Abstract
To retrospectively explore the characteristics of plasma amino acids (PAAs) in children with autism spectrum disorder and their clinical association via case-control study. A total of 110 autistic and 55 healthy children were recruited from 2014 to 2018. The clinical phenotypes included severity of autism, cognition, adaptability, and regression. Compared with the control group, autistic children had significantly elevated glutamate, γ-Amino-n-butyric acid, glutamine, sarcosine, δ-aminolevulinic acid, glycine and citrulline. In contrast, their plasma level of ethanolamine, phenylalanine, tryptophan, homocysteine, pyroglutamic acid, hydroxyproline, ornithine, histidine, lysine, and glutathione were significantly lower. Elevated neuroactive amino acids (glutamate) and decreased essential amino acids were mostly distinct characteristics of PAAs of autistic children. Increased level of tryptophan might be associated with severity of autism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by impairments in social interaction and communication, repetitive behaviors and/or restricted interests(Association, 2013). The overall prevalence of ASD has been consistently increasing in recent years. The early study on autism conducted in the 1960s reported that its prevalence was about 4/10,000(Lotter, 1966), whereas the prevalence of ASD had significantly risen from 13.4 to 1000 children in 2010(CDC, 2014) to 27.9 in 2016 in the USA(Xu et al., 2019). A recent meta-analysis indicated that the prevalence of ASD in China was 26.5/10,000(Liu et al., 2018), although this was significantly lower than the reported prevalence abroad. In 2020, the prevalence of ASD among children aged 6–12 years was about 0.7% in China(Zhou et al., 2020).
Children with ASD might be disabled and required life-long care(Cheuk et al., 2011), which burdens patients, families, and the public-health system. Although the pathogenesis of ASD is still not clear, it has been widely recognized that ASD occurs due to a combination of genetic and environmental factors, with the former being predominant (Bai et al., 2019). However, it has been found that maternal folic acid supplementation strategies, such as intake timing and intake dosage, may aid in reducing in the risk of ASD in offspring(Liu, Zou, Sun, Wu, & Chen, 2021).
In recent years, the metabolic abnormalities in ASD have attracted increasing attention, especially abnormalities related to amino acids. Amino acids are organic compounds containing amino and carboxyl groups, which are the basic protein units that have an essential role in regulating the immune system, cell signaling and metabolism, neurotransmission and so on(Vargason et al., 2018). Although previous studies have found the differences in plasma amino acid levels between autistic children and healthy children(Aldred, Moore, Fitzgerald, & Waring, 2003; D’Eufemia et al., 1995; Hoshino et al., 1984), there are still some disagreements over abnormal plasma amino acid levels between autistic children and healthy children. These inconsistent results in plasma amino acid levels between autistic children and healthy children might be due to the following reasons: firstly, most previous studies were small sample size studies, besides a few relatively large sample sizes in case-control studies (i.e., the sample size in ASD group ≥ 50 cases)(Adams et al., 2011; Cai, Ding, Zhang, Xue, & Wang, 2016; Naushad, Jain, Prasad, Naik, & Akella, 2013; Vargason et al., 2018; ** peers. Res Autism Spectr Disord, 50, 60–72. doi: https://doi.org/10.1016/j.rasd.2018.03.004 ." href="/article/10.1007/s10803-022-05829-z#ref-CR60" id="ref-link-section-d174067502e2131">2018; ** brain. The Journal Of Physiological Sciences: Jps, 66(5), 375–379. doi: https://doi.org/10.1007/s12576-016-0442-7 ." href="/article/10.1007/s10803-022-05829-z#ref-CR26" id="ref-link-section-d174067502e2857">2016; Owens & Kriegstein, 2002). Increased γ-aminobutyric acid and glycine in autistic children can disturb the excitation/inhibition balance in brain, which might lead to autism(Marotta et al., 2020; Zheng, Wang, Li, Rauw, & Baker, 2017).
Essential amino acids in the human body must be supplied by food; however, some autistic children may have eating difficulties and gastrointestinal symptoms, and be very picky about the taste and color of food(Kral, Eriksen, Souders, & Pinto-Martin, 2013). Accordingly, decreased essential amino acids (lysine, tryptophan, phenylalanine, histidine) in autistic children might be partially due to insufficient food intake or poor eating habits. Besides, we also found that the plasma levels of ethanolamine and glutathione (reduced) in the ASD group were decreased, which is consistent with the previous studies(Bala et al., 2016; Geier et al., 2009; James et al., 2004). Ethanolamine is involved in synthesizing phosphatidylethanolamine, and reduced ethanolamine in autistic children might cause chronic oxidative stress via decreased phosphatidylethanolamine synthesis(Wang et al., 2014). Glutathione is a tri-peptide involved in the redox balance of glutathione in the intracellular environment. The intracellular environment is maintained by a high glutathione (reduced)/glutathione (oxidative) ratio(Schafer & Buettner, 2001), which regulates a wide range of cell functions, including the scavenging of oxygen free radicals, cell membrane integrity, signal transduction, and so on(Dickinson et al., 2003). Therefore, reduced glutathione in autistic children may disrupt the redox balance of glutathione, thus further aggravating oxidative stress.
Interestingly, we also found that the plasma levels of sarcosine and δ-aminolevulinic acid were elevated. Sarcosine is an intermediate product of glycine metabolism, and the increased sarcosine level in our study might be due to increased glycine level, although Adams et al (Adams et al., 2011) found no significant difference in the sarcosine plasma level between the autistic children and neurotypical children in their study. However, most measurements of secondary plasmas amino acids and amino acid metabolites, including sarcosine being below the detection limit of 0.05 umoles/100ml and the large range age span of recruited subjects (5-16y) limited the interpretation of the outcomes of their study(Adams et al., 2011). Vargason et al(Vargason et al., 2018) also measured the level of Sarcosine, but omitted it from further analysis due to the subjects’ intervention issue. In addition, besides children, they(Vargason et al., 2018) also recruited adult subjects, and therefore, the age span of included subjects was large (11.8 ± 8.5y), which made it difficult to compare their PPA’s outcomes with current study and other studies that only included children’ subjects, as the level of plasma amino acids substantially varies with age(Lepage et al., 1997).
The plasma level of δ-aminolevulinic acid or pyroglutamic acid was not reported in the previous large sample sizes case-control studies (Table 5). In vivo, δ-aminolevulinic acid as the precursor of heme, is produced by glycine and succinyl-CoA under the δ-amino-γ-levulinic acid (ALA) synthetase(McLeod, Mack, & Brown, 1991), and δ-aminolevulinic acid must activate mitochondria so that it can convert into heme in the cell(Malik & Djaldetti, 1979). Most studies have indicated mitochondrial dysfunction and oxidative stress as the neuropathological basis of autism (Gorman et al., 2015; Rossignol & Frye, 2012). Therefore, the elevated δ-aminolevulinic acid in autistic children might cause mitochondrial dysfunction and oxidative stress. However, a recent animal model study showed that δ-aminolevulinic acid could inhibit oxidative stress and ameliorate autistic-like behaviors for the prenatal valproic acid-exposed rats(Matsuo, Yabuki, & Fukunaga, 2020), which implicate that accumulation of δ-aminolevulinic acid may result from autism-induced mitochondria dysfunction. The further studies needed regarding the relationship between δ-aminolevulinic acid and pathogenesis of autism.
Pyroglutamic acid is cyclized to form lactams from free amino groups of glutamate or glutamine. Pyroglutamic acid can antagonize nerve excitement by inhibiting glutamate(Abraham & Podell, 1981).The reduced plasma level of pyroglutamic acid in autistic children revealed in our study might further aggravate the neuroexcitatory toxicity of autism by reducing the inhibitory effect of pyroglutamate acid on glutamate.
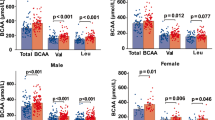
In addition, we found that the levels of homocysteine, hydroxyproline and ornithine in autism were reduced, which were contrary to previous researches’ results (Vargason et al., 2018; Zou et al., The Correlation Between Plasma Amino Acid and Clinical Phenotype The correlation between plasma amino acid and clinical phenotype was further analyzed using logistic regression analysis in the autistic children group. In terms of severity of autism, there were positively correlation between severity of autism and the plasma level of tryptophan (Table 4); the higher the plasma tryptophan level, the worse the severity of autism. The plasma level of tryptophan was reduced in the current study, which was in line with previous studies(Adams et al., 2011; Naushad et al., 2013; ** peers. Res Autism Spectr Disord, 50, 60–72. doi:
https://doi.org/10.1016/j.rasd.2018.03.004
." href="/article/10.1007/s10803-022-05829-z#ref-CR60" id="ref-link-section-d174067502e3103">2018
Cai et al.(Cai et al., 2016) found the plasma level of glutamate was positively associated with increasing severity of ASD. In their study, Cai and colleagues showed three possible mechanisms in their study, which were mostly related to the excitotoxicity of glutamate.
In 2020, Zou et al(Zou et al., 2020) also found that the level of homocysteine was positively correlated with the severity of autism, although they evaluated the severity of autistic children with ADOS-CSS (Table 5).
Regarding the regression, although there were no significant differences between plasma amino acids and regression in the current study, the level of glutathione might have a significant trend toward associate with regression (Table 4). Glutathione is a tripeptide, composed of glutamic acid, cysteine, and glycine, involved in the redox balance of glutathione in the intracellular environment. In addition, a previous study showed that the serum glutamate/glutamine ratio was elevated in ASD PIQ ≥ 70 group(**ng et al., 2021).
However, when the correlation was analyzed between plasma amino acids and other respective clinical phenotypes, including cognition and adaptability, there were no significant differences in the current study.
Strength and Limitation
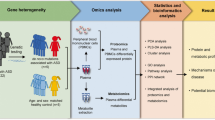
The present study was a relatively large-sample case-control study that recruiting more than 100 cases. We tried to exclude other confounding factors, such as acquired vitamin B12 deficiency, age and sex-matched between groups, to ensure the accuracy and reliability of the study. We also measured the level of amino acids by the advanced detection method of LC-MS/MS. Currently, we measured and analyzed the largest number of PAAs among case-control studies (Table 5). In addition, we further analyzed the correlation between plasma amino acid levels and a set of the clinical phenotypes of autistic children.
However, the present study has the following limitations: (1) this was retrospective research, so prospective large sample cohort studies are needed to further verify the findings of this study in the future; (2) the PAAs level was greatly affected by eating, it might be more accurate to dynamically monitor the changes of amino acids. Also, no information was collected on the patient’s underlying nutritional status; (3) there were no other neurodevelopmental groups, such as the ID group included in the current study, influencing outcomes’ representatives.
Conclusion
There were significant differences in seventeen amino acids between ASD and the control group; Elevated levels of neuroactive amino acids (glutamate) and decreased essential amino acids exhibited mostly distinct characteristics of plasma amino acid in autistic children. Increased level of tryptophan might be associated with the severity of autism.
References
Abraham, G. N., & Podell, D. N. (1981). Pyroglutamic acid. Non-metabolic formation, function in proteins and peptides, and characteristics of the enzymes effecting its removal. Mol Cell Biochem, 38 Spec No(Pt 1), 181–190. doi:https://doi.org/10.1007/bf00235695
Adams, J. B., Audhya, T., McDonough-Means, S., Rubin, R. A., Quig, D., Geis, E., & Lee, W. (2011). Nutritional and metabolic status of children with autism vs. neurotypical children, and the association with autism severity. Nutr Metab (Lond), 8(1), 34. doi:https://doi.org/10.1186/1743-7075-8-34.
Aldred, S., Moore, K. M., Fitzgerald, M., & Waring, R. H. (2003). Plasma amino acid levels in children with autism and their families. Journal Of Autism And Developmental Disorders, 33(1), 93–97. doi:https://doi.org/10.1023/a:1022238706604.
Association, A. P. (2013). The Diagnostic and Statistical Manual of Mental Disorders. 5th ed.Washington DC: American Psychiatric Publishing Inc,2013.
Bai, D., Yip, B. H. K., Windham, G. C., Sourander, A., Francis, R., Yoffe, R., & Sandin, S. (2019). Association of genetic and environmental factors with autism in a 5-Country cohort. JAMA Psychiatry, 76(10), 1035–1043. doi:https://doi.org/10.1001/jamapsychiatry.2019.1411.
Bala, K. A., Doğan, M., Mutluer, T., Kaba, S., Aslan, O., Balahoroğlu, R., & Kocaman, S. (2016). Plasma amino acid profile in autism spectrum disorder (ASD). European Review For Medical And Pharmacological Sciences, 20(5), 923–929. doi:https://doi.org/10.3390/children9040540.
Barger, B. D., Campbell, J. M., & McDonough, J. D. (2013). Prevalence and onset of regression within autism spectrum disorders: a meta-analytic review. Journal Of Autism And Developmental Disorders, 43(4), 817–828. doi:https://doi.org/10.1007/s10803-012-1621-x.
Bergwerff, C. E., Luman, M., Blom, H. J., & Oosterlaan, J. (2016). No tryptophan, tyrosine and phenylalanine abnormalities in children with Attention-Deficit/Hyperactivity disorder. PLoS One, 11(3), e0151100. doi:https://doi.org/10.1371/journal.pone.0151100.
Bhatia, P., & Singh, N. (2015). Homocysteine excess: delineating the possible mechanism of neurotoxicity and depression. Fundamental & Clinical Pharmacology, 29(6), 522–528. doi:https://doi.org/10.1111/fcp.12145.
Blaylock, R. L., & Strunecka, A. (2009). Immune-glutamatergic dysfunction as a central mechanism of the autism spectrum disorders. Current Medicinal Chemistry, 16(2), 157–170. doi:https://doi.org/10.2174/092986709787002745.
Boccuto, L., Chen, C. F., Pittman, A. R., Skinner, C. D., McCartney, H. J., Jones, K., & Schwartz, C. E. (2013). Decreased tryptophan metabolism in patients with autism spectrum disorders. Mol Autism, 4(1), 16. doi:https://doi.org/10.1186/2040-2392-4-16.
Bryn, V., Verkerk, R., Skjeldal, O. H., Saugstad, O. D., & Ormstad, H. (2017). Kynurenine Pathway in Autism Spectrum Disorders in Children. Neuropsychobiology, 76(2), 82–88. doi:https://doi.org/10.1159/000488157.
Cai, J., Ding, L., Zhang, J. S., Xue, J., & Wang, L. Z. (2016). Elevated plasma levels of glutamate in children with autism spectrum disorders. Neuroreport, 27(4), 272–276. doi:https://doi.org/10.1097/wnr.0000000000000532.
CDC, D. D. M. N. S. Y., & P., P. (2014). Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill Summ, 63(2), 1–21. doi:https://doi.org/10.15585/mmwr.ss6706a1
Cheuk, D. K., Wong, V., & Chen, W. X. (2011). Acupuncture for autism spectrum disorders (ASD). Cochrane Database Syst Rev(9), Cd007849. doi:https://doi.org/10.1002/14651858.CD007849.pub2
Choi, D. W. (1985). Glutamate neurotoxicity in cortical cell culture is calcium dependent. Neuroscience Letters, 58(3), 293–297. doi:https://doi.org/10.1016/0304-3940(85)90069-2.
D’Eufemia, P., Finocchiaro, R., Celli, M., Viozzi, L., Monteleone, D., & Giardini, O. (1995). Low serum tryptophan to large neutral amino acids ratio in idiopathic infantile autism. Biomedicine & Pharmacotherapy, 49(6), 288–292. doi:https://doi.org/10.1016/0753-3322(96)82645-x.
Davis, I., & Liu, A. (2015). What is the tryptophan kynurenine pathway and why is it important to neurotherapeutics? Expert Review Of Neurotherapeutics, 15(7), 719–721. doi:https://doi.org/10.1586/14737175.2015.1049999.
Dickinson, D. A., Moellering, D. R., Iles, K. E., Patel, R. P., Levonen, A. L., Wigley, A., & Forman, H. J. (2003). Cytoprotection against oxidative stress and the regulation of glutathione synthesis. Biological Chemistry, 384(4), 527–537. doi:https://doi.org/10.1515/bc.2003.061.
Froese, D. S., Fowler, B., & Baumgartner, M. R. (2019). Vitamin B(12), folate, and the methionine remethylation cycle-biochemistry, pathways, and regulation. Journal Of Inherited Metabolic Disease, 42(4), 673–685. doi:https://doi.org/10.1002/jimd.12009.
Gabriele, S., Sacco, R., & Persico, A. M. (2014). Blood serotonin levels in autism spectrum disorder: a systematic review and meta-analysis. European Neuropsychopharmacology, 24(6), 919–929. doi:https://doi.org/10.1016/j.euroneuro.2014.02.004.
Geier, D. A., Kern, J. K., Garver, C. R., Adams, J. B., Audhya, T., & Geier, M. R. (2009). A prospective study of transsulfuration biomarkers in autistic disorders. Neurochemical Research, 34(2), 386–393. doi:https://doi.org/10.1007/s11064-008-9782-x.
Gorman, G. S., Schaefer, A. M., Ng, Y., Gomez, N., Blakely, E. L., Alston, C. L., & McFarland, R. (2015). Prevalence of nuclear and mitochondrial DNA mutations related to adult mitochondrial disease. Annals Of Neurology, 77(5), 753–759. doi:https://doi.org/10.1002/ana.24362.
Guo, B. Q., Li, H. B., & Ding, S. B. (2020). Blood homocysteine levels in children with autism spectrum disorder: an updated systematic review and meta-analysis. Psychiatry Research, 291, 113283. doi:https://doi.org/10.1016/j.psychres.2020.113283.
Hoshino, Y., Yamamoto, T., Kaneko, M., Tachibana, R., Watanabe, M., Ono, Y., & Kumashiro, H. (1984). Blood serotonin and free tryptophan concentration in autistic children. Neuropsychobiology, 11(1), 22–27. doi:https://doi.org/10.1159/000118045.
Ito, S. (2016). GABA and glycine in the develo** brain. The Journal Of Physiological Sciences: Jps, 66(5), 375–379. doi:https://doi.org/10.1007/s12576-016-0442-7.
James, S. J., Cutler, P., Melnyk, S., Jernigan, S., Janak, L., Gaylor, D. W., & Neubrander, J. A. (2004). Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. American Journal Of Clinical Nutrition, 80(6), 1611–1617. doi:https://doi.org/10.1093/ajcn/80.6.1611.
James, S. J., Melnyk, S., Jernigan, S., Cleves, M. A., Halsted, C. H., Wong, D. H., & Gaylor, D. W. (2006). Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. Am J Med Genet B Neuropsychiatr Genet, 141b(8), 947–956. doi:https://doi.org/10.1002/ajmg.b.30366.
Jennings, L., & Basiri, R. (2022). Amino acids, B Vitamins, and Choline May independently and collaboratively influence the incidence and core symptoms of Autism Spectrum Disorder. Nutrients, 14(14), doi:https://doi.org/10.3390/nu14142896.
Jianduan, Z., Huishan, W., Shuhua, S., **aonan, H., Guoyan, L., Guangli, L., & Junxin, S. (2009). Reliability and validity of standardized chinese version of Urban Infant-Toddler Social and Emotional Assessment. Early Human Development, 85(5), 331–336. doi:https://doi.org/10.1016/j.earlhumdev.2008.12.012.
Kaluzna-Czaplinska, J., Jozwik-Pruska, J., Chirumbolo, S., & Bjorklund, G. (2017). Tryptophan status in autism spectrum disorder and the influence of supplementation on its level. Metabolic Brain Disease, 32(5), 1585–1593. doi:https://doi.org/10.1007/s11011-017-0045-x.
Kral, T. V. E., Eriksen, W. T., Souders, M. C., & Pinto-Martin, J. A. (2013). Eating Behaviors, Diet Quality, and gastrointestinal symptoms in Children with Autism Spectrum Disorders: a brief review. Journal of Pediatric Nursing, 28(6), 548–556. doi:https://doi.org/10.1016/j.pedn.2013.01.008.
Lepage, N., McDonald, N., Dallaire, L., & Lambert, M. (1997). Age-specific distribution of plasma amino acid concentrations in a healthy pediatric population. Clinical Chemistry, 43(12), 2397–2402.
Li, P., & Wu, G. (2018). Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth. Amino Acids, 50(1), 29–38. doi:https://doi.org/10.1007/s00726-017-2490-6.
Lim, C. K., Essa, M. M., de Paula Martins, R., Lovejoy, D. B., Bilgin, A. A., Waly, M. I., & Guillemin, G. J. (2016). Altered kynurenine pathway metabolism in autism: implication for immune-induced glutamatergic activity. Autism Research, 9(6), 621–631. doi:https://doi.org/10.1002/aur.1565.
Liu, X., Lin, S.F., Chen, W. X., Chan, F. F., Shen, S. Y., & Qiu, X. (2018). Prevalence of autism spectrum disorders among children in China:a systematic review and meta-analysis. Chinese Journal of Child Care, 26(04), 402–406. doi:https://doi.org/10.1177/13623613211045029.
Liu, X., Zou, M., Sun, C., Wu, L., & Chen, W. X. (2021). Prenatal folic acid supplements and offspring’s Autism Spectrum disorder: a Meta-analysis and Meta-regression. Journal Of Autism And Developmental Disorders. doi:https://doi.org/10.1007/s10803-021-04951-8.
Lotter, V. (1966). Epidemiology of autistic conditions in young children. Social Psychiatry, 1, 124–137.
Magera, M. J., Helgeson, J. K., Matern, D., & Rinaldo, P. (2000). Methylmalonic acid measured in plasma and urine by stable-isotope dilution and electrospray tandem mass spectrometry. Clinical Chemistry, 46(11), 1804–1810.
Malik, Z., & Djaldetti, M. (1979). 5-Aminolevulinic acid stimulation of porphyrin and hemoglobin synthesis by uninduced friend erythroleukemic cells. Cell Differ, 8(3), 223–233. doi:https://doi.org/10.1016/0045-6039(79)90049-6.
Manent, J. B., & Represa, A. (2007). Neurotransmitters and brain maturation: early paracrine actions of GABA and glutamate modulate neuronal migration. The Neuroscientist : A Review Journal Bringing Neurobiology, Neurology And Psychiatry, 13(3), 268–279. doi:https://doi.org/10.1177/1073858406298918.
Marotta, R., Risoleo, M. C., Messina, G., Parisi, L., Carotenuto, M., Vetri, L., & Roccella, M. (2020). The Neurochemistry of Autism. Brain Sci, 10(3), doi:https://doi.org/10.3390/brainsci10030163.
Matsuo, K., Yabuki, Y., & Fukunaga, K. (2020). 5-aminolevulinic acid inhibits oxidative stress and ameliorates autistic-like behaviors in prenatal valproic acid-exposed rats. Neuropharmacology, 168, 107975. doi:https://doi.org/10.1016/j.neuropharm.2020.107975.
McDougle, C., Naylor, S., Goodman, W., Volkmar, F., Cohen, D., & Price, L. (1993). Acute tryptophan depletion in autistic disorder: a controlled case study. Biological psychiatry, 33(7), 547–550. doi:https://doi.org/10.1016/0006-3223(93)90011-2.
McDougle, C. J., Naylor, S. T., Cohen, D. J., Aghajanian, G. K., Heninger, G. R., & Price, L. H. (1996). Effects of tryptophan depletion in drug-free adults with autistic disorder. Archives Of General Psychiatry, 53(11), 993–1000. doi:https://doi.org/10.1001/archpsyc.1996.01830110029004.
McLeod, R., Mack, D., & Brown, C. (1991). Toxoplasma gondii–new advances in cellular and molecular biology. Experimental Parasitology, 72(1), 109–121. doi:https://doi.org/10.1016/0014-4894(91)90129-k.
Melendez, J. A., Melathe, R. P., Rodriguez, A. M., Mazurkiewicz, J. E., & Davies, K. J. (1999). Nitric oxide enhances the manganese superoxide dismutase-dependent suppression of proliferation in HT-1080 fibrosarcoma cells. Cell Growth & Differentiation, 10(9), 655–664.
Muller, C. L., Anacker, A. M. J., & Veenstra-VanderWeele, J. (2016). The serotonin system in autism spectrum disorder: from biomarker to animal models. Neuroscience, 321, 24–41. doi:https://doi.org/10.1016/j.neuroscience.2015.11.010.
Naushad, S. M., Jain, J. M., Prasad, C. K., Naik, U., & Akella, R. R. (2013). Autistic children exhibit distinct plasma amino acid profile. Indian Journal Of Biochemistry & Biophysics, 50(5), 474–478.
Ormstad, H., Bryn, V., Verkerk, R., Skjeldal, O. H., Halvorsen, B., Saugstad, O. D., & Maes, M. (2018). Serum tryptophan, Tryptophan Catabolites and Brain-derived neurotrophic factor in subgroups of youngsters with Autism Spectrum Disorders. CNS Neurol Disord Drug Targets, 17(8), 626–639. doi:https://doi.org/10.2174/1871527317666180720163221.
Owens, D. F., & Kriegstein, A. R. (2002). Is there more to GABA than synaptic inhibition? Nature Reviews Neuroscience, 3(9), 715–727. doi:https://doi.org/10.1038/nrn919.
Peng, M. Z., Cai, Y. N., Shao, Y. X., Zhao, L., Jiang, M. Y., Lin, Y. T., & Liu, L. (2019). Simultaneous quantification of 48 plasma amino acids by liquid chromatography-tandem mass spectrometry to investigate urea cycle disorders. Clinica Chimica Acta, 495, 406–416. doi:https://doi.org/10.1016/j.cca.2019.05.011.
Proenza, A. M., Crespí, C., Roca, P., & Palou, A. (2001). Gender related differences in the effect of aging on blood amino acid compartmentation*. Journal Of Nutritional Biochemistry, 12(7), 431–440. doi:https://doi.org/10.1016/s0955-2863(01)00157-7.
Rossignol, D. A., & Frye, R. E. (2012). Mitochondrial dysfunction in autism spectrum disorders: a systematic review and meta-analysis. Molecular Psychiatry, 17(3), 290–314. doi:https://doi.org/10.1038/mp.2010.136.
Schafer, F. Q., & Buettner, G. R. (2001). Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radical Biology And Medicine, 30(11), 1191–1212. doi:https://doi.org/10.1016/s0891-5849(01)00480-4.
Shimmura, C., Suda, S., Tsuchiya, K. J., Hashimoto, K., Ohno, K., Matsuzaki, H., & Mori, N. (2011). Alteration of plasma glutamate and glutamine levels in children with high-functioning autism. PLoS One, 6(10), e25340. doi:https://doi.org/10.1371/journal.pone.0025340.
Stone, T. W., & Darlington, L. G. (2002). Endogenous kynurenines as targets for drug discovery and development. Nature Reviews. Drug Discovery, 1(8), 609–620. doi:https://doi.org/10.1038/nrd870.
Tanianskii, D. A., Jarzebska, N., Birkenfeld, A. L., O’Sullivan, J. F., & Rodionov, R. N. (2019). Beta-aminoisobutyric acid as a Novel Regulator of Carbohydrate and lipid metabolism. Nutrients, 11(3), doi:https://doi.org/10.3390/nu11030524.
Van der Leek, A. P., Yanishevsky, Y., & Kozyrskyj, A. L. (2017). The Kynurenine Pathway as a Novel link between Allergy and the gut Microbiome. Frontiers In Immunology, 8, 1374. doi:https://doi.org/10.3389/fimmu.2017.01374.
Vargason, T., Kruger, U., McGuinness, D. L., Adams, J. B., Geis, E., Gehn, E., & Hahn, J. (2018). Investigating plasma amino acids for differentiating individuals with Autism Spectrum Disorder and typically develo** peers. Res Autism Spectr Disord, 50, 60–72. doi:https://doi.org/10.1016/j.rasd.2018.03.004.
Wang, S., Zhang, S., Liou, L. C., Ren, Q., Zhang, Z., Caldwell, G. A., & Witt, S. N. (2014). Phosphatidylethanolamine deficiency disrupts α-synuclein homeostasis in yeast and worm models of Parkinson disease. Proc Natl Acad Sci U S A, 111(38), E3976–3985. doi:https://doi.org/10.1073/pnas.1411694111.
Xu, G., Strathearn, L., Liu, B., O’Brien, M., Kopelman, T. G., Zhu, J., & Bao, W. (2019). Prevalence and treatment patterns of Autism Spectrum Disorder in the United States, 2016. JAMA Pediatr, 173(2), 153–159. doi:https://doi.org/10.1001/jamapediatrics.2018.4208.
**ng, Y., Lv, Q. Q., You, C., Zou, X.B., & Deng, H. (2021). Reduction of essential amino acid levels and sex-specific alterations in serum amino acid concentration profiles in children with autism spectrum disorder. Psychiatry Research, 297, 113675. doi:https://doi.org/10.1016/j.psychres.2020.113675.
Yüksel, C., & Öngür, D. (2010). Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biological Psychiatry, 68(9), 785–794. doi:https://doi.org/10.1016/j.biopsych.2010.06.016.
Zheng, H. F., Wang, W. Q., Li, X. M., Rauw, G., & Baker, G. B. (2017). Body fluid levels of neuroactive amino acids in autism spectrum disorders: a review of the literature. Amino Acids, 49(1), 57–65. doi:https://doi.org/10.1007/s00726-016-2332-y.
Zhou, H., Xu, X., Yan, W., Zou, X., Wu, L., Luo, X., & Team, L. N. S. (2020). Prevalence of Autism Spectrum Disorder in China: a Nationwide Multi-center Population-based study among children aged 6 to 12 years. Neuroscience Bulletin, 36(9), 961–971. doi:https://doi.org/10.1007/s12264-020-00530-6.
Zou, M., Li, D., Wang, L., Li, L., **e, S., Liu, Y., & Wu, L. (2020). Identification of amino acid dysregulation as a potential biomarker for Autism Spectrum Disorder in China. Neurotoxicity Research, 38(4), 992–1000. doi:https://doi.org/10.1007/s12640-020-00242-9.
Zuo, Q. H. (2016). Social Adaptation Scale for Infants-Junior Middle School Students. Bei**g: Hua**a Press.
Funding
This study was supported by the grants of Guangzhou Science and technology plan “City School (College) joint funding project” (202102010232), and partly supported by the major Scientific and Technological Projects of Brain Science and Brain-like Research of Guangzhou (202007030002).
Author information
Authors and Affiliations
Contributions
All authors contributed to the collection and interpretation of data. Wen-**ong Chen was responsible for concept and design. Yi-Ru Chen, Min-Zhi Peng, **an Liu contributed to draft the manuscript. **an Liu and Yi-Ru Chen contributed to statistical analysis. Wen-**ong Chen had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis, and critically revised the manuscript. Wen-**ong Chen, Yi-Ru Chen, Min-Zhi Peng and **an Liu contributed equally to this work and are considered as a co-first author.
Corresponding author
Ethics declarations
Conflict of Interest
All authors report no conflicit of interest.
Ethical Approval
This study was approved by the Ethics Committee of Guangzhou Women and Children’s Medical Center.
Informed Consent
Written and signed consents were obtained from patients’ parents or guardians.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wen-**ong Chen, Yi-Ru Chen, Min-Zhi Peng, and **an Liu contributed equally to the article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, WX., Chen, YR., Peng, MZ. et al. Plasma Amino Acid Profile in Children with Autism Spectrum Disorder in Southern China: Analysis of 110 Cases. J Autism Dev Disord 54, 1567–1581 (2024). https://doi.org/10.1007/s10803-022-05829-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-022-05829-z