Abstract
Rapid solidification of a FeSi stoichiometric intermetallic compound was studied using the glass fluxing method. The recalescence process was in situ observed for the first time by the infrared high-speed high-resolution cameras. The dendrite envelope was found to be non-isothermal during the recalescence process. The growth velocity increased first, then decreased and finally held nearly constant. The average dendrite growth velocity for the recalescence process increased monotonically with undercooling and was described well by the dendrite growth model for a stoichiometric intermetallic compound. At low undercooling, the microstructure transition from coarse dendrites to refined grains was consistent with the dendrite fragmentation model and the chemical superheating model. At high undercooling, dendrite deformation triggers stress accumulation upon rapid solidification, thus providing the driving force for recrystallization. However, there were no evidences for annealing twins accompanied by recrystallization as well as random textures due to recrystallization nucleation. From the local misorientation map, the grain refinement mechanism was suggested to be stress-induced dendrite fragmentation. This study is helpful for not only understanding the intrinsic mechanisms of microstructure transitions in theory but also controlling microstructures and performance of intermetallic compounds in practical applications.















Similar content being viewed by others
Notes
The compound FeSi crystallizes in cubic B20 structure, and the corresponding space group is P213. Each primitive unit cell in the B20 structure contains eight atoms (four Fe atoms and four Si atoms), and any one of the atoms is surrounded by seven nearest neighbor atoms of the opposite element. Both Fe and Si atoms occupy the Wyckoff position of 4a (x, x, x). The coordinates of the position are (u, u, u), (0.5 + u, 0.5 − u, −u), (−u, 0.5 + u, 0.5 − u), (0.5 − u, −u, 0.5 − u), where uFe = 0.173a and uSi = 0.842a with a = 449 pm [38].
This camera is equipped with a refrigerated mercury cadmium telluride detector with the ability to produce a clear thermal image of 640 × 512 pixels and a temperature difference of less than 25 mK.
The second recalescence event is so weak that it cannot be captured by the high-speed cameras.
Because we aim to study grain refinement mechanisms and dendrite growth kinetics of the FeSi intermetallic compound, further identifying the crystal structure of the secondary phase is not shown here.
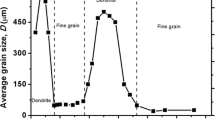
It should be noted that the thermal undercooling ΔTT adopted here is for an isothermal dendrite under a steady-state condition. The dendrite envelope recorded by the infrared high-speed camera shows that it is under a non-steady-state condition and is a non-isothermal one; see Fig. 4.
\( \Delta t_{\text{pl}} \) is defined in the cooling curves as the time difference between the highest recalescence temperature and the inflection point after recalescence. It should be noted that if the overall solidification is adopted, the cooling history can be predicted, according to which \( \Delta t_{\text{pl}} \) can be obtained theoretically [22]. In this case, dendrite fragmentation in undercooled melts can be described in a self-consistent way.
A plenty of sub-grains were also found within the coarse grains for rapid solidification in an undercooled CoNi equiatomic alloy [55].
References
Liu CT, Stiegler JO (1989) Ductile ordered intermetallic alloys. Science 226:636–642
Yang T, Zhao YL, Tong Y et al (2018) Multicomponent intermetallic nanoparticles and superb mechanical behaviors of complex alloys. Science 362:933–937
Hornfeck W, Kobold R, Kolbe M, Conrad M, Herlach D (2018) Quasicrystal nucleation and Z module twin growth in an intermetallic glass-forming system. Nat Commun 9:4054
Zhang Z, Hu XW, Jiang XX, Li YL (2019) Influences of Mono-Ni(P) and Dual-Cu/Ni(P) plating on the interfacial microstructure evolution of solder joints. Metall Mater Trans A 50A:480–492
Wei XX, Xu X, Kang JL, Ferry M, Li JF (2017) Phase selection in solidification of undercooled Co–B alloys. J Mater Sci Technol 33:352–358
Yang C, Gao J, Zhang YK, Kolbe M, Herlach DM (2011) New evidence for the dual origin of anomalous eutectic structures in undercooled Ni–Sn alloys: in situ observations and EBSD characterization. Acta Mater 29:3915–3926
Ahmad R, Cochrane RF, Mullis AM (2012) Disorder trap** during the solidification of βNi3Ge from its deeply undercooled melt. J Mater Sci 47:2411–2420. https://doi.org/10.1007/s10853-011-6062-y
Koch CC (1998) Rapid solidification of intermetallic. Int Mater Rev 33:201–219
Chen Z, Zhang Y, Wang S, Zhang JY, Zhang P (2018) Microstructure and mechanical properties of undercooled Fe80C5Si10B5 eutectic alloy. J Alloys Compd 747:846–853
Herlach DM (1994) Non-equilibrium solidification of undercooled metallic melts. Mater Sci Eng, R 12:177–272
Wang S, Chen Z, Feng LC, Liu YY et al (2018) Nano-phase formation accompanying phase separation in undercooled CoCrCuFeNi-3 at.% Sn high entropy alloy. Mater Charact 144:516–521
Liu L, Wei XX, Huang QS, Li JF et al (2012) Anomalous eutectic formation in the solidification of undercooled Co–Sn alloys. J Cryst Growth 358:20–28
Eckler K, Cochrane RF, Herlach DM, Feuerbacher B (1992) Evidence for a transition from diffusion-controlled to thermally controlled solidification in metallic alloys. Phys Rev B 45:5019–5022
Hartmann H, Hollandmoritz D, Galenko PK et al (2009) Evidence of the transition from ordered to disordered growth during rapid solidification of an intermetallic phase. EPL 87:40007
Assadi H, Barth M, Greer AL, Herlach DM (1998) Kinetics of solidification of intermetallic compounds in the Ni–Al system. Acta Mater 46:491–500
Assadi H, Greer AL (1996) Site-ordering effects on element partitioning during rapid solidification of alloys. Nature 383:150–152
Assadi H, Reutzel S, Herlach DM (2006) Kinetics of solidification of B2 intermetallic phase in the Ni–Al system. Acta Mater 54:2793–2800
Assadi H, Oghabi M, Herlach DM (2009) Influence of ordering kinetics on dendritic growth morphology. Acta Mater 57:1639–1647
Herlach DM (2015) Dendrite growth kinetics in undercooled melts of intermetallic compounds. Crystals 5:355–375
Wang J, Guo T, Li JS, Jia WJ, Kou HC (2018) Microstructure and mechanical properties of non-equilibrium solidified CoCrFeNi high entropy alloy. Mater Chem Phys 210:192–196
Moir SA, Eckler K, Herlach DM (1998) Evolution of microstructure resulting from dendrite growth in undercooled Fe–Cr–Ni melts. Acta Metall 46:4029–4036
Wang HF, Liu F, Tan YM (2011) Modeling grain refinement for undercooled single-phase solid-solution alloys. Acta Mater 59:4787–4797
Cochrane RF, Battersby SE, Mullis AM (2001) The mechanisms for spontaneous grain refinement in undercooled Cu–O and Cu–Sn melts. Mater Sci Eng, A 304:262–266
Schwarz M, Karma A, Eckler K, Herlach DM (1994) Physical mechanism of grain refinement in solidification of undercooled melts. Phys Rev Lett 73:1380–1383
Karma A (1998) Model of grain refinement in solidification of undercooled melts. Int J Non-Equilib Pr 11:201–233
Li JF, Liu YC, Lu YL, Yang GC, Zhou YH (1998) Structural evolution of undercooled Ni–Cu alloys. J Cryst Growth 192:462–470
Li JF, Jie WQ, Yang GC, Zhou YH (2002) Solidification structure formation in undercooled Fe–Ni alloy. Acta Mater 50:1797–1807
Lu SY, Li JF, Zhou YH (2007) Grain refinement in the solidification of undercooled Ni–Pd alloys. J Cryst Growth 309:103–111
Zhou JK, Li JG (2008) Grain refinement in bulk undercooled Fe81Ga19 magnetostrictive alloy. J Alloys Compd 461:113–116
Wang HF, Liu F, Yang GC (2010) Experimental study of grain refinement mechanism in undercooled Ni–15at.% Cu alloy. J Mater Res 25:1963–1974
Castle EG, Mullis AM, Cochrane RF (2014) Evidence for an extensive, undercooling-mediated transition in growth orientation, and novel dendritic seaweed microstructures in Cu–8.9 wt% Ni. Acta Mater 66:378–387
Castle EG, Mullis AM, Cochrane RF (2014) Mechanism selection for spontaneous grain refinement in undercooled metallic melts. Acta Mater 77:76–84
Haque N, Cochrane RF, Mullis AM (2017) The role of recrystallization in spontaneous grain refinement of rapidly solidified Ni3Ge. Metall Mater Trans A 48A:5424–5431
Haque N, Cochrane RF, Mullis AM (2016) Rapid solidification morphologies in Ni3Ge: spherulites, dendrites and dense-branched fractal structures. Intermetallics 76:70–77
Haque N, Cochrane RF, Mullis AM (2018) Disorder-order morphologies in drop-tube processed Ni3Ge: dendritic and seaweed growth. J Alloys Compd 744:740–749
Lai C, Zhang JB, Zhang F et al (2017) Growth velocities and growth orientations in an undercooled melt of Ni31Si12 intermetallic compound. J Alloys Compd 712:241–249
Zhang JB, Wang HF, Zhang F, Lu XL, Zhang YC, Zhou Q (2019) Growth kinetics and grain refinement mechanisms in an undercooled melt of a CoSi intermetallic compound. J Alloys Compd 781:13–25
Salamon M, Mehrer H (1999) Diffusion in the B20-type phase FeSi. Phil Mag A 79:2137–2155
Lacaze J, Sundman B (1991) An assessment of the Fe–C–Si system. Metall Trans A 22A:2211–2223
Biswas K, Phanikumarz G, Holland-moritz D, Herlach DM, Chattopadhyayy K (2007) Disorder trap** and grain refinement during solidification of undercooled Fe-18 at% Ge melts. Phil Mag 87:3817–3837
Barth M, Wei B, Herlach DM (1995) Crystal growth in undercooled melts of the intermetallic compounds FeSi and CoSi. Phys Rev B 51:3422–3428
Wang HF, Liu F, Herlach DM (2014) Modeling the growth kinetics of a multi-component stoichiometric compound. J Mater Sci 49:1537–1543. https://doi.org/10.1007/s10853-013-7835-2
Wang H, Herlach DM, Liu RP (2014) Dendrite growth in Cu50Zr50 glass-forming melts, thermodynamics vs. kinetics. EPL 105:36001
Kessler DA, Koplik J, Levine H (1988) Pattern selection in fingered growth phenomena. Adv Phys 37:255–339
Brener EA, Mel’nikov VI (1991) Pattern selection in two-dimensional dendritic growth. Adv Phys 40:53–97
Lipton J, Kurz W, Trivedi R (1987) Rapid dendrite growth in undercooled alloys. Acta Metall 35:957–964
Zhang JB, Wang HF, Kuang WW et al (2018) Rapid solidification of non-stoichiometric intermetallic compounds: modeling and experimental verification. Acta Mater 148:86–99
Hellawell A, Liu S, Lu SZ (1997) Dendrite fragmentation and the effects of fluid flow in castings. JOM 49(3):18–20
Humphreys FJ, Hatherly M (2004) Recrystallization and related annealing phenomena, 2nd edn. Elsevier, Oxford
Dahle AK, Thevik HJ, Arnberg L, John DHS (1999) Modeling the fluid-flow-induced stress and collapse in a dendritic network. Metall Mater Trans B 30:287–293
Zhang T, Liu F, Wang HF, Yang GC (2010) Grain refinement in highly undercooled solidification of Ni85Cu15 alloy melt: direct evidence for recrystallization mechanism. Scripta Mater 63:43–46
Mullis AM, Walker DJ, Batterby SE, Cochrane RF (2001) Deformation of dendrites by fluid flow during rapid solidification. Mater Sci Eng, A 245:304–306
Dragnevski K, Mullis AM, Walker DJ, Cochrane RF (2002) Mechanical deformation of dendrites by fluid flow during the solidification of undercooled melts. Acta Mater 50:3743–3755
Kozmel T, Vural M, Tin S (2015) EBSD analysis of high strain rate application Al–Cu based alloys. Mater Sci Eng, A 630:99–106
Zhang JB, Zhang YC, Zhang F, Cui DX, Zhao YM, Wu HX, Wang XZ, Zhou Q, Wang HF (2020) Dendrite growth and grain “coarsening” in an undercooled CoNi equiatomic alloy. J Alloys Compd. https://doi.org/10.1016/j.jallcom.2019.152529
Liu N, Chen C, Chang I, Zhou PJ, Wang XJ (2018) Compositional dependence of phase selection in CoCrCu0.1FeMoNi-based high-entropy alloy. Materials 11:1290–1300
Acknowledgements
This work was done under the Natural Science Foundation of China (No. 51975474), the Science Fund for Distinguished Young Scholars from Shaanxi province (2018-JC007), the Innovation Foundation for Doctor Dissertation of Northwestern Polytechnical University (No. CX201907) and the Fundamental Research Funds for the Central Universities. The authors appreciate Dr. Vipul Bhardwaj for reading and polishing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix: Secondary solidification
Appendix: Secondary solidification
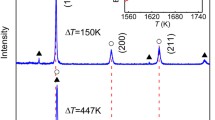
In this study, secondary solidification (i.e., eutectic solidification) happens after primary solidification of the FeSi intermetallic compound at undercooling of ΔT = 90 K, ΔT = 147 K, ΔT = 194 K and ΔT = 259 K; see Fig. 1. In a recent study on rapid solidification of a Ni31Si12 intermetallic compound in undercooled melts [36], secondary solidification was found when ΔT > 119 K. It seems that secondary solidification after primary solidification of an intermetallic compound appeared frequently in undercooled melts with a congruent composition.
Here, the kinetic phase diagram is used to illustrate the phase transition as the interface velocity increases. The kinetic phase diagram is the phase diagram for a given growth velocity, which shows the relationship between the interface temperature and compositions at a given growth velocity. For example, for the kinetic phase diagram calculated by Assadi [17], the kinetic congruent melting point of the Ni–Al alloy shifts toward to the Al-rich side. In the Co–Si kinetic phase diagram, the kinetic congruent melting point shifts toward to the Si-rich side with the increasing growth velocity [37].
Generally, the non-equilibrium interface condition has two effects on the kinetic phase diagram. These are shown schematically for a non-stoichiometric Fe–Si intermetallic compound in Fig. 16. First, the kinetic congruent melting point deviates from the equilibrium one due to disorder trap** and kinetic undercooling; see the red solid line in Fig. 16. Second, the solidus and liquidus gradually coincide due to the solute trap** effect; see the non-solid lines in Fig. 16. Therefore, the congruent composition can be within the solid–liquid two-phase zone in the kinetic phase diagrams. After primary rapid solidification of the FeSi intermetallic compound, the remained melt can be solidified into eutectics; see Figs. 7a and 16. The amount of metastable phase within the inter-dendrites can be seen from Fig. 5, which first increases and then decreases with undercooling. The metastable phase completely disappears when the undistributed solidified microstructure occurs at ΔT = 298 K. In other words, when the undercooling is high enough, complete solute trap** takes place, thus resulting in diffusionless solidification. According to the microstructure evolution of the FeSi intermetallic compound, the schematic diagram of the metastable phase formation and disappearance with the growth velocity is shown at the bottom of Fig. 16.
In the previous study of Lai et al. [36], the second phase β2-Ni3Si is a stable phase in the phase diagram. In this study, however, it is an unknown metastable phase. It should be noted that the formation of such a metastable phase is quite different from phase selection due to either thermodynamics [56] or kinetics [10]. For the latter, the mechanism can be nucleation controlled or growth controlled [10]. The result is that the stable phase is replaced by another stable phase or metastable phase. For the undercooled melts with a congruent composition, however, the intermetallic compound is always solidified primarily and the secondary phase is always a minor one.
Rights and permissions
About this article
Cite this article
Zhang, J., Zhang, F., Luo, X. et al. Rapid solidification of a FeSi intermetallic compound in undercooled melts: dendrite growth and microstructure transitions. J Mater Sci 55, 4094–4112 (2020). https://doi.org/10.1007/s10853-019-04265-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-04265-2