Abstract
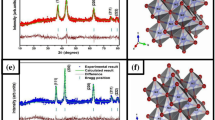
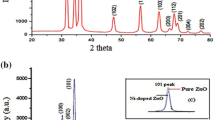
NiO was synthesized simply via thermal decomposition of nickel ascorbate, which resulted from semi-solid reaction of nickel acetate and ascorbic acid. The prepared precursor was characterized by elemental, thermal and spectral analyses. The precursor was decomposed at 700 °C producing cubic NiO with average size 50 nm as indicated from XRD and TEM. FESEM indicated the formation of macroporous NiO. Magnetic parameters (saturation magnetization (Ms), remanence (Mr) and coercivity (Hc)) of NiO nanoparticles and its precursor were determined and compared with the bulk nickel. Zeta potential measurements at different pH values indicated that NiO is negatively charged. Adsorption of ponceau xylidine dye onto NiO nanoparticles at different parameters as pH, amount of adsorbent and time of adsorption was studied. It was found that the adsorption behavior obeys Langmuir isotherm and intra-particle diffusion model. NiO can remove 97.2% of ponceau xylidine from aqueous media. NiO showed higher adsorption than some other reported adsorbents. In additional to that, the antimicrobial effect of NiO-NPs on Staphylococcus aureus, Candida albicans and Escherichia coli was investigated. NiO nanoparticles exhibited good effects against the tested species.












Similar content being viewed by others
References
N.M. Hosny, Single crystalline Co3O4: synthesis and optical properties. J. Mater. Chem. Phys. 144, 247–251 (2014)
H. Derikvandi, A. Nezamzadeh-Ejhieh, Increased photocatalytic activity of NiO and ZnO in photodegradation of a model drug aqueous solution: effect of coupling, supporting, particles size and calcination temperature. J. Hazard. Mater. 321, 629–638 (2017)
M. Karimi-Shamsabadi, A. Nezamzadeh-Ejhieh, Comparative study on the increased photoactivity of coupled and supported manganese-silver oxides onto a natural zeolite nano-particles. J. Mol. Catal A 418–419, 103–114 (2016)
N.M. Hosny, Synthesis, characterization and optical band gap of NiO nanoparticles derived from anthranilic acid precursors via a thermal decomposition route. Polyhedron 30, 470–476 (2011)
N.M. Hosny, E. Othman, F.I. ElDossoki, [Cd(anthranilate)2]H2O as a precursor of CdO nanoparticles. J. Mol. Struct. 1195, 723–732 (2019)
N.M. Hosny, M. Badr, F.I. El-Dossoki, Copolymer of m-phenylenediamine and Anthranilic Acid (P(mPDA-co-AA): new precursor of MnO nanoparticles. Polym. Plast. Technol. Mater. 58, 1178–1190 (2009)
D.S. Saidina, M.Z. Abdullah, M. Hussin, Metal oxide nanofluids in electronic cooling: a review. J. Mater. Sci. Mater. Electron. 31, 4381–4398 (2020)
I. Anastopoulos, A. Hosseini-Bandegharaei, J. Fu, A.C. Mitropoulos, G.Z. Kyzas, Use of nanoparticles for dye adsorption: review. J. Disper. Sci. Technol. 43, 836–847 (2018)
C.H. Wei, Q. Ru, X. Kang, H. Hou, C.H. Cheng, D. Zhang, Self-template synthesis of double shelled ZnS-NiS197 hollow spheres for electrochemical energy storage. Appl. Surf. Sci. 435, 993–1001 (2018)
C.H. Wei, Q. Chen, C.H. Cheng, R. Liu, Q. Zhang, L. Zhang, Mesoporous nickel cobalt manganese sulfide yolk-shell hollow spheres for high-performance electrochemical energy storage. Inorg. Chem. Front. 6, 1851–1860 (2019)
X. Peng, Mechanisms for the shape-control and shape-evolution of colloidal semiconductor nanocrystals. Adv. Mater. 15, 459–463 (2003)
M. Parashar, V.K. Shukla, R. Singh, Metal oxide nanoparticles via sol-gel method: a review on synthesis, characterization and applications. J. Mater. Sci: Mater. Electron. 31, 3729–3749 (2020)
N. Revaprasadu, S.N. Mlondo, Use of metal complexes to synthesize semiconductor nanoparticles. Pure Appl. Chem. 78, 1691–1702 (2006)
S.N. Mlondo, N. Revaprasadu, P. Christian, M. Helliwell, P. O’Brien, Cadmium thiosemicarbazide complexes as precursors for the synthesis of nanodimensional crystals of CdS. Polyhedron 28, 2097–2102 (2009)
H. Yang, Q. Tao, X. Zhang, A. Tang, J. Ouyang, Solid-state synthesis and eectrochemical property of SnO2/NiO nanomaterials. J. Alloys Compd. 459, 98–102 (2008)
C. Díaz-Guerra, A. Remón, J.A. García, J. Piqueras, Cathodoluminescence and photoluminescence spectroscopy of NiO. Phys. Status Solidi 163, 497–503 (1997)
J. Bahadur, D. Sen, S. Mazumder, S. Ramanathan, Effect of heat treatment on pore structure in nano-crystalline NiO: a small angle neutron scattering study. J. Solid State Chem. 181, 12271–21235 (2008)
T. Nathan, A. Aziz, A.F. Noor, S.R.S. Prabaharan, Nanostructured NiO for electrochemical capacitors: synthesis and electrochemical properties. J. Solid State Electrochem. 12, 1003–1009 (2008)
I. Bazin, A. Ibn Hadj Hassine, Y. Haj Hamouda, W. Mnif, A. Bartegi, M. Lopez Ferber, M. De Waard, C. Gonzalez, Estrogenic and anti-estrogenic activity of 23 commercial textile dyes. Ecotoxicol. Environ. Saf. 85, 131 (2012)
M. Hashem, E. Saion, N.M. Al-Hada, H.M. Kamari, A.H. Shaari, Z. Talib, S.B. Paiman, M.A. Kamarudeen, Fabrication and characterization of semiconductor nickel oxide (NiO) nanoparticles manufactured using a facile thermal treatment. Results Phys. 6, 1024–1030 (2016)
Y.C. Wong, Y.S.W. Szeto, H. Cheung, G. McKay, Adsorption of acid dyes on chitosan—equilibrium isotherm analyses. Process. Biochem. 39, 695–704 (2004)
Z. Ni, Sh **a, L. Wang, F. **ng, G. Pan, Treatment of methyl orange by calcined layered double hydroxides in aqueous solution: adsorption property and kinetic studies. J. Colloid Interface Sci. 316, 284–291 (2007)
N. Roya, R.B. Gholam, M.A. Mohammad, M. Hamed, Decolourization of synthetic wastewater by nickel oxide nanoparticles. Int. J. Environ. Health Eng. 1, 1–25 (2013)
K. Ravindhrnath, M. Ramamoorty, Nickel based nano particles as adsorbents in water purification methods—a review. Orient. J. Chem. 33, 1603–1613 (2017)
A.R. Shahverdi, A. Fakhimi, H.R. Shahverdi, S. Minaian, Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomed. Nanotechnol. Biol. Med. 3, 168–171 (2007)
A. Abbaszadegan, Y. Ghahramani, A. Gholami, B. Hemmateenejad, S. Dorostkar, M. Nabavizadeh, H. Sharghi, The effect of charge at the surface of silver nanoparticles on antimicrobial activity against gram-positive and gram-negative bacteria: a preliminary study. J. Nanomater. 8, 720654 (2015)
B.M. Lund, Foodborne diesease due to Bacillus and Clostridium species. Lancet 336, 982 (1990)
A.I. Vogel, A Text Book of Quantitative Inorganic Analyses, 2nd edn. (Longman, London, 1961)
R.H. Baltz, A.L. Demain, J.E. Davies, Manual of Industrial Microbiology and Biotechnology, 3rd edn. (ASM Press, Washington, DC, 2010)
K. Nakamoto, Infrared spectra of inorganic and coordination compounds, 2nd edn. (Wiley, New York, 1970)
H.A. Dabbagh, F. Azami, H. Farrokhpour, A.N. Chermahini, UV-vis, NMR and FT-IR spectra of tautomers of vitamin C experimental and DFT calculations. J. Chil. Chem. Soc. 59, 2588–2594 (2014)
T.V. Long, A.W. Herlinger, E.F. Epstein, I. Bernal, T.V. Long, A.W. Herlinger, E.F. Epstein, I. Bernal, Syntheses, structures, and laser Raman and infrared spectra of Co(NH3)6CuCl5, [Co(NH3)5OH2] CuCl5, Co(NH3)6CdCl5, Co(NH3)6ZnCl5, and Co(NH3)6ZnCl4(NO3). Inorg. Chem. 9, 459–464 (1970)
G. Gliemann, Polarized absorption spectra of tetracyanoplatinate(II) single crystals. Ber. Bunsenges. Für Phys. Chem. 89, 940–948 (1985)
A.B.P. Lever, Inorganic Electronic Spectroscopy, 1st edn. (Elsevier, Amsterdam, 1984)
H.P. Klug, L.E. Alexander, X-ray Diffraction Procedures for Polycrystalline and Amorphous Materials, 2nd edn. (Wiley, New York, 1974)
N.M. Hosny, Solvothermal synthesis, thermal and adsorption properties of metal-organic frameworks Zn and CoZn(DPB). J. Therm. Anal. Calorim. 122, 89–95 (2015)
J. Hwang, V. Dravid, M. Teng, J. Host, B. Elliott, D. Johnson, T. Mason, Magnetic properties of graphitically encapsulated nickel nanocrystals. J. Mater. Res. 12, 1076–1082 (1997)
T. Fardin, The study of structural and magnetic properties of NiO nanoparticles. Opt. Photon. J. 60, 164–169 (2016)
J.T. Richardson, D.I. Yiagas, B. Turk, K. Forster, M.V. Twigg, Origin of super paramagnetism in nickel oxide. J. Appl. Phys. 70, 6977–6982 (1991)
E. Tombácz, pH-dependent surface charging of metal oxides. Per. Pol. Chem. Eng. 53(2), 77–86 (2009)
M. Arshadi, F. SalimiVahid, J.W.L. Salvacion, M. Soleymanzadeh, Adsorption studies of methyl orange on an immobilized Mn-nanoparticle: kinetic and thermodynamic. RSC Adv. 4, 16005–16017 (2014)
I. Langmuir, The constitution and fundamental properties of solids and liquids. J. Am. Chem. Soc. 39, 1848–1906 (1917)
H.M.F. Freundlich, Over the adsorption in solution. Phys. Chem. 57, 385–471 (1906)
M.I. Temkin, V. Pyzhev, Kinetics of ammonia synthesis on promoted iron catalyst. Acta. Physicochim. URSS 12, 327–356 (1940)
S.K. Lagergren, About the theory of so-called adsorption of soluble substances. Sven. Vetenskapsakad. Handingarl 24, 1–39 (1898)
Y.S. Ho, G. McKay, Sorption of dye from aqueous solution by peat. Chem. Eng. J. 70, 115–124 (1998)
W.J. Weber, J.C. Morris, Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 89, 31–38 (1963)
G. McKay, H.S. Blair, J.R. Gardner, The adsorption of dyes onto chitin in fixed bed columns and batch adsorbers. J. Appl. Polym. Sci. 29, 1499–1514 (1984)
Y.S. Ho, G. McKay, A comparison of chemisorption kinetic models applied to pollutant removal on various sorbents. Process. Saf. Environ. Prot. 76, 332–340 (1998)
Y.S. Ho, G. McKay, Comparative sorption kinetic studies of dye and aromatic compounds onto fly ash. J. Environ. Sci. Heal. A 34, 1179–1204 (1999)
M.N. Zafar, Q. Dar, F. Nawaz, M.N. Zafar, M. Iqbal, M.F. Nazar, Effective adsorptive removal of azo dyes over spherical ZnO nanoparticles. J. Mater. Res. Technol. 8, 713–1725 (2019)
M. Batool, W.M.D. Daoush, F. Hashmi, N. Mehboob, Z. Qureshi, Kinetic isotherm studies of azo dyes by metallic oxide nanoparticles adsorbent. Arch. Org. Inorg. Chem. Sci. 3, 426–435 (2018)
G. Kheraldeen-Kara, M. Rabbani, Experimental study of methylene blue adsorption from aqueous solutions onto Fe3O4/NiO nano mixed oxides prepared by ultrasonic assisted co-precipitation. J. Nanostruct. 9, 287–300 (2019)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hosny, N.M., Gomaa, I., Abd El-Moemen, A. et al. Synthesis, magnetic and adsorption of dye onto the surface of NiO nanoparticles. J Mater Sci: Mater Electron 31, 8413–8422 (2020). https://doi.org/10.1007/s10854-020-03376-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-03376-w