Abstract
The room temperature aerosol deposition method is especially promising for the rapid deposition of ceramic thick films, making it interesting for functional components in energy, mobility, and telecommunications applications. Despite this, a number of challenges remain, such as an enhanced electrical conductivity and internal residual stresses in as-deposited films. In this work, a novel technique that integrates a sacrificial water-soluble buffer layer was used to fabricate freestanding ceramic thick films, which allows for direct observation of the film without influence of the substrate or prior thermal treatment. Here, the temperature-dependent chemical and structural relaxation phenomena in freestanding BaTiO3 films were directly investigated by characterizing the thermal expansion properties and temperature-dependent crystal structure as a function of oxygen partial pressure, where a clear nonlinear, hysteretic contraction was observed during heating, which is understood to be influenced by lattice defects. As such, aliovalent do** and atmosphere-dependent annealing experiments were used to demonstrate the influence of local chemical redistribution and oxygen vacancies on the thermal expansion, leading to insight into the origin of the high room temperature conductivity of as-deposited films as well as greater insight into the influence of the induced chemical, structural, and microstructural changes in room temperature deposited functional ceramic thick films.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The aerosol deposition method (AD) is a room temperature deposition process based on accelerating micron-sized particles through a specifically designed nozzle toward a substrate, resulting in particle fracture and subsequent impact consolidation into a nanograined coating [1]. During the deposition process, the kinetic energy of the particles is initially based on the pressure difference between the aerosol generator and the deposition vacuum chamber as well as the nozzle geometry, particle size, and particle morphology. The resulting thick films are nanograined with densities in excess of 97% [2,3,4,5,6,7]. In addition to being a room temperature process, it also requires no process additives, such as binders or plasticizers, common with traditional high-temperature ceramic processing methods. As such, AD allows for the integration of ceramics, metals, and glass coatings on various substrates, including low cost and low melting temperature metals, [8] in addition to multilayered ceramic and metallic composite structures [9]. For electrical applications, [10, 11] ceramic films can be directly deposited on a metallic or metallized substrate, thus providing an integrated electrode [4, 8, 12]. For these reasons, the AD method is gaining significant interest for the industrial applications, such as in semiconductors, [13] solid-state lithium ion battery [14], dielectric energy storage capacitors [5], and solid-oxide fuel cells [15].
Despite these advantages, however, there are a number of technical challenges that remain unresolved. In particular, previous investigations have demonstrated that the impact consolidation process induces large internal residual stresses of up to approximately 2 GPa dependent on various factors, such as the carrier gas, as reported by Schubert et al., [16] that can significantly influence the functional properties [17]. Similarly, other investigations using focused ion beam digital image correlation as well as micro-synchrotron X-Ray diffraction have also demonstrated internal stresses on the order of 500–800 MPa [8, 18]. In fact, these large stresses can lead to the delamination of films, in particular during thermal treatment, which is a significant issue for successful integration into application [19]. Although the origins of these internal stresses remain under debate, they are understood to be primarily due to particle impact during deposition resulting in the transformation of kinetic energy into plastic deformation and fracture of the particles [20]. In addition, numerous studies have shown that as-deposited ceramic films also display significant electrical conductivity, which leads to lossy polarization–electric field hysteresis behavior and increased dielectric loss [8, 21, 22]. Interestingly, however, some investigations have shown that under certain deposition conditions a slim polarization–electric field response without the apparent influence of significant electric conductivity can be observed in as-processed films, although it is important to note that various factors can play an important role, such as measurement frequency as well as maximum applied electric field. Here, for example, Imanaka and Akedo observed a slim P-E loop and relatively low dielectric loss in as-processed BaTiO3 ceramics films deposited with O2 as the carrier gas [9]. Interestingly, a response similar to linear dielectrics with comparatively low maximum polarization is observed before thermal treatment [23,24,25]. The origins of this response, however, remain unclear.
Thermal treatment of as-deposited films has been shown to enhance the functional properties of AD films in part through the reduction of internal residual stresses and electrical conductivity, [26,27,28] which increases the dielectric permittivity and polarizability [8, 10] in addition to reducing the dielectric loss and providing better aging behavior [29]. Although grain growth has also been suggested as a possible mechanism to enhance the functional response, studies have typically required significantly higher annealing temperatures on the order to 1000 °C to achieve increased grain size [30]. Here, there is extensive work by Exner et al. [26] showing the influence of thermal treatment on the microstructure as well as the electronic and ionic conductivity of AD films. In contrast, thermal post-treatment at significantly lower temperatures has been found to dramatically improve functional properties of ferroelectric films. For example, previous work has shown that annealing BaTiO3 and Pb(Mg1/3Nb2/3)O3–PbTiO3 films deposited on metallic and glass substrates at 300–500 °C in air is sufficient to significantly decrease internal stress and electrical conductivity [18, 31]. Here, however, the influence of annealing atmosphere and its interplay with thermal effects has not been comprehensively studied.
Although there is an apparent improvement in electrical properties and a reduction in internal stress of ceramic films after annealing, the origins of this behavior remain unclear. Importantly, the interactions between the film and the substrate must be also considered. The thermal treatment of ceramic AD films is typically limited by the temperature stability of the substrate as well as the coefficient of thermal expansion (CTE) mismatch between the ceramic film and the substrate, which can result in delamination due to additional internal stress build up that can fully detach the film [19, 32,33,34]. Therefore, the film-substrate interactions are understood to be an important factor to improve the functional properties of ceramics films, as shown by Han et al. on Pb(Zr,Ti)O3 films [35]. Despite this, the presence of a substrate prevents direct observation of the temperature-induced changes of AD film that are important to understand the nature of the change in residual stress and electrical properties, necessitating a freestanding film in the as-deposited state. Previous methods have achieved freestanding AD films through thermal treatment at elevated temperature utilizing the CTE mismatch to induce film delamination [19, 32,33,34]. These methods, however, expose the film to elevated temperatures that alter the as-processed state, in terms of the crystal and defect structure as well as the internal stress state.
In this work, we demonstrate a simple technique to create freestanding BaTiO3 thick films without requiring heat-treatment. By depositing a ceramic film on a water-soluble sacrificial buffering layer of sodium chloride (NaCl), freestanding ceramic films can be produced in various geometries and thicknesses without the need for thermal treatment. NaCl is established in thick and thin film technology as a suitable sacrificial material, [36] and it has been reported as a substrate for pulsed-laser-deposition albeit with the limitation of heat treatment, [37] self-supporting Au thin films, [38] a nanogap former, [39] and as a high-temperature sacrificial substrate [40]. In the field of aerosol deposition, NaCl has been used to reveal the film substrate interface [41] and as a sacrificial material for nanostructuring polymer surfaces [42]. Using this method, this investigation provides for the first time a direct observation of the thermal expansion and temperature-dependent crystal structure of an AD film in the as-processed state without the influence of an attached substrate, where possible origins of the thermal processes responsible for the observed electrical and mechanical film response are discussed. Here, a sharp drop in the thermal expansion related to a significant macroscopic contraction in the freestanding film is observed, corresponding to a related change in the crystal structure determined using ex situ and in situ temperature-dependent X-ray diffraction (XRD). Both Raman spectroscopy and transmission electron microscopy (TEM) are used to help elucidate the role of various possible mechanisms, providing information on the crystallinity and the crystallographic phase transition of the freestanding AD film. In addition, as defects in the parent composition have been found to play a central role, both donor (Nb5+)- and acceptor (Fe3+)-doped BaTiO3 [43,44,45,46,47,48,49,50] are also deposited and contrasted to stoichiometric BaTiO3, revealing oxygen vacancy concentration-dependent variations in the thermal expansion response. In particular, the influence of oxygen partial pressure of the carrier gas during deposition and during annealing was specifically considered to help distinguish between internal defect redistribution and oxygen reincorporation.
Experimental procedure
BaTiO3 ceramic powders were synthesized using the conventional solid-state reaction method. In addition to undoped BaTiO3, 2 mol% Nb-doped BaTiO3 and 2 mol% Fe-doped BaTiO3 were also prepared. Raw powders of TiO2 (Alfa Aesar, 99.6% purity) and BaCO3 (Alfa Aesar, 99.8% purity) with dopants Nb2O5 (Alfa Aesar, 99.9% purity) or Fe2O3 (Alfa Aesar, 99.9% purity) were stoichiometrically weighed and mixed, homogenized for 24 h, and subsequently calcined at 1100 °C for 6 h. The calcined powders were milled for 15 h using 5 mm yttria-stabilized zirconia (YSZ) milling balls in a rolling mill at 70 rpm to obtain a particle size d50 of 1.2 µm (d10 = 0.4 µm, d90 = 2.8 µm) appropriate for our AD setup.
All compositions (BaTiO3, BaTiO3–2Nb, and BaTiO3–2Fe) were deposited on mirror polished 10 × 10 mm SUS 304 stainless steel substrates using a deposition scan rate of 5 mm/s and a carrier gas (N2, 99.999% purity) flow rate of 4 L/min with a nozzle-to-substrate distance of 7 mm. In this work, a slot nozzle with an orifice size of 1 mm × 10 mm was used for the processing of all AD films using the same parameters mentioned above. Only the number of scans was varied to achieve a targeted film thickness. Films for TEM and scanning electron microscopy (SEM) were fabricated at a thickness of 15 µm using 50 scans, whereas freestanding films for Raman spectroscopy, XRD, and CTE measurements were fabricated with a 45 µm thickness using 250 scans on a pre-treated substrate with a sacrificial NaCl layer described below.
In order to obtain freestanding films, a water-soluble sacrificial layer with an approximate thickness of 2 μm was formed via AD on the SUS 304 stainless steel substrate using commercially available NaCl. This thickness was found to be sufficient to provide a continuous base layer for further deposition of the intended freestanding film material without significantly affecting the particle–substrate interaction during deposition, compared to deposition without a sacrificial layer. Details describing the powder preparation of NaCl can be found in our previous work [41]. Prior to the deposition of BaTiO3, the desired film area was masked with adhesive tape to prevent overlap onto the stainless steel substrate. BaTiO3 films of ~ 45 µm thickness were deposited on the sacrificial layer and subsequently removed by means of submersion in distilled water in an ultrasonic bath (Fig. 1). The films were left to soak for 30 min, then treated with ultrasound for 1 min and subsequently filtered from the solution. The obtained freestanding films were dried at room temperature and stored in a desiccator.
Structural characterization was performed with ex-situ XRD (Bruker D8 Eco, Bruker AXS GmbH, Germany) and a temperature-dependent in-situ X-ray powder diffractometer (X'Pert PRO with X’Celerator detector, PANalytical B.V., Netherlands) equipped with an HTK-1200 N heating stage (Anton Paar GmbH, Germany). Both setups are equipped with a Cu source and position sensitive line detector. A 2 K/min heating rate was used for the in situ temperature-dependent measurements. The sample was kept at each targeted temperature step for 5 min before starting diffraction data collection. Diffraction data were collected between 20 to 80° 2θ with a step size of 0.02° and for 0.2 s at each step.
In order to quantify the influence of annealing temperature and atmosphere on the thermal expansion properties, freestanding films with different defect concentrations were annealed in nitrogen, air, and vacuum atmospheres up to 500 °C. Freestanding BaTiO3 films with a sample size of approximately 0.6 × 0.5 mm2 were then placed in a temperature and atmosphere-controlled heating stage (HFS 600 E, Linkam Scientific Instruments Ltd., UK). It is important to note that the achievable purity of the measurement atmosphere in the hot-stage setup is crucial considering the sample size. A continuous inflow of N2 ensures an overpressure of 20 mbar above the ambient atmosphere to account for leakage and temperature related gas volume changes. Imaging was provided via a confocal microscope (Keyence VK-X160K, Keyence Deutschland GmbH, Germany) using a 20× magnification objective, where, importantly, the sample size was selected to fit within the objective field of view to avoid the need to stitch individual images together, reducing software-induced error. The thermal expansion was determined by evaluating six distinctive natural markers, resulting in 15 point-to-point connections distributed over the sample surface (see supplement A1). The influence of film bending was taken into account by measuring a set of undoped BaTiO3 films both on the top surface of the film and the former film-substrate interface. No significant deviation could be observed, indicating that the bending of 45 µm films is negligible within the imaged frame at 20× magnification. Therefore, subsequent films were measured with their AD-top surface pointing to the objective with the smoother film–substrate interface providing a better thermal connection to the heating stage below. Temperature profiles for all measurements, including high-temperature XRD (HT-XRD) and Raman spectroscopy, were performed from room temperature to 500 °C with dwelling time of 1 h. The heating and cooling rate were 5 K/min.
In advance of TEM preparation, BaTiO3 was deposited on a glass substrate using identical parameters to those used to generate freestanding BaTiO3. The glass substrate served as a carrier providing mechanical film stability during milling. BaTiO3 slices with a remaining film thickness of approximately 50 nm were prepared via ion milling and subsequently analyzed using transmission electron microscopy (TEM, JEM-ARM200F, JEOL Ltd., Japan). The temperature profile for annealing the slices was identical to that used for CTE measurements.
A wavelength dispersive Raman spectrometer was used based on a setup described by Veber et al. [51] which was comprised of a 488 nm laser source, a spectrometer (iHR 320, HORIBA Jobin Yvon GmbH, Bensheim, Germany), an 1800 g/mm grating, and a 2048 × 70 pixel CCD camera (Sincerity UV–VIS, HORIBA Jobin Yvon GmbH, Germany). Temperature control was provided in situ by a differential scanning calorimeter (DSC 8500, PerkinElmer, Waltham, MA, USA) with the sample located in a gold crucible. Raman spectra were collected in 10 K steps from 30–500 °C using a 10× magnification objective for a spatial resolution of approximately 10 µm. The exposure time for each spectrum was 60 s with a total of three exposures per temperature step. The heating rate between steps was 15 K/min with 2 min holding time before measurement. The specimens were fabricated to a thickness of 45 µm, well above the optical penetration depth indicated for Raman measurements in non-absorbing ceramic samples [52].
Results and discussion
Microstructure and crystal structure of freestanding BT films
As previously observed, AD results in a cratered film surface due to the impact of deposited particles, [2, 5] which can be also seen in the freestanding films in this study (Fig. 2a). Notably, upon removal of the water-soluble base layer, it is possible to directly view the bottom surface of the AD film that was formerly at the film/substrate interface (Fig. 2b), revealing that the surface is not smooth, as expected by using a mirror-polished substrate, but has small indents that protrude into the film. This can be attributed to the nature of the deposition process and the presence of the NaCl sacrificial layer before dissolution in water. It shows a replication of the surface structure previously reported on the deposition and subsequent removal of NaCl in our previous work focused on the investigation of the film-substrate interface, where the AD process-induced plastic deformation on the surface of the metal substrate through a shot-peening-like effect that increased the substrate surface roughness [41].
The crystal structure of AD films differs from bulk ceramics due to the nanograined microstructure and internal residual stress, resulting in peak broadening without any evident change in crystallographic phase [19]. This effect is shown in Fig. 3, where bulk tetragonal BaTiO3 shows characteristic peak splitting in the 200pc reflection not observed in the BaTiO3 films. This is a common feature of AD-films, attributable to the crystallite size of the films being in the range of 30 nm [53]. For BaTiO3 nanopowders in the range of 100 nm crystallite size, splitting of 200pc reflections into 002/200, a signature of tetragonal phase, is reported [54]. However, such a feature is not present in our case, further showing that the crystallite size of the as-processed freestanding BaTiO3 film is below 100 nm [54]. In order to determine the effect of the various processing steps on the crystal structure of the films, XRD spectra were taken on a BaTiO3 film deposited directly on a SUS 304 substrate, a BaTiO3 film deposited on the NaCl buffering layer on a SUS 304 substrate, the as-processed freestanding BaTiO3 film after removal from the substrate, and the freestanding BaTiO3 film after annealing at 500 °C in vacuum (Fig. 3). Interestingly, all as-processed, unannealed BaTiO3 films displayed the same peak broadening characteristic of nanograined AD films without an apparent 2θ peak shift, indicating that the presence of the 2 µm NaCl sacrificial layer as well as the removal of the BaTiO3 film did not significantly affect the internal stress state. Changes in microstructure, such as grain growth, are not expected in as-processed samples without thermal treatment. This is particularly significant as it demonstrates that the internal stress is not primarily due to the film/substrate interaction, rather the internal stress is locked into the resulting nanograined microstructure produced during deposition.
Following annealing of AD films at 500 °C, however, a decrease in peak broadening as well as a significant shift in peak positions is observed (Fig. 3). Assuming a pseudocubic structure before heat treatment, peak splitting of the 200pc reflection would also be an indication of partial restoration of tetragonal phase or significant increase in crystallite size; however, this is not observed, indicating that sharpened reflections and decrease in peak width in annealed state possibly originate from a change in internal residual stress [12, 18]. Similar observations have been made for AD films annealed while still attached to the substrate [28]. Thus, the origin and macroscopic consequences for thermal expansion of this thermally activated structural relaxation remain unclear as the substrate presence during annealing seems to be not mandatory to alter the internal stress state of the AD film. It is also noteworthy that annealing of the film was performed in vacuum, ruling out structural changes due to oxygen reincorporation, indicating that internal processes, such as defect reorientation, are also important.
Influence of annealing on dielectric and ferroelectric behavior
As previously noted, as-processed AD films show an enhanced electrical conductivity that can be significantly reduced with thermal treatment. In Fig. 4a, the temperature-dependent dielectric behavior was characterized during a heating/cooling cycle in air from the initial as-processed state for a BaTiO3 film on a SUS304 substrate without a NaCl sacrificial layer, where a significant increase in the dielectric permittivity and a corresponding decrease in the dielectric loss can be observed after heating to 500 °C. As the dielectric response has both intrinsic and extrinsic components, it is expected that the apparent reduction in internal residual stress leads to an enhancement in the observed relative permittivity as domain walls become less mechanically clamped and more mobile. Typically, this behavior would be expected to be accompanied by an increase in the dielectric loss, as extrinsic contributions, such as domain wall motion, are hysteretic in nature. However, a decrease in the loss tangent is observed here, which is understood to be due to a change in the defect structure and concentration. Analogous to the dielectric response, large field ferroelectric measurements on a BaTiO3 film on a SUS304 substrate without a NaCl sacrificial layer (Fig. 4b) also show a decrease in the observed hysteresis after annealing in air. It is important to note that these measurements were performed on the same sample, where a clear slimming of the polarization–electric field behavior and an increase in the maximum polarization are found after annealing. In combination with the temperature-dependent dielectric behavior and the XRD data, this response is consistent with a reduction in the electrical conductivity of the film. As such, to get better insight of the temperature-dependent changes, the macroscopic thermal expansion of AD processed freestanding films was investigated.
Thermal expansion of freestanding BaTiO3 films
During annealing, temperature-dependent processes are induced that lead to significant changes in the macroscopic dielectric and ferroelectric response of the AD-BaTiO3 film (Fig. 4) as well as the observed crystal structure (Fig. 3). Importantly, these effects are not observed in bulk BaTiO3 made from the same starting powder, demonstrating that this is related to the aerosol deposition process. During film deposition, the impact consolidation process results in the fracturing of accelerated particles, which bond together. The mechanical and chemical nature of the bonding, however, remains unclear [41]. Although this dynamic process results in large internal stresses [16, 55] as well as elevated temperatures, [56] it is not expected that the starting deposited material changes composition during AD, e.g., through volatilization of particular elements at elevated local temperatures. For this reason, the observed macroscopic properties are likely related to the defect rich grain boundaries that are created from fracture surfaces during the deposition process. At elevated temperatures, it is expected that both defect reorganization and oxygen reincorporation at the grain boundaries are possible, in addition to the formation of a grain boundary space-charge layer, [57] although the relative contributions remain unclear.
Importantly, however, phenomena related to the creation or filling of vacancies, such as oxygen vacancies, can lead to observable changes in the material volume [58]. As such, the temperature-dependent thermal expansion of a freestanding AD film was characterized in air by confocal microscopy. In Fig. 5a, the thermal expansion of an as-processed freestanding film is shown during the first heating/cooling cycle in air, revealing an initial increase and subsequent sharp drop in thermal expansion starting at approximately 200 °C with a remanent thermal expansion change of approximately −1.1%. Interestingly, the second heating/cooling cycle (Fig. 5b) displayed a thermal expansion behavior similar to the bulk material response (blue line, Fig. 5b), with an observed CTE of approximately \(11.1 \times 10^{ - 6} K^{ - 1}\). These data reveal that the AD-process-induced change in negative thermal expansion of the ceramic film is an irreversible effect and is not related to the interaction with the substrate.
The densification of the nanosized grain structure of the films does not explain this observed effect, as sintering shrinkage of nanocrystalline BaTiO3 with comparable grain sizes in the range of 30–40 nm was observed at approximately 800 °C, considerably below the 200 °C found here [59]. In addition, thermal expansion effects may coincide with a change in unit cell volume at structural phase boundaries [60]. In the case of polycrystalline BaTiO3, the Curie point (\(T_{{\text{c}}}\)) at approximately 120 °C separates the lower temperature ferroelectric tetragonal phase and the high temperature paraelectric cubic phase, where the smaller unit cell of the cubic phase results in in a sharp drop in thermal expansion at \(T_{{\text{c}}}\), [60,61,62] which is observable in the bulk BT data shown in Fig. 5b. The volume change associated this the ferroelectric-paraelectric phase transformation, however, is significantly smaller than that observed here and is also reversible, meaning that it does not explain the irreversible drop in thermal expansion observed in the as-processed BaTiO3 freestanding film. Previous investigations on nanograined BaTiO3 structures show positive CTE values for both tetragonal and cubic phases that can be present simultaneously even below TC [5.
There are a number of potential mechanisms responsible for the observed nonlinear irreversible thermal expansion, including: (i) recrystallization of amorphous grain boundary phase, (ii) change in grain orientation (grain rotation), (iii) sintering-like densification, (iv) reincorporation of oxygen at grain boundaries, (v) reorganization of grain boundary defects, and (vi) the space charge effect. Due to the nanograined nature of AD films, they contain a high fraction of grain boundaries per volume, which can be non-crystalline, [56, 71] in addition to highly deformed grains [71]. As such, a volume change [72] due to the recrystallization of an amorphous grain boundary phase or defect mediated grain reorientation is possible, both of which can induce a significant shrinkage. On the other hand, due to the AD process itself, where fractured particle surfaces are randomly recombined and consolidated in a rapid, dynamic process, cationic and anionic vacancies along the grain boundary are expected [73]. Here, a redistribution of defects at the grain boundary or a reincorporation of oxygen from the surrounding atmosphere can also result in a significant decrease in the apparent unit cell volume [57, 74]. Such significant chemical strains are well-known in oxides. For example, Swallow et al. demonstrated a high-temperature actuator through the electric field controlled electrochemical oxygen exchange and resulting volume change in PrxCe1−xO2−δ [75]. These two contributions are expected to be closely interrelated, as reincorporation of oxygen can limit the available defects for internal distribution. In addition, the concentration of defects in the starting material also plays an important role, which can be adjusted through aliovalent do** of the deposited BaTiO3 powder, in addition to variations in oxygen partial pressure during annealing and measurement to control possible oxygen reincorporation. Finally, dopant-induced oxygen vacancies may be present in the grains segregating electrons to the grain boundaries, [74] resulting in increased electrical resistivity [76]. It is also possible that the temperature-induced defect recombination during the first heating cycle is responsible for decreased electrical conductivity in the annealed film. Importantly, however, the temperature region of defect recombination is of particular interest as diffusion of oxygen along grain boundaries is kinetically more favorable than through the lattice [77].
In order to address these mechanisms, local structural investigations were performed and will be presented in the subsequent sections. Specifically, in situ temperature-dependent XRD and Raman spectroscopy were used to characterize the change in the internal stress state and local structure of the freestanding films, in addition to TEM, which was performed to directly observe the grain orientation before and after annealing. Importantly, the oxygen vacancy content of the AD films was adjusted by aliovalent do** the deposited starting material, in addition to controlling the oxygen partial pressure at elevated temperatures.
In situ temperature-dependent structural investigations
In order to better understand the structural origins of the observed thermal expansion, in situ temperature-dependent XRD was performed on an undoped BaTiO3 film utilizing the identical heating and cooling profile as in thermal expansion measurements. Due to high-temperature XRD heating stage limitations, the measurement atmosphere was restricted to ambient air. Provided that the origin of the thermal expansion anomaly is due to a change in lattice structure, temperature-dependent XRD data is expected to exhibit a change in peak width and peak positions with potential of quantifying the temperature-dependent changes in lattice parameters.
Figure 6a shows the temperature-dependent evolution of the diffraction patterns for a BaTiO3 film during heating from 23 to 500 °C and subsequent cooling back to 23 °C following a dwell at 500 °C for 1 h. In Fig. 6b, c, the intensities of the 111pc and 200pc reflections are highlighted. By utilizing the selected peak fitting scheme, the variation in 200pc lattice stain and the peak width (FWHM) was characterized as a function of annealing temperature (Fig. 6d, e, respectively), where a remanent change in both was found. This data indicates that the decrease in 200pc lattice strain and FWHM is significant above 150 °C (Fig. 6d, e), matching the onset temperature of the sharp drop observed in thermal expansion measurements (Fig. 5). In addition, a remanent unit cell volume reduction of 2.3% has been calculated (see supplementary section A2).
In situ XRD of BaTiO3-AD film as a function of temperature: a overview with highlighted reflections, b, c intensities of the 111pc and 200pc reflections at discrete temperatures. Temperature-dependent evolution of the lattice strain d and, peak width e based on the 200pc peak position. The dashed lines in d and e are added to guide the eye
Changes in peak width and position are attributable to a change in the crystallite size and strain. Similarly, a variation in lattice spacing is indicated by the temperature-dependent shift of the 200pc reflection, hence suggesting a change in lattice strain. It should be noted here that in the case of AD films attached to substrate, temperature-induced change in lattice spacings (stress-relaxation) of the films could be influenced by the substrate. For example, BaTiO3 films deposited on SUS 304 substrate, given that the CTE of the substrate (\(23.1 \times 10^{ - 6} K^{ - 1}\)) is significantly larger than that observed in either an annealed freestanding BT film (\(11.1 \times 10^{ - 6} K^{ - 1}\)) or bulk BT (\(9.8 \times 10^{ - 6} K^{ - 1}\)), would lead to an increase in the tensile stress in the film during heating. This mismatch is expected to influence the stress relaxation process. Nevertheless, it is evident that, without a substrate present, the hysteretic behavior in lattice parameters and thermal expansion is an intrinsic feature in freestanding AD films (Fig. 5a) and is not entirely related to mechanical interaction from a CTE mismatch with the substrate material. Although, the data presented here clearly indicates the change in crystal structure parameters with increasing temperature, it is not possible to separate the possible contributions, such as change in internal stress, crystallite size (crystallinity) and/or oxygen incorporation to the structural change.
There is no clear evidence of a tetragonal-to-cubic phase transition during annealing within the resolution limitation of the XRD instrument used in this work, likely due to the presence of nanograins. Importantly, Raman spectroscopy has been successfully utilized to investigate the phase transition in nanograined BaTiO3 as this technique probes the chemical bonds, which is advantageous for the investigation of the local structure [78]. The presence of a tetragonal phase from Raman spectrum is indicated by the sharp E(LO + TO), B1 band occurring around 305 cm−1 and disappearing with transition to cubic state, though the band around 515 cm−1 is simultaneously attributed to the tetragonal and cubic phase [79,80,81,82]. The sharpness of the 305 cm−1 band is influenced by size effects of the grain structure as elaborated by Hoshina et al. [83], finding an increase in linewidth for decreasing particle size. A decrease in intensity with increasing temperature is also observed [84].
The Raman spectra of as-processed and 500 °C annealed freestanding BaTiO3 films as well as bulk BaTiO3 are shown in Fig. 7. It is evident that the Raman bands of the as-processed film do not match with those of the bulk state, which, considering that the grain size of the freestanding film is below 100 nm, suggests a size effect that can be correlated to the aforementioned studies on nanocrystalline BaTiO3. However, the band around the 305 cm−1 of the as-processed film does not correspond to that of the tetragonal phase, nor that of cubic phase BaTiO3 of comparable grain size [85]. Interestingly, the room temperature spectrum of the freestanding film after annealing clearly represents the tetragonal spectrum obtained with nanograined BaTiO3. In addition, the 515 cm−1 band is shifted to lower wavenumbers with annealing from 522 cm−1 in the as-processed state to 510 cm−1 after annealing. Sakashita et al. [86] investigated the influence of internal stress on the Raman spectrum of BaTiO3, demonstrating that a shift of 515 cm−1 band to higher wavenumbers is related to increasing internal stress. The reported variation in Raman band was limited up to 60 MPa, however, by a linear extrapolation, an approximate decrease of 150 MPa can be expected in our freestanding film due to annealing. As such, our data indicates a thermally induced relaxation of internal stress in freestanding film even without the presence of substrate.
Raman spectra of freestanding BaTiO3 AD film before and after annealing as well as of the bulk ceramic sample. Peaks of interest regarding the cubic-to-tetragonal phase transition of BaTiO3 are highlighted. The Raman spectrum of bulk BaTiO3 collected at 500 °C, i.e., at cubic phase is shown for reference
Due to the nanograined nature of the films and the resolution limits of XRD, the structural phase transition could not be directly observed. As such, in situ temperature-dependent Raman spectra were measured during the annealing process to resolve the temperature-dependent structural phase transition behavior and evolution of the internal stress change. The heating (Fig. 8a) and cooling cycles (Fig. 8b) show significantly different responses, where the observed Raman spectra displayed irreversible temperature-dependent changes. Specifically, the slope of the room temperature spectrum between approximately 200 and 300 cm−1 varies significantly during annealing, indicating that the as-processed state might not be solely comprised of the tetragonal phase but also include a significant influence of AD process-induced lattice distortion. As reported by Hayashi et al., the tetragonal-to-cubic transition in BaTiO3 is marked by a loss in the sharp 305 cm−1 band and a broadening and splitting of the 515 cm−1 band [85]. Therefore, a structural phase transformation between the cubic and tetragonal states can be established during heating between 100 °C and 150 °C, although it is important to note that the presence of compressive internal stresses can shift the phase transition temperature in BaTiO3. Under uniaxial and biaxial compressive stress, TC would be shifted to higher temperatures, [87, 88] whereas a hydrostatic stress would shift TC to lower temperatures [89]. Conversely, the formation of a 305 cm−1 peak can be observed in Fig. 8b during cooling between 150 °C and 100 °C, indicating a cubic-to-tetragonal phase transition around bulk TC in freestanding BaTiO3 for both cycles.
The shift of the 515 cm−1 peak during the initial heating/cooling cycle, identified by its centroid position in Fig. 9, provides information on the temperature-dependent change in internal residual stress during annealing [86]. In particular, the initial as-processed 515 cm−1 peak value is significantly higher than both the bulk BaTiO3 and annealed film values at room temperature, demonstrating a significant internal residual stress. In fact, the stress calibration data provided by Sakshita et al. [86] applies a maximum stress of 60 MPa to BaTiO3 corresponding to a shift to 519 cm−1, well exceeded by the as-processed film., i.e., 522 cm−1. This is a strong indication that the as-processed film is deformed by the impact loading during the AD-process resulting in a lattice distortion [20, 56, 71, 90]. During thermal treatment, as can be seen in Fig. 9, the heating and cooling curves converge at approximately 150 °C, which is consistent with the observed sharp decrease in thermal strain (Fig. 5). At temperatures above 200 °C, a difference between heating and cooling cycle cannot be established by Raman spectroscopy, although it is also important to note that the temperature-dependent shift of the 515 cm−1 peak with increasing temperature is of higher magnitude than reported for non-AD processed barium titanate, [84, 85] thus indicating that at a given temperature, AD-processed BT films feature a higher increase in thermal stress compared to bulk. Importantly, during cooling the position of the 515 cm−1 peak displays a remanent value, where the annealed, room temperature value is similar to bulk BT. This indicates that heat treatment allows the deposition-induced residual internal stress of the AD film to relax [86] and that the irreversible effect responsible for the thermal expansion macroscopically observed in as-processed films is linked to the internal stress state at the microscale.
Temperature-dependent TEM investigation of BT films
Simulation of particle impact during the aerosol deposition process [91] and related cold spraying processes [92] indicate that AD films feature a disordered and plastically deformed microstructure. Sarobol et al. provide evidence that the fractured particles are plastically deformed by dislocation sliding and feature significant microstrain [71]. Importantly, although room temperature dislocation motion along certain preferred slip planes in perovskite oxides, such as SrTiO3, [93] have been experimentally observed, analogous dislocation motion in BaTiO3 was found to be only possible at elevated temperatures around 1150 °C due to significant brittleness [94]. As such, although dislocations in the resulting BaTiO3 films are expected, it suggested that their influence, both due to the brittleness of BaTiO3 and the limited slip systems required for plastic deformation in polycrystalline ceramics, is likely less critical than other factors in the temperature range investigated here. However, additional studies are required to determine the potential influence of plastic flow-related densification mechanisms in AD ceramic films.
In addition to dislocation motion, grain rotation and grain boundary sliding can serve as a mechanism for plasticity and were found to occur at room temperature in nanocrystalline materials as demonstrated by Wang et al. [95] during uniaxial mechanical loading of Pt thin films. The possibility of grain reorientation has been theoretically discussed by Ask et al. [72]. In addition to this, recrystallization of amorphous phases at grain boundaries could also serve as a mechanism for change in volume. Akedo et al. [96] for example, observed recrystallization in AD-deposited PZT starting at 500 °C, which is, however, much higher than the temperature required to induce the observed relaxation observed here in as-processed freestanding films. Similar studies on nanocrystalline SrTiO3 support the theory of recrystallization and grain growth being kinetically unfavorable below 900 °C [97]. In this study, the onset temperature for changes in lattice parameters, i.e., stress relaxation observed with Raman and XRD, is approximately 150 °C, making recrystallization an unlikely contributing factor.
In order to directly investigate these effects, scanning transmission electron microscopy (STEM) with nanodiffraction of an as-processed and 500 °C annealed BaTiO3 AD film was performed, as shown in Fig. 10. Here, both grain (Spot B) and grain boundaries (Spot A and C) were observed (Fig. 10a), as indicated by uniform white contrast and dark lines, respectively. Importantly, the nanobeam electron diffraction patterns show significant variation depending on the area of investigation, i.e., grain (Spot B) or grain boundary (Spot A and C), where the existence of non-crystalline regions at around grain boundaries was found at Spots A and C. Considering the existence of nanograins and large fraction of grain boundary volume in the AD-processed films, it is expected that the structure and chemistry of grain boundary will play crucial role in the observed macroscopic responses. Critically, the observation of crystallite size before and after ex situ 500 °C annealing (Fig. 10b, c) highlights that there is no evident change in crystallite size within this temperature range, suggesting that grain growth is not an important factor in BaTiO3 AD films for annealing temperatures up to 500 °C. Although this thermal treatment did not result in an observable change in the crystallite size, previous observations of the process-induced plastic deformations in PZT AD films [56] suggest that overall volume changes may be accomplished by grain reorientation or grain boundary sliding during thermal treatment.
a Annular dark-field (ADF) STEM image and nanobeam electron diffraction patterns of BaTiO3 film highlighting the variations in crystallinity between grain boundaries (spot A and C) and grains (spot B). Variation in bright-field (BF) TEM images before b and after c thermal treatment at 500 °C for 1 h
Although, ex situ TEM data (Fig. 10b, c) indicates that annealing up to 500 °C does not significantly influence crystallite size, the distinction of individual grains is difficult due to the disordered nanograins at the given resolution. Moreover, a seemingly amorphous structure identified at a grain boundary might be comprised of smaller crystallites. It also provides no information about the influence of defects on mobility of the hypothesized grain rotation mechanism. Therefore, it remains unclear if room temperature-induced plastic deformation, [34] grain rotation, [95] or grain sliding can induce the observed thermal expansion at annealing temperatures below 500 °C. In order to address this, additional in situ high temperature HRTEM investigations are required to quantify the total fraction of the crystalline phase or relaxation in the crystal lattice on an atomic scale as a function of temperature.
Influence of aliovalent do** on the CTE of AD-processed freestanding BT films
As previously noted, the AD process induces residual stresses that can be reduced through thermal treatment, which corresponds to a significant irreversible shrinkage. Importantly, however, our work has clearly demonstrated that the relaxation of internal stress and structural change in AD films are not solely dependent on the film/substrate interaction, i.e., CTE mismatch between the substrate and film, as they also occur in as-processed freestanding films. In oxide-based materials, oxygen vacancies, apart from their intrinsic or dopant-induced occurrence, are suggested to be generated at the surface of fractured particles during the AD deposition process [73]. Through the deposition process, it is understood that both cationic and anionic defects are formed at the grain boundaries due to dynamic fracture and random reorganization of newly created nanoparticles. This is expected to lead to three primary types of changes in the defect concentration during annealing: (i) Local reorganization of defects at the grain boundary through diffusion processes, (ii) Reincorporation of oxygen to fill oxygen vacancies, and (iii) Electron transfer creating a space charge layer [57]. Other mechanisms, such as recrystallization, grain boundary rotation, and densification/grain growth, are also expected to influence the defect concentrations, although their individual contributions remain unclear and require additional more detailed local microscopy investigations.
Oxygen vacancies increase the observed unit cell volume above that of stoichiometric BaTiO3 through cationic repulsion [98, 99]. Therefore, reincorporating oxygen onto an oxygen site through thermal treatment will reduce the unit cell volume, leading to shrinkage in BaTiO3 films. This, as previously noted, can occur through internal redistribution of A, B, and O defects as well as externally through reincorporation of atmospheric oxygen. Conversely, the formation of a space charge layer through electron transfer is thought to reduce the driving force for local reorganization and oxygen reincorporation [100]. The formation of an acceptor-type defect altering the oxidation state from Ti4+ to Ti3+ might lead to a positive lattice strain due to the difference in ionic radii, although the extent of such local strain remains unclear [101]. Importantly, however, although the local redistribution of ions and the formation of a space charge layer can occur in a low oxygen partial pressure environment, the reincorporation of oxygen at the grain boundaries requires the presence of oxygen in the surrounding environment. In addition, the oxygen vacancy concentration and the presence of aliovalent dopants in the starting composition are also critical to controlling this process.
It is well-known that aliovalent B-site do** can be used to alter the oxygen vacancy concentration as well as potentially induce cation vacancies, where donor and acceptor do** decrease and increase the oxygen vacancy concentration, respectively [43,44,45,46,47,48,49,50]. This, for example, has significant effects on the macroscopic ferroelectric and piezoelectric properties of ferroelectrics [102, 103]. As such, B-site donor- and acceptor-doped BaTiO3 were used to directly investigate the observed thermal expansion effects. Figure 11 shows the thermal expansion of Fe-doped, undoped, and Nb-doped freestanding BaTiO3 films during the heating and cooling from the as-processed state to 500 °C in both air and N2. Importantly, all thermal expansion curves show an initial increase during heating, followed by a subsequent decrease above approximately 150–200 °C, and a remanent shrinkage, in agreement with previous Raman and XRD data. Interestingly, the temperature-dependent thermal expansion behavior was found to be independent of do** when measured in N2 within the measurement resolution, yielding negative thermal expansion values of approximately − 0.6, − 0.7 and − 0.7% for BaTiO3–2Nb, BaTiO3, and BaTiO3–2Fe, respectively. With an increase in oxygen partial pressure in samples characterized in air, however, a significant increase in magnitude of the irreversible contribution were observed, where remanent thermal expansion values of − 0.8, − 1.1 and − 1.3% were found for BaTiO3–2Nb, BaTiO3, and BaTiO3–2Fe, respectively.
The observed remanent change in the thermal expansion in N2, which was found for all compositions, clearly indicates that oxygen exchange with the surrounding atmosphere is not the only factor contributing to volumetric change. In addition, this effect is apparently independent of the dopant concentration in N2, within the resolution of these measurements, strongly suggesting the important role of a local redistribution of elements and defects. However, with the increase in the oxygen partial pressure, all compositions display an increase in the remanent thermal expansion value, demonstrating that oxygen reincorporation is also a contribution to the observed thermal shrinkage in AD films. Here, deviations between air and N2 annealed samples began at approximately 200–250 °C, in agreement with both XRD and Raman spectroscopy data, considerably lower than previous molecular dynamics models by Dawson [104] and experiments on single crystal BaTiO3 by Kessel et al. [105], that showed significant oxygen exchange is not expected below 650 °C. Importantly, this increase in remanent thermal expansion was found to become larger with an increase in oxygen vacancy concentration through do**. Considering the potential occurrence of a space charge effect, creating an electron-rich barrier layer at the grain boundaries, [57] it was shown by Watanabe et al. [100] that the presence of space charges along the grain boundary effectively blocks oxygen diffusion into the grains. Therefore, the space charge effect must be either negligible, which may be attributable to the grain size of AD films reducing the space charge effect in comparable size regions, [57] or there is a different conduction path, such as low-density amorphous regions as reported by Cao et al. [106] on aerosol-deposited TiN layers investigated with high resolution TEM. It is important to note here that these various effects do not operate independently of one another, such that local redistribution can reduce the driving force for reincorporation and vice versa. These data, however, do show that an increase in the oxygen vacancy concentration of the starting material does play an important role in controlling the shrinkage response.
In order to separate structural and chemical effects on the thermal expansion, an ex situ annealing to 500 °C was performed in vacuum prior to thermal expansion measurement in air for BaTiO3, BaTiO3–2Nb, and BaTiO3–2Fe (Fig. 12). The aim of this measurement was to allow for local, internal processes, e.g., redistribution, to occur, but to eliminate the possibility of oxygen reincorporation from the surrounding environment. Following this, the thermal expansion response was characterized in air, which should provide a direct measurement of the contribution of oxygen reincorporation. For reference, the data for a film annealed in the air is also provided (Fig. 12a), which did not display any remanent shrinkage.
In good agreement with the previous results, the highest remanent shrinkage (− 0.5%) can be observed for the Fe-doped films (Fig. 12d), which has the highest oxygen vacancy concentration. The BaTiO3–2Nb film (Fig. 12b) shows the least amount of atmosphere dependent remanent shrinkage (− 0.1%), consistent with the reduced oxygen vacancy concentration, with undoped BaTiO3 (Fig. 12c) between these values (− 0.2%). For an undoped BaTiO3 film annealed in air instead of vacuum, the oxygen exchange process can be assumed to be complete as no deviation in thermal expansion can be observed in the subsequent heating/cooling cycle (Fig. 12a). As all internal redistribution processes are expected to have been completed during the first heating cycle in vacuum, the values observed in Fig. 12 represent a residual, chemical contribution from the reincorporation of oxygen. It is important to note that the residual shrinkage values observed during this ex situ measurement were approximately 0.1% smaller than those found during the in situ measurement, i.e., −0.4, −0.2, and −0.6% for BaTiO3, BaTiO3–2Nb, and BaTiO3–2Fe (Fig. 11). This indicates that the order in which the mechanisms occur, e.g., simultaneously or in series, has an effect on the macroscopic response. It is likely that local chemical redistribution at the grain boundary or the formation of a space charge can reduce oxygen exchange as well as grain boundary diffusion of oxygen, limiting the influence of the atmospheric partial pressure.
The atmosphere dependent thermal expansion data of doped BaTiO3 demonstrates that as-processed AD-films inherit a significant concentration of AD-process-induced defects, including oxygen vacancies, which supported by XRD, Raman spectroscopy, and TEM data revealing corresponding changes in the crystal structure and internal stress state. Accumulated point defects at the grain boundaries are suggested to be a major contributing factor to an increased electronic conductivity in as-processed AD-films, and reducing their concentration is expected to be the primary contribution to the observed increase in electrical resistivity. These observations coincide with a structural relaxation based on a reduction in unit cell volume that is clearly visible by both direct optical observation and indirect methods such as in-situ temperature XRD and Raman spectroscopy. Separating chemical and structural relaxation, however, is left with a degree of uncertainty as both mechanisms may occur simultaneously by self-diffusion of oxygen defects [100] at elevated temperatures without extrinsic atmosphere contribution.
Conclusion
In this work, we successfully demonstrated the room temperature fabrication of freestanding AD films that preserves the as-processed state of the film. With the role of the CTE mismatch between film and substrate being crucial to understand internal stress relaxation in AD films, we were able to determine the thermal expansion response of AD thick films of BaTiO3. A negative thermal expansion was observed during the first annealing cycle, which was dependent on the oxygen partial pressure of the annealing atmosphere and the oxygen vacancy concentration in the film. This data clearly demonstrated that both internal redistribution effects as well as oxygen reincorporation play an important role. Importantly, oxygen exchange was found to start at approximately 200–250 °C, far below observations in bulk BaTiO3, implying that significant oxygen mobility exists in AD films. These macroscopic measurements were supported by both in situ Raman spectroscopy and XRD investigations that revealed irreversible temperature-dependent structural changes during the heating, concurrent with the ordering and tilting of the crystallites. Ex situ TEM, however, did not reveal a change in crystallite size. This work provides important information on influence of low temperature annealing of room temperature deposited ferroelectric films but also highlights the necessity to study structural relaxation mechanisms in AD films at grain boundary level.
References
Akedo J, Ichiki M, Kikuchi K, Maeda R (1998) Jet molding system for realization of three-dimensional micro-structures. Sens Actuators Phys 69:106–112. https://doi.org/10.1016/S0924-4247(98)00059-4
Hanft D, Exner J, Schubert M, Stöcker T, Fuierer P, Moos R (2015) An overview of the aerosol deposition method: process fundamentals and new trends in materials applications. J Ceram Sci Technol 6:147–181. https://doi.org/10.4416/JCST2015-00018
Akedo J (2008) Room temperature impact consolidation (RTIC) of fine ceramic powder by aerosol deposition method and applications to microdevices. J Therm Spray Technol 17:181–198. https://doi.org/10.1007/s11666-008-9163-7
Akedo J, Lebedev M (2000) Piezoelectric properties and poling effect of Pb(Zr, Ti)O3 thick films prepared for microactuators by aerosol deposition. Appl Phys Lett 77:1710–1712. https://doi.org/10.1063/1.1309029
Akedo J (2020) Room temperature impact consolidation and application to ceramic coatings: aerosol deposition method. J Ceram Soc Jpn 128:101–116. https://doi.org/10.2109/jcersj2.19196
Sadl M, Tomc U, Ursic H (2021) Investigating the feasibility of preparing metal-ceramic multi-layered composites using only the aerosol-deposition technique. Materials. https://doi.org/10.3390/ma14164548
Schubert M, Hanft D, Nazarenus T, Exner J, Schubert M, Nieke P, Glosse P, Leupold N, Kita J, Moos R (2019) Powder aerosol deposition method—novel applications in the field of sensing and energy technology. Funct Mater Lett 12:1930005. https://doi.org/10.1142/S1793604719300056
Khansur NH, Eckstein U, Benker L, Deisinger U, Merle B, Webber KG (2018) Room temperature deposition of functional ceramic films on low-cost metal substrate. Ceram Int 44:16295–16301. https://doi.org/10.1016/j.ceramint.2018.06.027
Imanaka Y, Akedo J (2010) Embedded capacitor technology using aerosol deposition. Int J Appl Ceram Technol 7:E23–E32. https://doi.org/10.1111/j.1744-7402.2009.02359.x
Hatono H, Ito T, Matsumura A (2007) Application of BaTiO 3 film deposited by aerosol deposition to decoupling capacitor. Jpn J Appl Phys 46:6915–6919. https://doi.org/10.1143/JJAP.46.6915
Imanaka Y, Akedo J (2007) Microwave capacitor film using aerosol deposition and its application. Earozoru Kenkyu 22:14–19. https://doi.org/10.11203/jar.22.14
Kim E-S, Liang J-G, Wang C, Cho M-Y, Oh J-M, Kim N-Y (2019) Inter-digital capacitors with aerosol-deposited high-K dielectric layer for highest capacitance value in capacitive super-sensing applications. Sci Rep 9:680. https://doi.org/10.1038/s41598-018-37416-7
Sun J, Kanungo B, Cho T, Zhang Y (2017) Aerosol deposition coating for semiconductor chamber components. US Patent US9708713B2
Suzuki M, Akedo J, Takashi T (2018) Method for producing lithium solid state battery. US Patent US20180090785A1
Echigo M, Ohnishi H, Manabe K, Yamazak O, Minami K, Akedo J, Suzuki T (2019) Electrochemical element, solid oxide fuel cell, and methods for producing the same. US Patent US10347929B2
Schubert M, Exner J, Moos R (2014) Influence of carrier gas composition on the stress of Al2O3 coatings prepared by the aerosol deposition method. Mater Basel Switz. https://doi.org/10.3390/ma7085633
Baba S, Akedo J (2005) Thickness dependence of aerosol-deposited Pb(Zr, Ti)O3 films on stainless-steel sheet annealed by CO2 laser radiation. J Cryst Growth 275:e1247–e1252. https://doi.org/10.1016/j.jcrysgro.2004.11.148
Khansur NH, Eckstein U, Riess K, Martin A, Drnec J, Deisinger U, Webber KG (2018) Synchrotron x-ray microdiffraction study of residual stresses in BaTiO3 films deposited at room temperature by aerosol deposition. Scr Mater 157:86–89. https://doi.org/10.1016/j.scriptamat.2018.07.045
Hoshina T, Furuta T, Kigoshi Y, Hatta S, Horiuchi N, Takeda H, Tsurumi T (2010) Size effect of nanograined BaTiO3 ceramics fabricated by aerosol deposition method. Jpn J Appl Phys. https://doi.org/10.1143/JJAP.49.09MC02
Saunders R, Johnson SD, Schwer D, Patterson EA, Ryou H, Gorzkowski EP (2021) A self-consistent scheme for understanding particle impact and adhesion in the aerosol deposition process. J Therm Spray Technol 30:523–541. https://doi.org/10.1007/s11666-021-01164-4
Park C-K, Lee S, Lim J-H, Ryu J, Choi D, Jeong D-Y (2018) Nano-size grains and high density of 65PMN-35PT thick film for high energy storage capacitor. Ceram Int 44:20111–20114. https://doi.org/10.1016/j.ceramint.2018.07.303
Ryu J, Choi J-J, Hahn B-D, Park D-S, Yoon W-H, Kim K-H (2007) Fabrication and ferroelectric properties of highly dense lead-free piezoelectric (K0.5Na0.5) NbO3 thick films by aerosol deposition. Appl Phys Lett 90:152901. https://doi.org/10.1063/1.2720751
Hahn BD, Park DS, Choi JJ, Ryu JH, Yoon WH, Kim DY (2007) Influence of Zr/Ti ratio on electrical properties of PZT thick films deposited by aerosol deposition process. In: 2007 Sixteenth IEEE International Symposium on the Applications of Ferroelectrics. 457–459 https://doi.org/10.1109/ISAF.2007.4393297
Lee J, Lee S, Choi M-G, Kang SB, Lim J-H, Kim H-J, Jeong D-Y, Kong Y-M, Lee J-P (2015) Structural and ferroelectric properties of (K, Na, Li)(Nb, Ta)O3—CaZrO3 thick films by aerosol deposition. J Korean Phys Soc 66:1101–1105. https://doi.org/10.3938/jkps.66.1101
Park C-K, Kang D-K, Lee S-H, Kong Y-M, Jeong D-Y (2017) Phase evolution and electrical properties of pzt films by aerosol-deposition method. J Korean Inst Electr Electron Mater Eng 30:541–545. https://doi.org/10.4313/JKEM.2017.30.9.541
Exner J, Nazarenus T, Hanft D, Kita J, Moos R (2020) What happens during thermal post-treatment of powder aerosol deposited functional ceramic films? Explanations based on an experiment-enhanced literature survey. Adv Mater. https://doi.org/10.1002/adma.201908104
Popovici D, Tsuda H, Akedo J (2009) Postdeposition annealing effect on (Ba0.6,Sr0.4)TiO3 thick films deposited by aerosol deposition method. J Appl Phys 105(6):061638. https://doi.org/10.1063/1.3086197
Yao Z, Wang C, Li Y, Kim H-K, Kim N-Y (2014) Effects of starting powder and thermal treatment on the aerosol deposited BaTiO3 thin films toward less leakage currents. Nanoscale Res Lett. https://doi.org/10.1186/1556-276X-9-435
Schubert M, Münch C, Schuurman S, Poulain V, Kita J, Moos R (2018) Thermal treatment of aerosol deposited NiMn2O4 NTC thermistors for improved aging stability. Sensors. https://doi.org/10.3390/s18113982
Kawakami Y, Watanabe M, Arai K-I, Sugimoto S (2016) Piezoelectric Properties and microstructure of BaTiO3 films on heat-resistant stainless-steel substrates deposited using aerosol deposition. Trans Mater Res Soc Jpn 41:279–283. https://doi.org/10.14723/tmrsj.41.279
Sadl M, Condurache O, Bencan A, Dragomir M, Prah U, Malic B, Deluca M, Eckstein U, Hausmann D, Khansur NH, Webber KG, Ursic H (2021) Energy-storage-efficient 0.9Pb(Mg1/3Nb2/3)O3–0.1PbTiO3 thick films integrated directly onto stainless steel. Acta Mater 221:117403. https://doi.org/10.1016/j.actamat.2021.117403
Furuta T, Hatta S, Kigoshi Y, Hoshina T, Takeda H, Tsurumi T (2011) Dielectric properties of nanograined BaTiO3 ceramics fabricated by aerosol deposition method. Trans Tech Publ Ltd. https://doi.org/10.4028/www.scientific.net/KEM.485.183
Ryu J, Priya S, Park C-S, Kim K-Y, Choi J-J, Hahn B-D, Yoon W-H, Lee B-K, Park D-S, Park C (2009) Enhanced domain contribution to ferroelectric properties in freestanding thick films. J Appl Phys 106:024108. https://doi.org/10.1063/1.3181058
Sarobol P, Chandross M, Holmes TD, Miller AS, Kotula PG, Hall AC (2016) Aerosol deposition: room temperature solid-state deposition of ceramics. Sandia National Laboratories, SAND2016-2870
Han G, Ryu J, Yoon W-H, Choi J-J, Hahn B-D, Kim J-W, Park D-S, Ahn C-W, Priya S, Jeong D-Y (2011) Stress-controlled Pb(Zr0.52 Ti 0.48)O3 thick films by thermal expansion mismatch between substrate and Pb(Zr 0.52 Ti 0.48)O3 film. J Appl Phys 110:124101. https://doi.org/10.1063/1.3669384
Lee DK, Kim S, Oh S, Choi J-Y, Lee J-L, Yu HK (2017) Water-soluble epitaxial NaCl thin film for fabrication of flexible devices. Sci Rep 7:8716. https://doi.org/10.1038/s41598-017-09603-5
Thema FT, Beukes P, Ngom BD, Manikandan E, Maaza M (2015) Free standing diamond-like carbon thin films by PLD for laser based electrons/protons acceleration. J Alloys Compd 648:326–331. https://doi.org/10.1016/j.jallcom.2015.06.277
Miyamoto Y, Fujii Y, Yamano M, Harigai T, Suda Y, Takikawa H, Kawano T, Nishiuchi M, Sakaki H, Kondo K (2016) Preparation of self-supporting Au thin films on perforated substrate by releasing from water-soluble sacrificial layer. Jpn J Appl Phys 55:07LE05. https://doi.org/10.7567/JJAP.55.07LE05
Min K, Jeon WJ, Kim Y, Choi J-Y, Yu HK (2018) Spontaneous nano-gap formation in Ag film using NaCl sacrificial layer for Raman enhancement. Nanotechnology 29:105502. https://doi.org/10.1088/1361-6528/aaa746
Graham C, Frances MMM, Maniyara RA, Wen Y, Mazumder P, Pruneri V (2020) NaCl substrates for high temperature processing and transfer of ultrathin materials. Sci Rep 10:7253. https://doi.org/10.1038/s41598-020-64313-9
Khansur NH, Eckstein U, Li Y, Hall DA, Kaschta J, Webber KG (2019) Revealing the effects of aerosol deposition on the substrate-film interface using NaCl coating. J Am Ceram Soc 102:5763–5771. https://doi.org/10.1111/jace.16489
Lim K-W, Peddigari M, Park CH, Lee HY, Min Y, Kim J-W, Ahn C-W, Choi J-J, Hahn B-D, Choi J-H, Park D-S, Hong J-K, Yeom J-T, Yoon W-H, Ryu J, Yi SN, Hwang G-T (2019) A high output magneto-mechano-triboelectric generator enabled by accelerated water-soluble nano-bullets for powering a wireless indoor positioning system. Energy Environ Sci 12:666–674. https://doi.org/10.1039/C8EE03008A
Brzozowski E, Castro MS, Foschini CR, Stojanovic B (2002) Secondary phases in Nb-doped BaTiO3 ceramics. Ceram Int 28:773–777. https://doi.org/10.1016/S0272-8842(02)00042-1
Cui B, Yu P, Tian J, Guo H, Chang Z (2007) Preparation and characterization of niobium-doped barium titanate nanocrystalline powders and ceramics. Mater Sci Eng A 454–455:667–672. https://doi.org/10.1016/j.msea.2006.11.115
Dechakupt T, Tangsritrakul J, Ketsuwan P, Yimnirun R (2011) Microstructure and electrical properties of niobium doped barium titanate ceramics. Ferroelectrics 415:141–148. https://doi.org/10.1080/00150193.2011.577386
Mishra A (2012) Iron-doped BaTiO3: Influence of iron on physical properties. Int J Mater Sci Appl 1:14. https://doi.org/10.11648/j.ijmsa.20120101.13
Paunovic V, Mitic VV, Djordjevic M, Prijic Z (2020) Niobium do** effect on BaTiO3 structure and dielectric properties. Ceram Int 46:8154–8164. https://doi.org/10.1016/j.ceramint.2019.12.043
Singh D, Dixit A, Dobal PS (2021) Ferroelectricity and ferromagnetism in Fe-doped barium titanate ceramics. Ferroelectrics 573:63–75. https://doi.org/10.1080/00150193.2021.1890464
Stojanovic BD (2002) Microstructure of doped barium titanate prepared from polymeric precursors. Bol Soc Esp Cerám Vidr 41:190–193
Yaseen H, Baltianski S, Tsur Y (2006) Effect of incorporating method of niobium on the properties of doped barium titanate ceramics. J Am Ceram Soc 89:1584–1589. https://doi.org/10.1111/j.1551-2916.2006.00966.x
Veber A, Cicconi MR, Reinfelder H, Ligny D (2018) Combined differential scanning calorimetry, Raman and Brillouin spectroscopies: a multiscale approach for materials investigation. Anal Chim Acta 998:37–44. https://doi.org/10.1016/j.aca.2017.09.045
Robins LH, Kaiser DL, Rotter LD, Schenck PK, Stauf GT, Rytz D (1994) Investigation of the structure of barium titanate thin films by Raman spectroscopy. J Appl Phys 76:7487–7498. https://doi.org/10.1063/1.357978
Deng X, Wang X, Wen H, Kang A, Gui Z, Li L (2006) Phase transitions in nanocrystalline barium titanate ceramics prepared by spark plasma sintering. J Am Ceram Soc 89:1059–1064. https://doi.org/10.1111/j.1551-2916.2005.00836.x
Lee H-W, Moon S, Choi C-H, Kim DK (2012) Synthesis and size control of tetragonal barium titanate nanopowders by facile solvothermal method. J Am Ceram Soc 95:2429–2434. https://doi.org/10.1111/j.1551-2916.2012.05085.x
Cicconi MR, Khansur NH, Eckstein U, Werr F, Webber K, De Ligny D (2019) Determining the local pressure during aerosol deposition using glass memory. J Am Ceram Soc 103:2443–2452. https://doi.org/10.1111/jace.16947
Akedo J (2006) Aerosol deposition of ceramic thick films at room temperature: densification mechanism of ceramic layers. J Am Ceram Soc 89:1834–1839. https://doi.org/10.1111/j.1551-2916.2006.01030.x
Wu Y, Bowes PC, Baker JN, Irving DL (2020) Influence of space charge on the conductivity of nanocrystalline SrTiO3. J Appl Phys 128:014101. https://doi.org/10.1063/5.0008020
Enriquez E, Chen A, Harrell Z, Lü X, Dowden P, Koskelo N, Janoschek M, Chen C, Jia Q (2016) Oxygen vacancy-driven evolution of structural and electrical properties in SrFeO3-δ thin films and a method of stabilization. Appl Phys Lett 109:141906. https://doi.org/10.1063/1.4964384
Zych Ł, Wajler A, Kwapiszewska A (2016) Sintering behaviour of fine barium titanate (BaTiO3) powders consolidated with the pressure filtration method. J Ceram Sci Technol 7:277–287. https://doi.org/10.4416/JCST2016-00016
He Y (2004) Heat capacity, thermal conductivity, and thermal expansion of barium titanate-based ceramics. Thermochim Acta 419:135–141. https://doi.org/10.1016/j.tca.2004.02.008
Bland JA (1959) The thermal expansion of cubic barium titanate (BaTiO3) from 350 °C to 1050 °C. Can J Phys 37:417–421
Sawada S, Shirane G (1949) Specific heat and thermal expansion of BaTiO3. J Phys Soc Jpn 4:52–56. https://doi.org/10.1143/JPSJ.4.52
Han M, Rong Y, Li Q, **ng X, Kang L (2015) Thermal expansion of nano-sized BaTiO3. CrystEngComm 17:1944–1951. https://doi.org/10.1039/c4ce02328e
Ablitt C, Craddock S, Senn MS, Mostofi AA, Bristowe NC (2017) The origin of uniaxial negative thermal expansion in layered perovskites. Npj Comput Mater 3:1–8. https://doi.org/10.1038/s41524-017-0040-0
Chen J, Hu L, Deng J, **ng X (2015) Negative thermal expansion in functional materials: controllable thermal expansion by chemical modifications. Chem Soc Rev 44:3522–3567. https://doi.org/10.1039/c4cs00461b
Wang F, **e Y, Chen J, Fu H, **ng X (2013) First-principles study on negative thermal expansion of PbTiO3. Appl Phys Lett 103:221901. https://doi.org/10.1063/1.4833280
Chen J, **ng XR, Liu GR, Li JH, Liu YT (2006) Structure and negative thermal expansion in the PbTiO3–BiFeO3 system. Appl Phys Lett 89:101914. https://doi.org/10.1063/1.2347279
Chen J, Nittala K, Forrester JS, Jones JL, Deng J, Yu R, **ng X (2011) The role of spontaneous polarization in the negative thermal expansion of tetragonal PbTiO3-based compounds. J Am Chem Soc 133:11114–11117. https://doi.org/10.1021/ja2046292
Hu P, Chen J, Sun X, Deng J, Chen X, Yu R, Qiao L, **ng X (2009) Zero thermal expansion in (1–x)PbTiO3–xBi(Mg, Ti)1/2O3 piezoceramics. J Mater Chem 19:1648. https://doi.org/10.1039/b816822a
**ng J, Radovic M, Muliana A (2016) Thermal properties of BaTiO3/Ag composites at different temperatures. Compos Part B Eng 90:287–301. https://doi.org/10.1016/j.compositesb.2015.12.014
Sarobol P, Chandross M, Carroll JD, Mook WM, Bufford DC, Boyce BL, Hattar K, Kotula PG, Hall AC (2016) Room temperature deformation mechanisms of alumina particles observed from in situ micro-compression and atomistic simulations. J Therm Spray Technol 25:82–93. https://doi.org/10.1007/s11666-015-0295-2
Ask A, Forest S, Appolaire B, Ammar K (2020) Microstructure evolution in deformed polycrystals predicted by a diffuse interface cosserat approach. Adv Model Simul Eng Sci. https://doi.org/10.1186/s40323-020-00146-5
Akedo J, Lebedev M, Baba S (2003) Aerosol deposition method for preparation of lead zirconate titanate thick layer at low temperature –improvement of electrical properties by irradiation of fast atom beam and plasma–. Jpn J Appl Phys 42:5931–5935. https://doi.org/10.1143/JJAP.42.5931
Chiang Y-M, Takagi T (1990) Grain-boundary chemistry of barium titanate and strontium titanate: i, high-temperature equilibrium space charge. J Am Ceram Soc 73:3278–3285. https://doi.org/10.1111/j.1151-2916.1990.tb06450.x
Swallow JG, Kim JJ, Maloney JM, Chen D, Smith JF, Bishop SR, Tuller HL, Van Vliet KJ (2017) Dynamic chemical expansion of thin-film non-stoichiometric oxides at extreme temperatures. Nat Mater 16:749–754. https://doi.org/10.1038/nmat4898
Waser R (1994) Charge transport in perovskite-type titanates: space charge effects in ceramics and films. Ferroelectrics 151:125–131. https://doi.org/10.1080/00150199408244732
Navickas E, Huber TM, Chen Y, Hetaba W, Holzlechner G, Rupp G, Stöger-Pollach M, Friedbacher G, Hutter H, Yildiz B, Fleig J (2015) Fast oxygen exchange and diffusion kinetics of grain boundaries in Sr-doped LaMnO3 thin films. Phys Chem Chem Phys PCCP 17:7659–7669. https://doi.org/10.1039/c4cp05421k
Smith MB, Page K, Siegrist T, Redmond PL, Walter EC, Seshadri R, Brus LE, Steigerwald ML (2008) Crystal structure and the paraelectric-to-ferroelectric phase transition of nanoscale BaTiO3. J Am Chem Soc 130:6955–6963. https://doi.org/10.1021/ja0758436
Filipovic S, Andjelkovic LJ, Jeremic D, Vulic P, Nikolic AS, Markovic S, Paunovic V, Levic S, Pavlovic VB (2020) Structure and properties of nanocrystalline tetragonal BaTiO3 prepared by combustion solid state synthesis. Sci Sinter 52:257–268. https://doi.org/10.2298/SOS2003257F
Al-Naboulsi T, Boulos M, Tenailleau C, Dufour P, Zakhour M, Guillemet-Fritsch S (2016) Elaboration and characterization of barium titanate powders obtained by the mechanical activation of barium nitrate and titanate oxide, and electrical properties of the ceramics sintered by SPS. J Ceram Process Res 17:870–875
Shiratori Y, Pithan C, Dornseiffer J, Waser R (2007) Raman scattering studies on nanocrystalline BaTiO3 Part I—isolated particles and aggregates. J Raman Spectrosc 38:1288–1299. https://doi.org/10.1002/jrs.1764
Shiratori Y, Pithan C, Dornseiffer J, Waser R (2007) Raman scattering studies on nanocrystalline BaTiO3 Part II—consolidated polycrystalline ceramics. J Raman Spectrosc 38:1300–1306. https://doi.org/10.1002/jrs.1763
Hoshina T, Kakemoto H, Tsurumi T, Wada S, Yashima M (2006) Size and temperature induced phase transition behaviors of barium titanate nanoparticles. J Appl Phys. https://doi.org/10.1063/1.2179971
Manika GC, Andrikopoulos KS, Psarras GC (2020) On the ferroelectric to paraelectric structural transition of BaTiO3 micro-/nanoparticles and their epoxy nanocomposites. Mol Basel Switz. https://doi.org/10.3390/molecules25112686
Hayashi H, Nakamura T, Ebina T (2013) In-situ Raman spectroscopy of BaTiO3 particles for tetragonal-cubic transformation. J Phys Chem Solids 74:957–962. https://doi.org/10.1016/j.jpcs.2013.02.010
Sakashita T, Deluca M, Yamamoto S, Chazono H, Pezzotti G (2007) Stress dependence of the Raman spectrum of polycrystalline barium titanate in presence of localized domain texture. J Appl Phys. https://doi.org/10.1063/1.2747217
Schader FH, Aulbach E, Webber KG, Rossetti GA (2013) Influence of uniaxial stress on the ferroelectric-to-paraelectric phase change in barium titanate. J Appl Phys 113:174103. https://doi.org/10.1063/1.4799581
Forsbergh PW (1954) Effect of a two-dimensional pressure on the curie point of barium titanate. Phys Rev 93:686–692. https://doi.org/10.1103/PhysRev.93.686
Schader FH, Khakpash N, Rossetti GA, Webber KG (2017) Phase transitions in BaTiO3 under uniaxial compressive stress: experiments and phenomenological analysis. J Appl Phys 121:064109. https://doi.org/10.1063/1.4976060
Daneshian B, Gaertner F, Assadi H, Hoeche D, Weber W, Klassen T (2021) Size effects of brittle particles in aerosol deposition—molecular dynamics simulation. J Therm Spray Technol. https://doi.org/10.1007/s11666-020-01149-9
Ogawa H (2005) Molecular dynamics simulation on the single particle impacts in the aerosol deposition process. Mater Trans 46:1235–1239. https://doi.org/10.2320/matertrans.46.1235
Temitope Oyinbo S, Jen T-C (2020) Molecular dynamics simulation of dislocation plasticity mechanism of nanoscale ductile materials in the cold gas dynamic spray process. Coatings 10:1079. https://doi.org/10.3390/coatings10111079
Brunner D, Taeri-Baghbadrani S, Sigle W, Rühle M (2001) (2001) Surprising results of a study on the plasticity in strontium titanate. J Am Ceram Soc 84:1161–1163. https://doi.org/10.1111/j.1151-2916.2001.tb00805.x
Höfling M, Zhou X, Riemer LM, Bruder E, Liu B, Zhou L, Groszewicz PB, Zhuo F, Xu B-X, Durst K, Tan X, Damjanovic D, Koruza J, Rödel J (2021) Control of polarization in bulk ferroelectrics by mechanical dislocation imprint. Science 372:961–964
Wang L, Teng J, Liu P, Hirata A, Ma E, Zhang Z, Chen M, Han X (2014) Grain rotation mediated by grain boundary dislocations in nanocrystalline platinum. Nat Commun 5:4402. https://doi.org/10.1038/ncomms5402
Akedo J, Lebedev M (1999) Microstructure and electrical properties of lead zirconate titanate (Pb(Zr52/Ti48)O3) thick films deposited by aerosol deposition method. Jpn J Appl Phys 38:5397–5401. https://doi.org/10.1143/JJAP.38.5397
Hu J, Shen Z (2012) Grain growth by multiple ordered coalescence of nanocrystals during spark plasma sintering of SrTiO3 nanopowders. Acta Mater 60:6405–6412. https://doi.org/10.1016/j.actamat.2012.08.027
Serrano S, Duque C, Medina P, Stashans A (2012) Oxgen-vacancy defects in PbTiO3 and BaTiO3 crystals: a quantum chemical study. In: Krumins A, Millers D, Muzikante I, Sternbergs A, Zauls V (Eds) SPIE. 287–294 https://doi.org/10.1117/12.515777
Zulueta YA, Dawson JA, Leyet Y, Guerrero F, Anglada-Rivera J, Nguyen MT (2016) Influence of titanium and oxygen vacancies on the transport and conducting properties of barium titanate. Phys Status Solidi B 253:345–350. https://doi.org/10.1002/pssb.201552366
Watanabe K, Sakaguchi I, Hishita S, Ohashi N, Haneda H (2011) Visualization of grain boundary as blocking layer for oxygen tracer diffusion and a proposed defect model in non doped BaTiO 3 ceramics. Appl Phys Exp 4:055801. https://doi.org/10.1143/APEX.4.055801
Liu J, Liu L, Zhang J, ** L, Wang D, Wei J, Ye Z-G, Jia C-L (2020) Charge effects in donor-doped perovskite ferroelectrics. J Am Ceram Soc 103:5392–5399. https://doi.org/10.1111/jace.17270
Morozov MI, Einarsrud M-A, Tolchard JR, Geiger PT, Webber KG, Damjanovic D, Grande T (2015) In-situ structural investigations of ferroelasticity in soft and hard rhombohedral and tetragonal PZT. J Appl Phys 118:164104
Zhang XL, Chen ZX, Cross LE, W.A. (1983) Schulze, dielectric and piezoelectric properties of modified lead titanate zirconate ceramics from 4.2 to 300 K. J Mater Sci 18:968–972. https://doi.org/10.1007/BF00551962
Dawson JA (2020) Dynamical insights into oxygen diffusion in BaTiO3 and SrTiO3. Phys Status Solidi B 257:1900422. https://doi.org/10.1002/pssb.201900422
Kessel M, Souza RA, Martin M (2015) Oxygen diffusion in single crystal barium titanate. Phys Chem Chem Phys PCCP 17:12587–12597. https://doi.org/10.1039/c5cp01187f
Cao F, Park H, Heo J, Kwon J, Lee C (2013) Effect of process gas flow on the coating microstructure and mechanical properties of vacuum kinetic-sprayed TiN layers. J Therm Spray Technol 22:1109–1119. https://doi.org/10.1007/s11666-013-9963-2
Acknowledgements
U.E., N.H.K., and K.G.W. gratefully acknowledge financial support for this work from the Deutsche Forschungsgemeinschaft (DFG) under WE4972/2 and GRK2495F. H.U., M.Š. and M.D. acknowledge the Slovenian Research Agency (project J2-3058, SLO-DE bilateral project BI-DE/20-21-012, young researcher project, research core funding P2-0105). M.D. acknowledges the research core funding P2-0105 and European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 101031415. This work was also partially supported by JSPS KAKENHI Grant Number JP19KK0124.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Till Froemling.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eckstein, U., Khansur, N.H., Bergler, M. et al. Room temperature deposition of freestanding BaTiO3 films: temperature-induced irreversible structural and chemical relaxation. J Mater Sci 57, 13264–13286 (2022). https://doi.org/10.1007/s10853-022-07467-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-022-07467-3



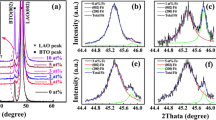



 indicates reflections originating from the SUS304 substrate. Bulk BaTiO3 given as reference to AD-induced changes
indicates reflections originating from the SUS304 substrate. Bulk BaTiO3 given as reference to AD-induced changes







