed and dried. The bright gray powder was calcined at 350 °C for 2 h.
Synthesis of Na2Ti3O7/MWCNT composite
The Na2Ti3O7/MWCNT composite was synthesized by hydrothermal route. Typically, 0.3 g of CNTs-TiO2 was stirred in 30 mL of 10 M NaOH solution for 10 min. The slurry was poured into a 50-mL Teflon-lined stainless-steel autoclave and maintained at 180 °C for 24 h. The autoclave was cooled naturally; then, the precipitates were recovered by centrifugation. The precipitates were washed several times with deionized water followed by washing with 0.1 M HCl, and again with deionized water until they reached neutralization and finally dried.
Synthesis of nitrogen-doped Na2Ti3O7/MWCNT composite
The nitrogen-doped Na2Ti3O7/MWCNTs were executed by a simple solid-state reaction using urea as a nitrogen source. At the outset, about 3 mL of ethanol was added to a mixture of 0.3 g of Na2Ti3O7/MWCNT composite and different rations of urea, as represented in Table 1. Each slurry was grounded manually in an agate mortar for 10 min, and then heat-treated at 350 °C for 3 h. Finally, the obtained N-doped Na2Ti3O7/MWCNTs were labeled as CNT-TNT0.5, CNT-TNT1.0, and CNT-TNT1.5. Figure 1 represents a scheme of the preparation procedure.
Membrane preparation
The membranes were constructed by using a simple vacuum filtration apparatus. A certain amount (10 mg) of each composite was ultrasonically dispersed in 50 mL of deionized water for 30 min and stirred for an additional 1 h to get a homogeneous solution. The obtained composite was filtered through a nitrocellulose filter membrane for 10 min. The achieved membrane was dried at 50 °C for 24 h for further testing and characterization. The membrane preparation process is presented in Figure 2a and b.
Experimental setup
Oil separation was carried out with a designed setup in Figure 2b; the oil/water mixture (1: 1.6weight) was flowing past the composite membrane matrix due to suction towards gravity force and the separated liquid was eventually collected in a conical flask. Water and different types of oil (including n-hexane, vacuum oil pump, engine oil, petroleum ether, and toluene) were colored with methylene blue. The separation time for 200 mL mixture solution was about 60 s by a vacuum pressure of 15 Micron Hgs.
Characterization techniques
The phase composition was identified by XRD using Malvern Panalytical Empyrean 3 with Cu Kα radiation at a wavelength of 1.54 Å. The FTIR spectra were acquired using an FTIR spectrometer (Vertex 70, Bruker); the spectra were collected in a spectral range of 4000–400 cm−1 with a spectral resolution of 5 cm−1. The microstructure was verified by scanning electron microscopy (SEM), Quattro S, Thermo Scientific. More details regarding the microstructure were verified by the high-resolution transmission electron microscope (HR-TEM) model JEOL JEM2100 electron microscope. The optical properties were performed at room temperature using a spectrophotometer (UV–Vis-NIR, JASCO V-570, Japan) equipped with an integrating sphere attachment (JASCO ISV) in the 200–2000 nm wavelength.
Oil/water separation experiment
n-Hexane was used as an oil model; then, different types of oils were utilized and tested. The water/oil combination was executed by mixing 15 g of n-hexane and 25 g of water. The prepared membrane was wetted before starting the separation experiment. The separation efficiency was estimated using the following equation:
where Mr and Mi are the mass of oil before and after separation respectively.
Results and discussion
Phase assessment
This section explores the structural specifications of the synthesized composites via different apparatus. A powerful and standard device is the X-ray diffraction which sheds light on the internal nature of the composite, the crystallite size, the observed microstrain, the dislocation density, and other significant parameters that unveil the structure’s nature. Figure 3 represents the XRD diffractograms of the CNT-TiO2, CNT-TNT, CNT-TNT0.5, CNT-TNT1.0, and CNT-TNT1.5 as remarked above each diffraction pattern performed within 5° to 75° at ambient conditions. CNTs have two peaks at the diffraction angles of around 25° and 42°, which are indexed to the (002) and (100) planes correspondingly, while the former is the strongest one and the latter owes much lower intensity [40]. This coincides with ICDD card no. #01–077-7164, and preceding reports [41]. The peaks of the anatase tetragonal TiO2 reside at 25.68°, 37.9°, 48.0°, 54.67°, 62.84°, and 69.75° which are attributed to (101), (004), (200), (105), (204), and (116) planes consecutively. Noticeably, the first peak overlaps with the highest peak for the CNTs [42, 43]. This matches with the previous reports and card no. # JCPDS card No. 21–1272 from papers [40, 44, 45] with a tiny negligible shift (< 1°), which reflects the purity of the synthesized composite and the feasibility of the synthesis procedure. The CNT-TNT has the apparent diffraction peaks at 9.59°, 25.07°, 29.50°, and 48.41° for the (100), (011), (300), and (020) planes correspondingly according to the ICDD card number 00–059-0666 for sodium titanate oxide. The characteristic peak of CNTs intensity decreases upon do**. Noticeably, the characteristic peak for titanium oxide at around 10° tends to be less intense and broader upon the treatment confirmed by origin program calculations for the full width at the half maximum (FWHM). Besides, all characteristic peaks exist and are in fine accordance with the preceding cards.
The crystallite size (D) for the synthesized composites is monitored using the usual Scherrer equation denoted as [46,47,48]:
k represents the sample’s shape factor, λ is the wavelength for the incident ray CuKα source with the value of 0.15406 nm, ∆ remarks the full width at the median of the highest value, and θ is the angle at which the diffraction occurs. Furthermore, the value of the microstrain could be determined as follows [49,50,51]:
The value of the microstrain has a meaning; a negative one remarks the presence of compressive strain in the particles [52]. Conversely, the positive value represents tensile strain [53]. The enhancement of the strain magnitudes reflects the propagation of grain walls [54]. Knowing the crystallite size, the dislocation density is estimated as follows [49, 50, 55]:
For further analysis of the composites, the degree of crystallinity might be produced based on the area under the peaks of the plot as follows [56,57,58]:
While AC is the area under crystalline peaks, AT is the elaborated area under the crystalline and non-crystalline peaks of the pattern.
Calculated parameters for these composites are tabulated in Table 1.
As can be seen from Table 1, the average particle size decreases, while the positive value of the microstrain indicates the occurrence of tensile strain [51]. The reduced crystallinity degree is due to the occurring hydrothermal treatment.
FTIR
The FTIR was used mainly to characterize the formed bonding between constituents of composite. Figure 4 displays the FTIR spectra of the as-prepared CNT-TiO2 nanoparticles, CNT-TNT, and nitrogen-doped CNT-TNT nanocomposite with do** ratios of 0.5, 1.0, and 1.5%. All the FTIR spectra revealed distinct peaks at about 3430, 681, and 487 cm−1 which are assigned to the stretching vibration bands of O–H, Ti–O-Ti groups of the TiO2 nanoparticles, and TNT [59, 60].
Furthermore, the peak that appeared at 1623 cm−1 is characteristic of the C = O group of the acid-treated CNT. A prominent peak appears at the spectra of CNT-TNT, and nitrogen-doped CNT-TNT nanocomposite appears at 901 cm−1; this peak is characteristic of the Ti–O-Na bond. This confirms the formation of TNT. Also, the spectra of nitrogen-doped CNT-TNT reveal a characteristic peak at 1365 cm−1 is assigned for the N–H vibration mode. It was observed that the peak intensity increases with increasing the do** ratio of nitrogen. This peak does not appear at the spectra of CNT-TiO2 and CNT-TNT, which confirms the successful do** of the CNT-TNT with nitrogen [61,62,63,64,65].
Morphological evaluation
The FE-SEM and HR-TEM instruments were used to investigate the morphology of the prepared materials. The general overview of the structure of the materials was examined through FE-SEM, while more detailed morphology was recognized from HR-TEM images. The FE-SEM images of CNT-TNT are illustrated in Fig. 5.
The SEM images of CNTs-TNTs exhibit a fiber-like structure that covers the outer diameter of CNTs. The images indicate that the CNTs are fully covered with TNT with noticeable agglomeration. It seems that the TNT was grown perpendicular to the outer surface of CNTs. More details regarding the microstructure were deduced from the HR-TEM as presented in Fig. 6. The HR-TEM images of CNTs-TiO2 indicated in Fig. 6a reveal that the CNTs have a tubular structure with a hollow cavity. The spherical TiO2 nanoparticles are attached externally to the CNTs with good distribution and less agglomeration. However, some TiO2 nanoparticles were grown out of the CNT surface, as indicated in Fig. 6b. The size of the spherical TiO2 nanoparticles was measured to be around 6 nm. The measured particle size from XRD was found to be in good agreement as obtained from HR-TEM.
After hydrothermal treatment, the TiO2 spherical particles were turned into nanotubes with an external diameter of about 7–9 nm as shown in Fig. 6c. The outer diameter of TNT is the same as the diameter of spherical TiO2 nanoparticles, indicating the transformation of TiO2 to TNT after hydrothermal treatment. The TNT seems to be agglomerated and covers the external surface of the CNTs. The TNT seems to be grown perpendicular to the outer surface of CNTs.
Optical studies
The electronic configuration of Ti3 is 3d1 4s2. It has five-fold degeneracy, and its ground state term symbol is 2D. The five-fold degeneracy is split into 2T2g and 2Eg states in an octahedral crystal field. Thus, only one electron transition, 2T2g → 2Eg, is expected in an octahedral crystal field. The separation between these energies is 10Dq, which is crystal field energy. Normally, the ground 2T2g state is split due to the Jahn–Teller effect, and hence, lowering of symmetry is expected for Ti(III) ion. This state splits into 2B2g and 2Eg states in tetragonal symmetry, and the exciting term 2Eg also splits into 2B1g and 2A1g levels. Thus, three bands are expected for tetragonal (C4v) symmetry. The energy level diagram in a tetragonal environment is shown in Fig. 7.
From the observed band positions, the crystal field parameter in the octahedral field is Dq (1205 cm−1), and tetragonal field parameters are Ds (1867 cm−1) and Dt (268 cm−1).
The optical properties were investigated using a UV–Vis-NIR reflectance spectrophotometer with an integrating sphere attachment in the 200–2000 nm wavelength range. Figure 8 shows the absorbance and reflectance of TiO2, TiO2-CNT, and N-doped TiO2-CNT nanocomposite.
The diffuse reflectance spectrum of TiO2, TiO2/CNT, and N-doped TiO2-CNT nanocomposite is reported in Fig. 8a. A relatively moderated reflectivity characterized the sample since the reflectance was ~ 50% for wavelengths above 400 nm, and at 800 nm it was about ~ 40%. Figure 8b also reports the absorbance spectrum. As clearly visible, the spectrum unveils absorbance values of about 0.3–0.6 for wavelengths above 400 nm.
However, the total absorption spectra will depend on the phase of crystal and microstructure shape morphology and the distribution of holes among available sites at a given time after the photogeneration. Figure 8 reveals the positions of the bands on the wavelength scale (nm) rather than their relative intensities, and the bands’ maxima are aligned. Ti(III) ion in anatase phase is characterized by three broad bands around 1440 nm (0.86 eV, 6945 cm−1), 830 nm (1.49 eV, 12,050 cm−1), and 550 nm (2.25 eV, 18,180 cm−1) which are due to the transitions from 2B2g → 2Eg, 2B2g → 2B1g, and 2B2g → 2A1g respectively. The inset in Fig. 8 illustrates two clear peaks near 216 and 263 nm related to GQDs of π → π* transition of sp2C hybridization of aromatic C = C bonds, which confirm the report for carbon materials such as graphene quantum dots [65, 66].
The interaction between the CNT and pure and N-doped TiO2 nanoparticles changes the energy level of the conduction band through the interaction of unpaired π electrons with the titanium atoms, causing a displacement of the conduction band edge toward the visible region, therefore a reduction in the composite band gap value. The band gap energies of the specimens are calculated using the Kubelka–Munk function [67, 68]:
F (R∞) is the so-called remission or Kubelka–Munk function. In the parabolic band structure, the band gap Eg and absorption coefficient α of a direct band gap semiconductor are related through the well-known equation [69,70,71,72].
where α is the linear absorption coefficient of the material, hν is the photon energy, and A is the proportionality constant. When the material scatters in a perfectly diffuse manner (or when it is illuminated at 60 ± incidence), the K-M absorption coefficient K becomes equal to 2α (K = 2α). Considering the K-M scattering coefficient S as constant concerning wavelength, we obtain the following expression [73, 74]:
Therefore, obtaining plotting the [F (R∞)hν]2 against hν, the band gap Eg of a powder sample can be extracted easily. Figure 9 displays the band gap energy of CNTs and pure and N-doped TiO2 nanoparticles after Kubelka–Munk treatment which the direct band gap energy of pure anatase structure TiO2 nanoparticles was 3.45 eV. After the composite process, the band gap energy of the CNT-TiO2-doped N nanocomposite was shifted to 3.71 eV, which depended on the result of the larger crystallites into an agglomeration of the nanocomposites. However, this behavior can be attributed to the chemical bond between the CNTs and the anatase structure TiO2 nanoparticles formed during the synthesis of the materials.
Water/oil separation evaluation
This study investigated the oil/water separation efficiency of the prepared composites using a simple vacuum filtration system. n-Hexane has been utilized as an oil model in all oil/water separation experiments. Different types of oils, including n-hexane, vacuum pump oil, engine oil, petroleum ether, and toluene, have been tested. Figure 10 represents the separation efficiency of the prepared samples using a mixture of water and n-hexane. Remarkably, all samples have an efficiency greater than 90%. The order of separation efficiency is CNTs-TNT1.0 ˃ CNTs-TNT0.5 ˃ CNTs-TiO2 ˃ CNTs-TNT 1.5 ˃ CNTs-TNT. The CNT-TNT1.0 sample has the highest separation efficiency of 99%; the lowest separation rate was achieved by the CNT-TNT sample.
The CNT-TiO2 sample performs better than the CNT-TNT sample. This situation was changed when titania was doped with nitrogen. This confirms that incorporating nitrogen atoms has a remarkable effect on enhancing the oil/water separation efficiency. Since sample No. 4 exhibited a remarkable separation efficiency over other prepared samples, it will be used for further separation experiments. Different oils were allowed to pass through sample No. 4 (CNTs-TNT1.0) to check the separation efficiency. The separation efficiency as a function of the oil type is represented in Fig. 10. Moreover, among all tested oils, the highest efficiency was achieved for n-hexane. Table 2 reveals close and recent structures directed towards water/oil separation and their efficiency and our structure.
Comparing to the studied structure by different research groups, the proposed membrane reveals the highest efficiency.
Conclusion
The preparation of carbon nanotubes is delivered in this report where doped and undoped samples with nitrogen are acquired using a solid-state hydrothermal procedure. The obtained structural results verified the existence of the intended titania nanotubes as well as the carbon nanotubes. The morphological studies of the samples using HR-TEM and SEM support the XRD results. The effective incorporation of the starting constitutes into the composite’s network is verified. The optical study further demonstrates a variation of the band gap energy due to the occurring interaction between the CNTs and TNTs with a raised band gap higher than 3 eV. Diverse oils of n-hexane, vacuum pump oil, engine oil, petroleum ether, and toluene were employed for the water/oil separation experiment. Among all the studied samples for the water/oil separation, sample 4 (CNTs-TNT1.0) achieved the highest successful separation efficiency (> 95% efficiency) compared to other composites. The proposed structure could function on industrial application for removing petroleum spill in nature.
Data availability
The presented data to regenerate these results are included in the manuscript.
References
Wang Y, Pan C, Chu W, Vipin AK, Sun L (2019) Environmental remediation applications of carbon nanotubes and graphene oxide: adsorption and catalysis. Nanomaterials 9:439. https://doi.org/10.3390/nano9030439
Ong YT, Ahmad AL, Hussein S, Zein S, Tan SH (2010) A review on carbon nanotubes in an environmental protection and green engineering perspective. Braz J Chem Eng 27:227–242
Abdel-Salam AI, Awad MM, Soliman TS, Khalid A (2022) The effect of graphene on structure and optical properties of CdSe nanoparticles for optoelectronic application. J Alloys Compd 898:162946. https://doi.org/10.1016/j.jallcom.2021.162946
Hussein Y, Kamoun EA, Loutfy SA, Amen R, Taha TH, Mansour AS, Abdel-Salam AI, Amer M (2021) Plant nanocellulose and its composite hydrogel membranes-based polyvinyl alcohol/hyaluronic acid for biomedical applications: extraction, characterization, and in vitro bioevaluation. J Appl Pharm Sci 11:049–060. https://doi.org/10.7324/JAPS.2021.110105
Wang N, Deng Z (2019) Synthesis of magnetic, durable and superhydrophobic carbon sponges for oil/water separation. Mat Res Bull 115:19–26. https://doi.org/10.1016/j.materresbull.2019.03.007
Bai X, Zhao Z, Yang H, Li J (2019) ZnO nanoparticles coated mesh with switchable wettability for on-demand ultrafast separation of emulsified oil/water mixtures. Sep Purif Technol 221:294–302. https://doi.org/10.1016/j.seppur.2019.04.003
Sarbatly R, Krishnaiah D, Kamin Z (2016) A review of polymer nanofibres by electrospinning and their application in oil–water separation for cleaning up marine oil spills. Mar Pollut Bull 106:8–16. https://doi.org/10.1016/j.marpolbul.2016.03.037
Al-Majed AA, Adebayo AR, Hossain ME (2012) A sustainable approach to controlling oil spills. J Enviro Manage 113:213–227. https://doi.org/10.1016/j.jenvman.2012.07.034
Baig U, Faizan M, Dastageer MA (2021) Polyimide based super-wettable membranes/materials for high performance oil/water mixture and emulsion separation: a review. Adv Colloid Interface Sci 297:102525. https://doi.org/10.1016/j.cis.2021.102525
Youssry M, Mussa A (2021) Controllable synthesis of sodium titanates using facile ball milling method. Ceram Int 47:14021–14032. https://doi.org/10.1016/j.ceramint.2021.01.271
Amy L, Favre S, Gau DL, Faccio R (2021) The effect of morphology on the optical and electrical properties of sodium titanate nanostructures. Appl Surf Sci 555:149610. https://doi.org/10.1016/j.apsusc.2021.149610
Nashed R, Girgis E, Shehata A, Abdel-Salam AI, Mohamed MB (2018) Remarkable enhancement of the photocurrent response of dye-sensitized solar cells using CuInSe2 nanocrystals. In: Asia Commun. Photonics Conf., Optica Publishing Group, Guangzhou, p ATh2F.4. https://doi.org/10.1364/acpc.2012.ath2f.4
Abdel-Salam AI, Mohsen Abdelaziz M, Emam AN, Mansour AS, Zikry AAF, Mohamed MB, Elbashar YH (2020) Anisotropic CuInSe2 nanocrystals: synthesis, optical properties and their effect on photoelectric response of dye-sensitized solar cell. Revista mexicana de física 66:14–22
Barbieriková Z, Lonc D, Ahrenkiel SP, Nedeljkovic JM (2020) Photocatalytic hydrogen evolution over surface-modified titanate nanotubes by 5-aminosalicylic acid decorated with silver nanoparticles ˇ arevic. 31:4683–4690. https://doi.org/10.1016/j.apt.2020.11.001
Zhang Y, Li G, Liu J, Wang T, Wang X, Liu B, Liu Y, Huo Q, Chu Z (2018) Synthesis of hierarchical hollow sodium titanate microspheres and their application for selective removal of organic dyes. J Colloid Interface Sci 528:109–115. https://doi.org/10.1016/j.jcis.2018.05.069
Hosny NM, Gomaa I, Abd El-Moemen A, Anwar ZM (2021) Adsorption of Ponceau Xylidine dye by synthesized Mn2O3 nanoparticles. Int J Environ Anal Chem:1–17.https://doi.org/10.1080/03067319.2021.2014470
Hosny NM, Gomaa I, Abd El-Moemen A, Anwar ZM (2020) Synthesis, magnetic and adsorption of dye onto the surface of NiO nanoparticles. J Mater Sci: Mater Electron 31:8413–8422. https://doi.org/10.1007/s10854-020-03376-w
Ding Y, Liu T, Jiang Y, Zhou J, Zhou Z, Sun J, Peng J (2022) Flexible fire-resistant and heat-insulating materials fabricated using sodium titanate nanobelts. Mater Today Nano 17:100161. https://doi.org/10.1016/j.mtnano.2021.100161
Mastoroudes BC, Markgraaff J, Wagener JB, Olivier EJ (2020) Synthesis of cesium, sodium and nitrogen derived titanates using the Pechini sol-gel method. Chem Phys 537:110816. https://doi.org/10.1016/j.chemphys.2020.110816
Fuentes S, Cabrera AL, Fuenzalida VM (2008) Structural characterization of single crystals of sodium titanate nanowires prepared by hydrothermal process. J Crystal Growth 310:3630–3637. https://doi.org/10.1016/j.jcrysgro.2008.05.020
Srivastava S, Mishra BK (2018) Electron transport through double-walled carbon nanotube quantum dots. J Nanopart Res 20:295. https://doi.org/10.1007/s11051-018-4398-9
Vijayalakshmi V, Sadanandan B, Venkataramanaiah Raghu A (2022) Single walled carbon nanotubes in high concentrations is cytotoxic to the human neuronal cell LN18. Res Chem 4:100484. https://doi.org/10.1016/j.rechem.2022.100484
Kumar S, Reddy KR, Reddy CV, Shetti NP, Sadhu V, Shankar MV, Reddy VG, Raghu AV, Aminabhavi TM (2021) Metal nitrides and graphitic carbon nitrides as novel photocatalysts for hydrogen production and environmental remediation BT - nanostructured materials for environmental applications. In: Keller V, Shankar MV (eds) Balakumar S. Springer International Publishing, Cham, pp 485–519
Kumari P, Malika K, Kumar L, Gupta R (2021) Recent advances in application of the graphene-based membrane for water purification. Mat Today Chem 22:100597. https://doi.org/10.1016/j.mtchem.2021.100597
Ghosal K, Mondal P, Bera S, Ghosh S (2021) FlatChem graphene family nanomaterials - opportunities and challenges in tissue engineering applications. FlatChem 30:100315. https://doi.org/10.1016/j.flatc.2021.100315
Wu N, Che S, Li HW, Wang CN, Tian XJ, Li YF (2021) A review of three-dimensional graphene networks for use in thermally conductive polymer composites: construction and applications. **nxing Tan Cailiao/New Carbon Mater 36:911–929. https://doi.org/10.1016/S1872-5805(21)60089-6
Morsy M, Yahia IS, Zahran HY, Ibrahim M (2019) Hydrothermal synthesis of CNTs/Co 3 O 4 @rGO mesopours nanocomposite as a room temperature gas sensor for VOCs. J Inorg Organomet Polym Mater 29:416–422. https://doi.org/10.1007/s10904-018-1011-8
Sami SK, Seo JY, Hyeon S-E, Shershah MSA, Yoo P-J, Chung C-H (2018) Enhanced capacitive deionization performance by an rGO–SnO 2 nanocomposite modified carbon felt electrode. RSC Adv 8:4182–4190. https://doi.org/10.1039/C7RA12764B
Chen L, Huang L, Lin Y, Sai L, Chang Q, Shi W, Chen Q (2018) Sensors and actuators B : chemical fully gravure-printed WO 3 / Pt-decorated rGO nanosheets composite film for detection of acetone. Sens Actuators, B Chem 255:1482–1490. https://doi.org/10.1016/j.snb.2017.08.158
Morsy M, Yahia IS, Zahran HY, Meng F, Ibrahim M (2019) Portable and battery operated ammonia gas sensor based on CNTs / rGO / ZnO nanocomposite. J Electron Mater 48:7328–7335. https://doi.org/10.1007/s11664-019-07550-7
Papers F, Morsy M, Secondary CA, Author C, Morsy M, Yahia I, Zahran H, Ibrahim M, Morsy M. Hydrothermal synthesis of CNTs / Co3O4 @ rGO mesopours nanocomposite for enhanced VOCs. J Inorg Organomet Polym Mater
Payan A, Fattahi M, Jorfi S, Roozbehani B, Payan S (2018) Synthesis and characterization of titanate nanotube/single-walled carbon nanotube (TNT/SWCNT) porous nanocomposite and its photocatalytic activity on 4-chlorophenol degradation under UV and solar irradiation. Appl Surf Sci 434:336–350. https://doi.org/10.1016/j.apsusc.2017.10.149
Santos SRA, Jardim IS, Bicalho HA, Binatti I, Sousa EMB, Peres AM, Resende RR, Lorençon E (2016) Multifunctional catalysts based on carbon nanotubes and titanate nanotubes for oxidation of organic compounds in biphasic systems. J Colloid Interface Sci 483:211–219. https://doi.org/10.1016/j.jcis.2016.08.025
Ibrahim MM, Ahmed SA, Khairou KS, Mokhtar M (2014) General carbon nanotube / titanium nanotube composites loaded platinum nanoparticles as high performance photocatalysts. Appl Catal A General 475:90–97. https://doi.org/10.1016/j.apcata.2014.01.030
Li H, Zhang B, Hong X, Wu Y, Wang T (2022) Optimizing the microstructure and properties of microfiltration carbon membranes enabled with PAN fibers for emulsified oil removal from wastewater. Chem Eng Res Des 184:566–576. https://doi.org/10.1016/j.cherd.2022.06.035
Li H, Wu Y, Huang H, Zhang B, Liang Y, Chen Y, Wang T (2022) Surface synthesis of a polyethylene glutaraldehyde coating for improving the oil removal from wastewater of microfiltration carbon membranes. J Water Process Eng 47:102724. https://doi.org/10.1016/j.jwpe.2022.102724
Morsy M, Helal M, El-Okr M, Ibrahim M (2014) Preparation, purification and characterization of high purity multi-wall carbon nanotube. Spectrochimica Acta - Part A 132. https://doi.org/10.1016/j.saa.2014.04.122
Morsy M, Helal M, El-Okr M, Ibrahim M (2015) Preparation and characterization of multiwall carbon nanotubes decorated with zinc oxide. Der Pharma Chemica 7(10):139–144. https://www.derpharmachemica.com/pharma-chemica/preparation-and-characterization-of-multiwall-carbonnanotubes-decorated-with-zinc-oxide.pdf
Okasha A, Gomaa F, Elhaes H, Morsy M, El-Khodary S, Fakhry A, Ibrahim M (2015) Spectroscopic analyses of the photocatalytic behavior of nano titanium dioxide, Spectrochim. Acta - Part A Mol Biomol Spectrosc 136:504–509. https://doi.org/10.1016/j.saa.2014.09.063
Yi Q, Wang H, Cong S, Cao Y, Wang Y, Sun Y, Lou Y, Zhao J, Wu J, Zou G (2016) Self-cleaning glass of photocatalytic anatase TiO2@carbon nanotubes thin film by polymer-assisted approach. Nanoscale Res Lett 11:457. https://doi.org/10.1186/s11671-016-1674-4
Gao B, Chen GZ, Li Puma G (2009) Carbon nanotubes/titanium dioxide (CNTs/TiO2) nanocomposites prepared by conventional and novel surfactant wrap** sol–gel methods exhibiting enhanced photocatalytic activity. Applied Catalysis B 89:503–509. https://doi.org/10.1016/j.apcatb.2009.01.009
Fan W, Lai Q, Zhang Q, Wang Y (2011) Nanocomposites of TiO2 and reduced graphene oxide as efficient photocatalysts for hydrogen evolution. J Phys Chem C 115:10694–10701. https://doi.org/10.1021/jp2008804
Adhikary SK, Rudžionis Ž, Tučkutė S, Ashish DK (2021) Effects of carbon nanotubes on expanded glass and silica aerogel based lightweight concrete. Sci Rep 11:2104. https://doi.org/10.1038/s41598-021-81665-y
Shaban M, Ashraf AM, Abukhadra MR (2018) TiO2 nanoribbons/carbon nanotubes composite with enhanced photocatalytic activity; fabrication, characterization, and application. Sci Rep 8:781. https://doi.org/10.1038/s41598-018-19172-w
Sim LC, Leong KH, Ibrahim S, Saravanan P (2014) Graphene oxide and Ag engulfed TiO2 nanotube arrays for enhanced electron mobility and visible-light-driven photocatalytic performance. J Mater Chem A 2:5315–5322. https://doi.org/10.1039/C3TA14857B
El Nahrawy AM, Hemdan BA, Mansour AM, Elzwawy A, AbouHammad AB (2022) Structural and opto-magnetic properties of nickel magnesium copper zircon silicate nano-composite for suppress the spread of foodborne pathogenic bacteria. Silicon 14:6645–6660. https://doi.org/10.1007/s12633-021-01295-x
El Nahrawy AM, Elzwawy A, Alam MM, Hemdan BA, Asiri AM, Karim MR, Hammad ABA, Rahman MM (2021) Synthesis, structural analysis, electrochemical and antimicrobial activities of copper magnesium zirconosilicate (Cu20Mg10Si40Zr(30–x)O:(x = 0,5,7,10) Ni2+) nanocrystals. Microchem J 163:10588. https://doi.org/10.1016/j.microc.2020.105881
Abdel-Salam AI, Attia SY, El-Hosiny FI, Sadek MA, Mohamed SG, Rashad MM (2022) Facile one-step hydrothermal method for NiCo2S4/rGO nanocomposite synthesis for efficient hybrid supercapacitor electrodes. Mater Chem Phys 277:125554. https://doi.org/10.1016/j.matchemphys.2021.125554
Bindu P, Thomas S (2014) Estimation of lattice strain in ZnO nanoparticles: X-ray peak profile analysis. J Theor App Phys 8:123–134. https://doi.org/10.1007/s40094-014-0141-9
El Nahrawy AM, Elzwawy A, Abou Hammad AB, Mansour AM (2020) Influence of NiO on structural, optical, and magnetic properties of Al2O3–P2O5–Na2O magnetic porous nanocomposites nucleated by SiO2. Solid State Sci 108:106454. https://doi.org/10.1016/j.solidstatesciences.2020.106454
Yousf N, Elzwawy A, Ouda E, Mansour SA, Duraia ESM (2021) Synthesis of 3D hollow structured MnCo2O4/CNTs nanocomposite and its magnetic properties. ECS J Solid State Sci Technol. https://doi.org/10.1149/2162-8777/ac44f7
Prabhu RR, Khadar MA (2008) Study of optical phonon modes of CdS nanoparticles using Raman spectroscopy. Bull Mater Sci 31:511–515. https://doi.org/10.1007/s12034-008-0080-7
Bharti B, Barman PB, Kumar R (2015) XRD analysis of undoped and Fe doped TiO2 nanoparticles by Williamson Hall method. AIP Conf Proc 1675. https://doi.org/10.1063/1.4929241
Wahba AM, Mohamed MB (2020) Correlating cation distribution with the structural and magnetic properties of Co0.5Zn0.5AlxFe2–xO4 nanoferrites. App Phys A 126:488. https://doi.org/10.1007/s00339-020-03692-2
El Nahrawy AM, Hemdan BA, Mansour AM, Elzwawy A, AbouHammad AB (2021) Integrated use of nickel cobalt aluminoferrite/Ni2+ nano-crystallites supported with SiO2 for optomagnetic and biomedical applications. Mater Sci Eng B 274:115491. https://doi.org/10.1016/j.mseb.2021.115491
Ouda E, Elzwawy A, Duraia E-SM (2021) A facile microwave irradiation synthesis of GO/CNTs hybrids doped with MnO2: structural and magnetic analysis. Appl Phys A 127:676. https://doi.org/10.1007/s00339-021-04842-w
Doumeng M, Makhlouf L, Berthet F, Marsan O, Delbé K, Denape J, Chabert F (2021) A comparative study of the crystallinity of polyetheretherketone by using density, DSC, XRD, and Raman spectroscopy techniques. Polym Test 93:106878. https://doi.org/10.1016/j.polymertesting.2020.106878
Shaheen TI, El-Gamal MS, Desouky SE, Hassan SED, Alemam AM (2021) Benign production of AgNPs/bacterial nanocellulose for wound healing dress: antioxidant, cytotoxicity and in vitro studies. J Cluster Sci. https://doi.org/10.1007/s10876-021-02190-6
Kudinalli L, Bhatta G, Subramanyam S, Chengala MD, Manjunatha U, Venkatesh K (2017) Low-temperature CO 2 adsorption on titania nanotubes ( TNTs ). Surf Interfaces 8:158–162. https://doi.org/10.1016/j.surfin.2017.06.001
Gusmão SBS, Ghosh A, Marques TMF, Ferreira OP, Lobo AO, Osajima JAO, Luz-Lima C, Sousa RRM, Matos JME, Viana BC (2019) One-pot synthesis of titanate nanotubes decorated with anatase nanoparticles using a microwave-assisted hydrothermal reaction. J Nanomater 2019. https://doi.org/10.1155/2019/4825432
Raghu AV, Gadaginamath GS, Priya M, Seema P, Jeong HM, Aminabhavi TM (2008) Synthesis and characterization of novel polyurethanes based on N1, N4-bis[(4-hydroxyphenyl)methylene]succinohydrazide hard segment. J Appl Polym Sci 110:2315–2320. https://doi.org/10.1002/app.27366
Raghu AV, Gadaginamath GS, Mathew N, Halligudi SB, Aminabhavi TM (2007) Synthesis, characterization, and acoustic properties of new soluble polyurethanes based on 2,2′-[1,4-phenylenebis(nitrilomethylylidene)diphenol and 2,2′-[4,4′-methylene-di-2-methylphenylene-1,1′-bis(nitrilomethylylidene)]diphenol. J App Polym Sci 106:299–308. https://doi.org/10.1002/app.26547
Morsy M, Abdel-Salam AI, Mostafa M, Elzwawy A (2022) Promoting the humidity sensing capabilities of titania nanorods/rGO nanocomposite via de-bundling and maximizing porosity and surface area through lyophilization. Micro Nano Eng 17:100163. https://doi.org/10.1016/j.mne.2022.100163
Morsy M, Elzwawy A, Abdel-salam AI, Mokhtar MM, El BAB (2022) The humidity sensing characteristics of PANI-titania nanotube-rGO ternary nanocomposite. Diam Relat Mater 126:109040. https://doi.org/10.1016/j.diamond.2022.109040
Hatefi R, Younesi H, Mashinchian-Moradi A, Nojavan S (2021) A facile decoration of anatase Fe3O4/TiO2 nanocomposite with graphene quantum dots: synthesis, characterization, and photocatalytic activity. Adv Powder Technol 32:2410–2422. https://doi.org/10.1016/j.apt.2021.05.020
Dhenadhayalan N, Lin K-C, Suresh R, Ramamurthy P (2016) Unravelling the multiple emissive states in citric-acid-derived carbon dots. J Phys Chem C 120:1252–1261. https://doi.org/10.1021/acs.jpcc.5b08516
Abdel-Khalek EK, Rayan DA, Askar AA, Maksoud MIAA, El-Bahnasawy HH (2021) Synthesis and characterization of SrFeO3-δ nanoparticles as antimicrobial agent. J Sol-Gel Sci Technol 97:27–38. https://doi.org/10.1007/s10971-020-05431-8
Rayan DA, Elseman AM, Rashad MM (2018) Remarkable impact of Ni2+ ion on the structural, optical, and magnetic properties of hexagonal wurtzite ZnS nanopowders. Appl Phys A 124:659. https://doi.org/10.1007/s00339-018-2084-5
Elbashar YH, Mohamed MA, Rayan D, Badr AM, Elshaikh HA (2020) Optical spectroscopic analysis of bandpass filter used for laser protection based on cobalt phosphate glass. J Opt 49:270–276. https://doi.org/10.1007/s12596-020-00611-9
Elbashar YH, Moslem SS, Rayan DA, Hassan HH (2019) The influence of nickel ions on the electric behaviour of P 2 O 5 – ZnO – Na 2 O glassy system. J Microw Power Electromagn Energy 53:184–194. https://doi.org/10.1080/08327823.2019.1643649
Abou Hammad AB, Elzwawy A, Mansour AM, Alam MM, Asiri AM, Karim MR, Rahman MM, El Nahrawy AM (2020) Detection of 3,4-diaminotoluene based on Sr0.3Pb0.7TiO3/CoFe2O4 core/shell nanocomposite: Via an electrochemical approach. New J Chem 44:7941–7953.https://doi.org/10.1039/d0nj01074j
Sadeq MS, Ibrahim A (2021) The path towards wide-bandgap and UV-transparent lithium phosphate glasses doped with cobalt oxide for optical applications. J Non Cryst Solids 569:120983. https://doi.org/10.1016/j.jnoncrysol.2021.120983
Abdelbasir SM, El-Sheikh SM, Rashad MM, Rayan DA (2018) Controlling the optical and magnetic properties of nanostructured cuprous oxide synthesized from waste electric cables. Electron Mater Lett 14:505–516. https://doi.org/10.1007/s13391-018-0056-8
Rashad MM, Mostafa AG, Mwakikunga BW (2017) Tunable optical properties of some rare earth elements-doped mayenite Ca 12 Al 14 O 33 nanopowders elaborated by oxalate precursor route. Appl Phys A 123:1–7. https://doi.org/10.1007/s00339-016-0654-y
Lei Z, Zheng P, Niu L, Yang Y, Shen J, Zhang W, Wang C (2019) Ultralight, robustly compressible and super-hydrophobic biomass-decorated carbonaceous melamine sponge for oil/water separation with high oil retention. App Surf Sci 489:922–929. https://doi.org/10.1016/j.apsusc.2019.06.025
Hong X, Zhang B, Zhang X, Wu Y, Wang T, Qiu J (2019) Tailoring the structure and property of microfiltration carbon membranes by polyacrylonitrile-based microspheres for oil-water emulsion separation. J Water Proc Eng 32:100973. https://doi.org/10.1016/j.jwpe.2019.100973
Zhao L, Du Z, Tai X, Ma Y (2021) One-step facile fabrication of hydrophobic SiO2 coated super-hydrophobic/super-oleophilic mesh via an improved Stöber method to efficient oil/water separation. Colloids Surf A 623:126404. https://doi.org/10.1016/j.colsurfa.2021.126404
Barani M, Bazgir S, Keyvan Hosseini M, Keyvan Hosseini P (2021) Eco-facile application of electrospun nanofibers to the oil-water emulsion separation via coalescing filtration in pilot- scale and beyond. Proc Safe Environ Protec 148:342–357. https://doi.org/10.1016/j.psep.2020.10.015
Zhou H, **e J, Yan F, Guo W, Gao P, Qin H, **ao H (2022) Porous Al2O3 ceramics with directional gradient pore structure modified by cobweb-bridged WO3 nanowires for oil/water emulsions separation. Ceram Int 48:18753–18764. https://doi.org/10.1016/j.ceramint.2022.03.150
Li C, Gao Z, Qi X, Han X, Liu Z (2022) Preparation and research of Mn-TiO2/ Fe membrane with high efficiency light-oil/water emulsion separation. Surf Interfaces 31:101995. https://doi.org/10.1016/j.surfin.2022.101995
Chen Z, Su X, Wu W, Chen S, Zhang X, Wu Y, **e H, Li K (2022) Superhydrophobic PDMS@TiO2 wood for photocatalytic degradation and rapid oil-water separation. Surf Coat Technol 434:128182. https://doi.org/10.1016/j.surfcoat.2022.128182
Luo Y, Wang K, Luo S, Zhao F, Wu H, Jiang K, Li Q, Fan S, Wang J (2018) Three-dimensional carbon nanotube/transition-metal oxide sponges as composite electrodes with enhanced electrochemical performance. ACS Appl Nano Mater. 1:2997–3005. https://doi.org/10.1021/acsanm.8b00606
Morsy M, Helal M, El-Okr M, Ibrahim M (2014) Preparation, purification and characterization of high purity multiwall carbon nanotube. Spectrochim Acta - Part A Mol Biomol Spectrosc 132:594–598. https://doi.org/10.1016/j.saa.2014.04.122
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Morsy, M., Abdel-Salam, A.I., Rayan, D.A. et al. Oil/water separation and functionality of smart carbon nanotube–titania nanotube composite. J Nanopart Res 24, 226 (2022). https://doi.org/10.1007/s11051-022-05597-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-022-05597-y