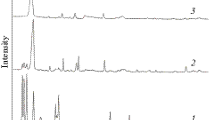
HfTaTiNbZrC5 high-entropy carbide micropowder has been synthesized using the technique of direct-current plasma arc discharge at atmospheric pressure. The synthesis was carried out in air atmosphere under conditions of the formation of a protective carbon monoxide gas layer. TiO2, ZrO2, Nb2O5, HfO2, and Ta2O5 oxide metal powders included in the composition of high-entropy carbide with characteristic grain dimensions of 5–10 μm have been used as raw feedstock. It has been shown that it takes at least 90–100 s for the formation of high-entropy carbide to occur from raw feedstock under the action of arc discharge plasma. The advantage of the proposed method of producing high-entropy carbide micropowder compared to other similar methods is the short time of synthesis of such powder with low energy expenditure (~960 kJ/g) and the possibility of using simple equipment for synthesis.
Similar content being viewed by others
References
J. W. Yeh et al., Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes, Adv. Eng. Mater., 6, Issue 5, 299–303 (2004).
Y. Zhang et al., Microstructures and properties of high-entropy alloys, Prog. Mater. Sci., 61, 1–93 (2014).
R. A. Andrievski, High-melting point compounds: New approaches and new results, Phys. Usp., 60, 276–289 (2017).
O. N. Senkov et al., Refractory high-entropy alloys, Intermetallics, 18, Issue 9, 1758–1765 (2010).
J. Chen et al., A review on fundamentals of high entropy alloys with promising high-temperature properties, J. Alloy. Compd., 760, 15–30 (2018).
W. Ji et al., Alloying behavior and novel properties of CoCrFeNiMn high-entropy alloy fabricated by mechanical alloying and spark plasma sintering, Intermetallics, 56, 24–27 (2015).
S. C. Middleburgh et al., Segregation and migration of species in the CrCoFeNi high entropy alloy, J. Alloy. Compd., 599, 179–182 (2014).
J. Zhoua, J. Zhanga, F. Zhanga, et al., High-entropy carbide: A novel class of multicomponent ceramics, Ceram. Int., 44, 22014–22018 (2018).
O. Cedillos-Barraza et al., Investigating the highest melting temperature materials: A laser melting study of the TaC–HfC system, Sci. Rep., 6, Issue 1, 1–11 (2016).
E. Castle, T. Csanadi, S. Grasso, J. Dusza, and M. Reece, Processing and properties of high-entropy ultra-high temperature carbides, Sci Rep., 8, 1–12 (2018).
B. Ye, T. Wen, and Y. Chu, High-temperature oxidation behavior of (Hf0.2Zr0.2Ta0.2Nb0.2Ti0.2)C high-entropy ceramics in air, J. Am. Ceram. Soc., 103, Issue 1, 500–507 (2020).
E. Chicardi et al., Synthesis of all equiatomic five-transition metals high-entropy carbides of the IVB (Ti, Zr, Hf) and VB (V, Nb, Ta) groups by a low-temperature route, Ceram. Int., 46, Issue 13, 21421–21430 (2020).
D. O. Moskovskikh et al., High-entropy (HfTaTiNbZr)C and (HfTaTiNbMo)C carbides fabricated through reactive high-energy ball milling and spark plasma sintering, Ceram. Int., 46, Issue 11, Part B, 19008–19014 (2020).
A. Y. Pak, A vacuum-free method for producing cubic titanium carbide in the plasma of low-voltage direct-current arc discharge, Tech. Phys. Lett., 44, Issue 12, 1192–1194 (2018).
D. V. Schur, A. G. Dubovoy, S. Yu. Zaginaichenko, V. M. Adejev, A. V. Kotko, V. A. Bogolepov, A. F. Savenko, and A. D. Zolotarenko, Production of carbon nanostructures by arc synthesis in the liquid phase, Carbon, 45, 1322–1329 (2007).
Von L. E. Toth, Transition Metal Carbides and Nitrides, Academic Press, New York–London (1974).
A. Ya. Pak, I. I. Shanenkov, G. Y. Mamontov, and A. I. Kokorina, Vacuumless synthesis of tungsten carbide in a selfshielding atmospheric plasma of DC arc discharge, Int. J. Refract. Mel. Hard Mater., 93, Article No. 105343 (2020).
W. H. Kan, Y. Zhang, X. Tang, et al., Precipitation of (Ti,Zr,Nb,Ta,Hf)C high entropy carbides in a steel matrix, Materialia, 9, Article No.100540 (2020).
B. Ye, T. Wen, K. Huang, C. Wang, and Y. Chu, First-principles study, fabrication, and characterization of (Hf0.2Zr0.2Ta0.2Nb0.2Ti0.2)C high-entropy ceramic, J. Am. Ceram. Soc., 102, 4344–4352 (2019).
Z. Zhang, S. Fu, F. Aversano, M. Bortolotti, H. Zhang, C. Hu, and S. Grasso, Arc melting: a novel method to prepare homogeneous solid solutions of transition metal carbides (Zr,Ta,Hf), Ceram. Int., 45, 9316–9319 (2019).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Inzhenerno-Fizicheskii Zhurnal, Vol. 94, No. 1, pp. 93–100, January–February, 2021.
Rights and permissions
About this article
Cite this article
Pak, A., Grinchuk, P.S., Mamontov, G.Y. et al. Production of HfTaTiNbZrC5 High-Entropy Carbide Micropowder in the Plasma of an Atmospheric Pressure Arc Discharge. J Eng Phys Thermophy 94, 88–94 (2021). https://doi.org/10.1007/s10891-021-02276-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10891-021-02276-y