Abstract
Salinity stress constitutes one of the main abiotic stresses that considerably reduces crop yield. An approach to enhance plant growth under salt stress involves the addition of humic substances (HS) to roots or leaves. Here, we evaluated the potential use of BLACKJAK®, an HS-based product, to enhance salt tolerance in lettuce (Lactuca sativa L.). For this aim, plants were exposed to salinity (100 mM NaCl), salinity + HS: radicular (R)-HS (0.40 mL/L and 0.60 mL/L) and foliar (F)-HS (7.50 mL/L and 10.00 mL/L), along with a control (without NaCl). Parameters related to plant growth, Na+ and K+ accumulation, photosynthetic activity, oxidative stress, enzymatic and non-enzymatic antioxidants, as well as proline levels were evaluated. Results showed that R and F-HS considerably enhanced salinity tolerance. In addition, F-HS offered a greater improvement of plant growth in relation to shoot fresh weight, shoot relative growth rate, and foliar area, being 10.00 mL/L the best dose. This tolerance could be associated with reduced Na+ translocation to the shoot and enhanced shoot K+ accumulation, decreasing Na+/K+ ratio. Furthermore, HS improved the net photosynthetic rate, Rubisco carboxylation efficiency, and photosystem II performance, and reduced ROS levels and lipid peroxidation. Hence, our data show the potential use of BLACKJAK® to improve lettuce tolerance to salinity, with foliar application slightly better than radicular to achieve this aim, especially at 10.00 mL/L dose.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the major abiotic stresses that considerably reduce plant growth and quality is soil salinity. This environmental problem results from higher salt concentrations, mainly sodium chloride (NaCl), in soil or irrigation water (Mubarak et al. 2021; Shaaban et al. 2023a). It is estimated that salinity affects more than 20% of irrigated land, mainly in arid and semi-arid areas such as the Mediterranean region (Singh et al. 2022a). In the future, a significant portion of these saline soils will undergo cultivation in response to the expanding world population, projected to reach 10 billion people by 2050, and the escalating demand for food (Shah et al. 2021). Several crops are continuously exposed to salinity conditions, which significantly reduce plant growth and crop productivity (Saudy et al. 2023b). In this way, annual economic losses due to salinity are estimated at 12 billion USD (Zuluaga et al. 2023). For this reason, different agronomic strategies, including the breeding and evaluation of new varieties (Abd El-Mageed et al. 2022) and the application of natural products (Salem et al. 2021), are being studied to increase plant tolerance to salinity.
Osmotic, ionic, and oxidative stress impair plant physiology during exposure to salinity. Besides, the accumulation of soluble salts, and the subsequent reduction in soil water potential, reduces roots’ water uptake (Valenzuela et al. 2022). Consequently, one of the responses to osmotic stress is stomatal closure to decrease water loss by transpiration (Makhlouf et al. 2022; Ali et al. 2024b), although it limits CO2 assimilation and photosynthesis performance (Marriboina and Attipalli 2020). At the cellular level, osmotic adjustment occurs through the synthesis and accumulation of compatible solutes and osmolytes to maintain cellular turgor (El-Metwally et al. 2022a; Ramadan et al. 2023a; Doklega et al. 2024). Proline, betaine, and glycine are examples of solutes synthesized by plants as an osmotic response to different adverse conditions (Hussain et al. 2021; Saudy et al. 2023a). In addition, salt stress results in an ionic imbalance associated with high cytosolic accumulation of Na+ and Cl−, resulting in leaf senescence (Shahzad et al. 2021; Liu et al. 2022). Because of the ionic similarity, Na+ tends to replace K+, and consequently many enzymatic activities involving K+ are disrupted. Thus, some vital processes, such as photosynthesis, are affected by Na+ toxicity (Navarro-León et al. 2021).
Reactive oxygen species (ROS), including superoxide radical (O2−) and hydrogen peroxide (H2O2), play an important role as stress response signal molecules, at low concentrations (Hadid et al. 2023; Shaaban et al. 2023b). Under high salinity, ROS overproduction is observed in plant cells, which triggers oxidative stress. Nucleic acids, enzyme activities, membrane lipids, photosynthetic apparatus, etc., are damaged by ROS, resulting in cell and plant death (Liu et al. 2022; Mangal et al. 2023). Lipid peroxidation results in malondialdehyde (MDA) generation, therefore, low MDA in plant cells is linked with enhanced salinity stress tolerance (Mousavi et al. 2022). To cope with oxidative stress, different enzymatic and non-enzymatic antioxidants are activated. Superoxide dismutase (SOD) reduces O2.− to H2O2 which is detoxified by ascorbate peroxidase (APX), whereas ascorbate (AsA), glutathione (GSH), and phenolic compounds are non-enzymatic antioxidants that contribute to ROS scavenging (Liu et al. 2022; Navarro-León et al. 2023). Therefore, the plant’s ability to detoxify ROS and maintain osmotic and ionic homeostasis is essential to overcome salt stress (Chen et al. 2022a, b).
One way to improve salinity stress tolerance is the application of biostimulants or biofertilizers to soil or plant. These natural products are classified in two main groups: microbial (beneficial bacteria and fungi) and non-microbial (protein hydrolysates, plant extracts, seaweed extracts, beneficial elements, biopolymers, chitosan, and humic substances) (Pizzeghello et al. 2020; Ali et al. 2024a; El-Metwally et al. 2022b; Abou El-Enin et al. 2023). Humic substances (HS), such as humic acids (HA), fulvic acids (FA), and humin, represent up to 70% of soil organic matter and have been employed as potential plant biostimulants (Saudy et al. 2020; Ore et al. 2023). HS are derived from the chemical and physical degradation of plant and animal waste present in soil, through the “humification” process. In terms of chemical structure, HS are defined as supramolecular colloids formed by the interaction between hundreds of small organic molecules through hydrophobic interactions and hydrogen bonds (Tiwari et al. 2023). HS positively affect primary and secondary metabolism, enhancing plant resistance to different adverse conditions such as salt stress. Thus, HS may enhance root growth and water uptake, nutrient bioavailability and absorption, photosynthesis performance, compatible solutes accumulation, and enzymatic and non-enzymatic antioxidant activity (Ramadan et al. 2023a; Lasheen et al. 2024). All of this translates into ROS scavenging, photosynthetic activity maintenance, preventing cytoplasmic ionic imbalances, and ensuring adequate cellular water status (García et al. 2016; Khaleda et al. 2017; Man-hong et al. 2020; Souza et al. 2021; Abu-Ria et al. 2023; Ramadan et al. 2023b). In this way, several researchers and companies extract HS from various sources such as leonardite, coal, and peat to develop HS-based products and improve plant growth and abiotic stress tolerance in different crops such as lettuce (Lactuca sativa L.).
Lettuce is a high-nutrient and low-calorie leafy vegetable with a high economic value (Abd-Elrahman et al. 2022). However, under salinity stress conditions, lettuce production may be reduced because of its sensitivity to salt, prompting researchers to explore strategies to mitigate salt stress damage (Liu et al. 2024). Hence, the objective of the present study was to assess the effectiveness of a HS-based product as a biostimulant to increase salinity stress tolerance in lettuce plants, identifying the possible mechanisms of action of this salt stress alleviation, as well as the differences between both modes of HS application (radicular and foliar).
Materials and methods
Plant material and cultivation conditions
Seeds of lettuce (L. sativa L. cv. Capitata) were previously germinated and grown in tray cells (3 cm × 3 cm × 3 cm). After 45 days, lettuce seedlings were transferred to pots with vermiculite:perlite (3:1) as substrate, and distributed randomly in a growth chamber under controlled environmental conditions: temperature 25/15 °C (day/night), relative humidity 70%, photoperiod 16/8 h, and 350 μmol m−2 s−1 photosynthetically active radiation (PAR). Lettuce plants were watered during the experiment with a complete nutritive solution composed of 1 mM KH2PO4, 1 mM NaH2PO4·2H2O, 4 mM KNO3, 2 mM MgSO4·7H2O, 3 mM Ca(NO3)2·4H2O, 5 µM Fe-chelate (Sequestrene; 138FeG100), 0.25 mM CuSO4·5H2O, 10 µM HBO3, 1 µM ZnSO4·7H2O, 2 µM MnCl2·4H2O, and 0.1 µM Na2MoO4·2H2O. The pH of this solution was adjusted to 5.5–6.
Treatments and experimental design
Treatments started 7 days after transplantation and were maintained for 30 days. A total of six treatments were conducted: (1) Control (nutritive solution only); (2) salinity (nutritive solution and 100 mM NaCl); (3) salinity + radicular-HS (R-HS) 0.40 mL/L (100 mM NaCl and 0.40 mL/L HS added to nutritive solution); (4) salinity + R-HS 0.60 mL/L (100 mM NaCl and 0.60 mL/L HS added to nutritive solution); (5) salinity + foliar-HS (F-HS) 7.50 mL/L (100 mM NaCl added to nutritive solution and spraying leaves with HS 7.50 mL/L); (6) salinity + F-HS 10.00 mL/L (100 mM NaCl added to nutritive solution and spraying leaves with HS 10.00 mL/L). Salinity stress conditions (100 mM NaCl) were established according to previous studies by our research group on lettuce plants grown under the same environmental conditions (Leyva et al. 2011). HS application was conducted using a leonardite-suspension concentrate (SC)-based product called BLACKJAK® provided by Sofbey S.A. (Mendrisio, Switzerland), with acidic pH (4–5) and 30% organic matter. In this way, radicular applications lowered the pH of nutritive solution to 4.5. A total of three HS applications with a periodicity of 10 days were performed. Radicular and foliar-HS doses were selected according to previous results obtained in lettuce plants, in terms of increases in biomass production, nutritional, and antioxidant quality under control conditions (Atero-Calvo et al. 2023). The experimental design comprised a randomized complete block featuring six treatments, each with three replications, and eight plants per replicate.
Plant sampling
Leaves and roots from plants of each treatment were sampled, weighed to measure fresh weight (FW), and subsequently rinsed with distilled water before being dried on filter paper. From each treatment, half of the leaves were preserved at − 45 °C for future biochemical analysis. The remaining half of the leaves and roots were dried by a forced-air oven at 70 °C to obtain dry weight (DW). To determine the relative growth rate (RGR), leaves from each treatment were sampled before the treatments started (initial time, Ti = 0 days), dried, and weighed (initial DW, DWi). At the end of the trial (final time, Tf = 30 days), leaves DW (final DW, DWf) from each treatment was employed to estimate RGR as (ln DWf – ln DWi)/(Tf-Ti) (Navarro-León et al. 2020c). Furthermore, salt tolerance index (STI) was also estimated as (Total FW of salinity plants/Total FW of control) × 100 (Navarro-León et al. 2020b). In addition, leaf area was determined using a LI-COR (LI-3000A) optical reader (LI-COR Inc. Nebraska, USA).
Na+ and K+ concentrations
Samples of dried leaves and roots (0.15 g) were used to estimate Na+ and K+ concentrations through a mineralization process by wet digestion (Wolf 2008). Dry leaves and roots were mineralized using a nitric acid (HNO3)/perchloric acid (HClO4) mixture (v/v) with H2O2 (30%). After added a total of 20 mL of mili-Q H2O at the end of the mineralization, the concentration of Na+ and K+ was measured by ICP-MS (X-Series II; Termo Fisher Scientific Inc., Waltham, MA, USA).
In addition, the distribution coefficient (DC) of Na+ and K+ was calculated as DC = [Ion] in leaves/[Ion] in roots.
Pigment concentrations
Leaves (0.1 g) were macerated in methanol (1 mL). After centrifugation (5000×g, 5 min), the supernatant was used to measure absorbance at 666, 653, and 470 nm. Chlorophyll a (Chl a), chlorophyll b (Chl b), and carotenoids concentrations were calculated following the equations proposed in Wellburn (1994).
Leaf gas exchange parameters
Parameters related to leaf gas exchange were estimated using a LICOR 6800 (IRGA: LICOR Inc. Nebraska, USA), an infra-red gas analyzer. System warmup tests were run before measurements following the manufacturer’s recommendations. The environmental conditions of leaf chamber were then adapted to the physiological requirements of lettuce culture, as described previously (Hidalgo-Santiago et al. 2021). Thus, all parameters were measured with CO2 concentration at 400 µmol mol−1, relative humidity at 70%, chamber fan mixing speed at 10,000 rpm, 25 °C leaf temperature, PAR at 350 μmol m−2 s−1. Gas exchange measurements were taken between 10.00 a.m. and 02.00 p.m., in fully expanded leaves at the midstem position in six plants per treatment. For each plant, a total of nine measurements were recorded and the mean was used for: net photosynthetic rate (A), transpiration rate (E), stomatal conductance (gs), and intercellular CO2 (Ci). The equation A/E was employed to determine the water use efficiency (WUE).
In addition, after each measurement of these parameters, the Rubisco maximum carboxylation rate (Vcmax) was estimated using a rapid A-Ci response curve (RACiR). For this purpose, increasing concentrations of CO2 (from 10 to 510 µmol mol−1) were applied to lettuce leaf. As CO2 concentration increased, A and Ci were recorded every 2 s for 7 min. Furthermore, before the first measurement, a RACiR curve was obtained with the chamber closed and without the leaf to correct data. Vcmax was estimated through the ‘Plantecophys’ package in R (Duursma 2015) by fitting previously the data following the Farquhar model (Farquhar et al. 1980).
Chl a fluorescence measurement
Six leaves from each treatment located in the middle section of the stem were exposed to darkness using a clip for 30 min. Subsequently, Chl a fluorescence parameters were estimated through a Chlorophyll Fluorimeter (Handy PEA, Hansatech Ltd., King’s Lynn, Norfolk, UK), by fluorescence induction at 650 nm of red light. These parameters were: Area (the region above the transient curve), Fv/Fm (maximum quantum yield of primary photochemistry), PIABS (performance index), ΦEo (maximum quantum yield of electron transport), Sm (energy needed to reduce reaction centres, N (the number of times that quinone A (QA) is reduced from time 0 to the time of maximum fluorescence), and ETo/RC (the electron flux from QA) (Strasser et al. 2004).
Lipid peroxidation and ROS concentrations
The MDA concentration was measured at 532 nm and 600 nm as described by Fu and Huang (2001). In addition, the electrolyte loss test was assayed to determine the electrolyte leakage (EL). Fresh leaves (0.3 g) were placed in tubes with 30 mL of distilled water. After the tubes were vortexed, a conductivity meter (Cond 8; XS Instruments, Italy), was employed to determine the initial conductivity (EC1). Afterwards, an incubation at 100 °C for 20 min was carried out, and the final conductivity (EC2) was measured. Using the formula EL = EC1/EC2 × 100, the EC percentage was determined (Soloklui et al. 2012).
The method described in Kubiś (2008) and based on the nitroblue tetrazolium (NBT) reduction at 580 nm was followed to estimate O2.− concentration, while H2O2 was assayed colorimetrically at 350 nm (Junglee et al. 2014).
Antioxidant enzyme activities
The SOD activity determination was performed according to Yu et al. (1998), which is based on the inhibition of NBT. The APX was determined following the method of Rao et al. (1996), measuring H2O2 reduction for 5 min at 290 nm.
AsA, GSH, total phenols, and flavonoids
Total and reduced AsA were determined by measuring at 525 nm the Fe3+ reduction by AsA. Dehydroascorbate (DHA) was estimated as total AsA–reduced AsA (Law et al. 1983). Total GSH and oxidized GSH (GSSG) were assayed following the methodology of Noctor and Foyer (1998), based on GSSG reduction to GSH through glutathione reductase, and the subsequent DTNB oxidation, measuring the absorbance at 412 nm. Reduced GSH was calculated as total GSH–GSSG.
Total phenols and flavonoids were measured according to (Rivero et al. 2001). Folin–Ciocâlteu reagent was employed to measure total phenols concentration at 725 nm, using a curve of caffeic acid, while flavonoids were estimated at 415 nm against a curve of rutin.
Proline concentration
0.1 g of leaves were macerated with 1.2 mL of 83% ethanol. After centrifugation (2800×g, 10 min), 1 mL of supernatant was mixture with 4 mL of distilled water, 2.5 mL of ninhidrine, and 2.5 mL of 95% acetic acid were added. After incubation at 100 °C for 45 min, 3 mL of benzene were added, and the absorbance was determined at 515 nm against a curve of proline (Pro) (Irigoyen et al. 1992).
Statistical analysis
The statistical procedure was performed using Statgraphics Centurion 16.1.03 software. A simple analysis of variance (ANOVA) at 95% confidence was conducted to evaluate the data obtained. The means were compared by the Fisher’s least significant difference test (LSD) and the significance levels were indicated as: *P < 0.05; **P < 0.01; ***P < 0.001; NS not significant.
Results
Plant biomass, RGR, and STI
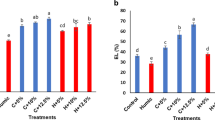
Salinity stress conditions without biostimulant application caused a significant reduction in shoot FW (− 75%), shoot DW (− 62%), foliar area (− 64%), shoot RGR (− 40%), root FW (− 38%), and root DW (− 67%) with respect to control treatment. Nevertheless, these reductions were significantly lower in plants subjected to HS: shoot FW (− 61%, − 65%, − 59%, − 55%), shoot DW (− 31%, − 34%, − 37%, − 31%), foliar area (− 53%, − 54%, − 48%, − 46%), shoot RGR (− 14%, − 14%, − 18%, − 14%), root FW (− 2%, − 19%, − 22%, − 18%), and root DW (17%, 6%, 0%, 0%), for R-HS 0.40 mL/L, 0.60 mL/L, and F-HS 7.50 mL/L and 10.00 mL/L, respectively. In addition, although no differences were observed between modes of application in terms of shoot DW, F-HS caused a higher increase in shoot FW, foliar area, and shoot RGR, compared to R-HS, showing a slight increase in these parameters at 10.00 mL/L dose. Concerning STI, it was enhanced in lettuce plants treated with HS (55%, 44%, 78%, 84%, for R-HS 0.40 mL/L, R-HS 0.60 mL/L, F-HS 7.50 mL/L, and F-HS 10.00 mL/L, respectively, compared to lettuce non-treated with HS), (Table 1; Fig. 1).
Na+ and K+concentrations, DC, and Na+/K+ ratio
Under salinity stress without biostimulant application, shoot and root Na+ concentrations, as well as Na+ DC increased (908%, 188%, and 250%, respectively), compared with control treatment (Fig. 2A–C). However, shoot Na+ increased was lower after HS application at R-HS 0.40 mL/L (813%), 0.60 mL/L (801%), and F-HS 10.00 mL/L (801%) (Fig. 2A). In addition, the increase in root Na+ concentration was significantly higher (Fig. 2B) and, subsequently, the increase in Na+ DC was lower in HS-treated plants (199%, 193%, 208%, and 184%, at R-HS 0.40 and 0.60 mL/L, and F-HS 7.50 and 10.00 mL/L, respectively), with F-HS 10.00 mL/L being the lowest value (Fig. 2C). Furthermore, salt stress reduced shoot K+ − 19%), being this reduction lower under biostimulant application (− 7%, − 7%, − 8%, and − 7% at R-HS 0.40 and 0.60 mL/L, and F-HS 7.50 and 10.00 mL/L, respectively) (Fig. 2D). Besides, F-HS 7.50 mL/L increased root K+ concentration (30%) (Fig. 2E). In this way, K+ DC was reduced by 100 mM NaCl application (− 25%), compared with control plants, although this reduction was lower with R-HS 0.40 (− 7%), R-HS0.60 mL/L (− 12%), and F-HS 10.00 mL/L (− 16%) (Fig. 2F). Thus, salinity stress without biostimulant increased Na+/K+ ratio in leaves (1140%), whereas this increase was significantly lower in HS-treated plants (869%, 877%, 979%, 886%, for R-HS 0.40 mL/L, R-HS 0.60 mL/L, F-HS 7.50 mL/L, and F-HS 10.00 mL/L, respectively) (Fig. 2G).
Effect of salinity stress and HS application on shoot Na+ (A), root Na+ (B), Na+ DC Distribution coefficient (C), shoot K+ (D), root K+ (E), K+ DC (F), and leaf Na+/K+ ratio (G). R-HS Radicular-Humic substances, F-HS Foliar-Humic substances. Values are expressed as means ± standard error (n = 6). Columns marked with the same letters were not significantly different based on the LSD test (p < 0.05)
Photosynthetic pigments concentration
The application of salinity stress without biostimulant did not affect Chl a, Chl b, and carotenoids concentrations compared with control treatment. Furthermore, R-HS 0.40 and 0.60 mL/L as well as F-HS 10.00 mL/L significantly enhanced Chl a (9%, 19%, and 9%, respectively) and b (8%, 16%, and 7%, respectively) concentrations, while R-HS 0.60 mL/L also increased carotenoids (7%), with respect to salt stress only. However, F-HS 7.50 mL/L significantly reduced photosynthetic pigments concentration (− 13%, − 11%, and − 19% for Chl a, b, and carotenoids, respectively), with respect to salt stress without biostimulant (Table 2).
Leaf gas exchange
In general, salinity conditions without biostimulant reduced A (− 59%), E (− 67%), Ci (− 20%), and gs (− 82%), compared with plants not supplied with 100 mM NaCl. Nevertheless, the reduction in A was lower under HS treatments (− 33%, − 32%, − 34%, and − 19%, at R-HS 0.40 and 0.60 mL/L and F-HS 7.50 and 10.00 mL/L, respectively). Furthermore, plants subjected to F-HS 10.00 mL/L did not show significant differences in A, E, and Ci, compared with control conditions, and increased gs with respect to stressed plants without HS. Besides, salinity stress without HS reduced Vcmax (− 46%), whereas HS application significantly enhanced it (12%, 14%, 19%, and 28% for R-HS 0.40 and 0.60 mL/L and F-HS 7.50 and 10.00 mL/L, respectively), compared with control conditions. Regarding WUE, a significant increase was found under salinity compared to conditions, presenting HS higher values (49%, 79%, 51%, and 67% at R-HS 0.40 and 0.60 mL/L and F-HS 7.50 and 10.00 mL/L, respectively) (Table 3).
Chl a fluorescence
Plants treated with 100 mM NaCl without HS application showed a significant decrease in area (− 42%), Fv/Fm (− 3%), PIABS (− 11%), ΦEo (− 5%), Sm (− 21%), N (− 37), and ETo/RC (− 24%), compared with control conditions. This reduction was significantly lower in plants treated with the leonardite-SC-based product. In addition, all HS doses increased PIABS compared to control treatment (20%, 10%, 6%, 28% for R-HS 0.40 and 0.60 mL/L and F-HS 7.50 and 10.00 mL/L, respectively), and did not affect ΦEo, with respect to control treatment, although the higher values of ΦEo were found after F-HS 10.00 mL/L application (Fig. 3, Table S1).
Effect of salinity stress and HS application on Chl a fluorescence parameters. Fv/Fm (Maximum quantum yield for primary photochemistry), PIABS (Performance index), ΦEo (Maximum quantum yield for electron transport), Sm (Energy needed to reduce reaction centres), N Number of times that quinone A is reduced from time 0 to time the maximum fluorescence is reached, ETo/RC The electron flux from quinone A. R-HS Radicular-Humic substances, F-HS Foliar-Humic substances. All parameters are represented on the same scale, for which data were normalized. Values are means from six data (n = 6)
Oxidative stress indicators
Salinity conditions without biostimulant application increased EL (171%) percentage and MDA (338%) concentration, although this increase was significantly lower in lettuce supplied with HS (128%, 127%, 116%, and 102% for EL, 241%, 257%, 133%, and 165% for MDA, under R-HS 0.40 and 0.60 mL/L, and F-HS 7.50 mL/L and 10.00 mL/L, respectively) (Fig. 4A, B). Consequently, O2.− generation was higher after 100 mM NaCl addition without HS (225%), whereas under HS lower O2.− was found (205%, 203%, 169%, and 160% for R-HS 0.40 and 0.60 mL/L, and F-HS 7.50 mL/L and 10.00 mL/L, respectively) (Fig. 4C). Similarly, H2O2 was increased under salt stress conditions (37%), with lower values in HS-treated plants (9% and 8% for F-HS 7.50 and 10.00 mL/L, respectively), whereas R-HS did not show differences compared with the control (Fig. 4D).
Effect of salinity stress and HS application on oxidative stress indicators: EL Electrolyte leakage (A), MDA (Malondialdehyde) (B), O2.− (Superoxide radical) (C), and H2O2 (Hydrogen peroxide) (D). R-HS Radicular-Humic substances, F-HS Foliar-Humic substances. Values are expressed as means ± standard error (n = 9). Columns marked with the same letters were not significantly different based on the LSD test (p < 0.05)
Antioxidant enzyme activities
SOD activity was significantly increased under 100 mM NaCl without HS (14%), compared with control treatment. However, plants treated with all HS doses showed a decrease in SOD activity (− 11%, − 22%, − 30%, and − 30% at R-HS 0.40 and 0.60 mL/L, and F-HS 7.50 and 10.00 mL/L, respectively) (Fig. 5A). Concerning APX, and comparing with control plants, salt stress conditions without HS as well as R-HS at 0.40 and 0.60 mL/L enhanced APX activity (27%, 20%, and 20%, respectively), whereas both F-HS did not affect it (Fig. 5B).
Effect of salinity stress and HS application on SOD Superoxide dismutase (A) and APX Ascorbate peroxidase (B) activities. R-HS Radicular-Humic substances, F-HS Foliar-Humic substances. Values are expressed as means ± standard error (n = 9). Columns marked with the same letters were not significantly different based on the LSD test (p < 0.05)
Non-enzymatic antioxidants
Total phenols and flavonoids were increased under salinity stress without biostimulant (184% and 88%, respectively) compared with the control treatment. Nevertheless, this increase was lower under HS application for both total phenols (141%, 100%, 73%, 108%) and flavonoids (54%, 45%, 28%, 39%) at R-HS 0.40 and 0.60 mL/L, and F-HS 7.50 and 10.00 mL/L, respectively. In addition, total AsA was also enhanced under salt stress (60%, 63%, 39%, 53%, and 58%, for non-treated plants with biostimulant, R-HS 0.40 and 0.60 mL/L, and F-HS 7.50 and 10.00 mL/L, respectively), compared with control conditions. Salinity treatment without HS also increased AsA concentration (23%). However, R-HS 0.40 mL/L and F-HS 7.50 mL/L did not affect AsA concentration, whereas R-HS 0.60 mL/L and F-HS 10.00 mL/L decreased it (− 28 and − 30%, respectively), compared with the control. Moreover, DHA was enhanced under salinity (with or without HS application), being this increment higher in HS-treated plants, and showing F-HS 10.00 mL/L the highest value (180%). Regarding total GSH, salinity without BLAKCJAK® application increased its concentration (92%) compared with the control, whereas this increment was lower in HS-treated plants (47%, 33%, 31%, and 32%, at R-HS 0.40 and 0.60 mL/L, and F-HS 7.50 and 10.00 mL/L, respectively). Besides, salinity stress without biostimulant and salinity stress with R-HS at both doses increased GSH (119%, 40%, and 46%, respectively), compared to control. In addition, salinity enhanced GSSG, showing salt stress without HS and R-HS 0.40 mL/L the largest increase (56% and 57%, respectively) (Table 4).
Pro concentration
Plants subjected to salinity stress conditions and not treated with leonardite-SC showed a significant increase in Pro accumulation (311%) compared with the control treatment. Similarly, plants treated with R-HS 0.60 mL/L also presented an increase in Pro with respect to control (148%), although this increase was lower. However, the remaining HS doses did not significantly affect Pro concentration with respect to control plants, and lower Pro levels were found in F-HS (Fig. 6).
Discussion
Biomass production, RGR, and STI
The main effect of salt stress is plant growth reduction, as observed in different vegetables, including lettuce plants (Leyva et al. 2011; Navarro-León et al. 2020a; Behera et al. 2022; Sardar et al. 2023). In the present study, salt stress reduced plant growth parameters, whereas the application of radicular and foliar-HS caused a lower reduction in lettuce growth, which resulted in higher STI. Moreover, better plant tolerance was observed in plants subjected to F-HS, particularly at 10.00 mL/L, in terms of FW (Table 1, Fig. 1). This fact is of great importance, considering that lettuce is a leafy vegetable mainly consumed fresh (Tan et al. 2020). Our results align with different studies that have shown the potential use of radicular and foliar HS as plant biostimulants to enhance salt tolerance. In this way, Moubarak et al. (2022) found that radicular HA application minimized the negative effect of salinity on lettuce growth. Similar results were shown in strawberry plants by adding HA to nutritive solution (Saidimoradi et al. 2019), in wheat subjected to foliar HA and FA (Sary and Hamed 2021), and in citrus seedlings treated with radicular FA (Zhang et al. 2023). Hence, our findings suggest that the leonardite-SC-based product used in the present study could be a good candidate for increasing lettuce tolerance to salinity stress, being foliar method slightly better than radicular, especially at 10.00 mL/L. These results could be attributed to different physiological mechanisms, including an enhancement in photosynthesis activity, decreasing ROS generation and ionic imbalances, and maintaining cell membrane stability (Acosta-Motos et al. 2017). In addition, among the physiological effects of HS, the induction of root plasma membrane (PM)-H+-ATPase, stands out as a mechanism of action to increase root growth, nutrient uptake, and shoot growth (Olaetxea et al. 2019), which could favour lettuce growth under salt stress. Thus, Souza et al. (2021) found an induction of root PM-H+-ATPase activity in tomato, thereby preventing salt damage. Thus, BLAKCJAK® application could increase PM-H+-ATPase activity, enhancing lettuce growth in saline conditions, although further research is needed.
Na + and K + concentrations and ratio
The toxic effects of salinity stress are primarily attributed to Na+, whose increase is correlated with a decrease in essential nutrients such as K+. Thus, ionic imbalances caused by salinity negatively impact cell metabolism and essential enzyme activities (Javed et al. 2022). Therefore, reducing Na+ uptake and translocation to leaves, while maintaining a low Na+/K+ ratio leads to increased tolerance to salinity (Navarro-León et al. 2020b). In our experiment, the application of root and foliar-HS caused a reduction in Na+ translocation from roots to leaves, especially at F-HS 10.00 mL/L (Fig. 2C). Furthermore, an increase in K+ translocation to the shoot was observed, in general, after BLACKJAK® application, resulting in higher K+ accumulation (Fig. 2E, F). Consequently, HS caused a lower Na+/K+ ratio (Fig. 2G), which might aid in salt tolerance.
As previously observed, the application of HS-based products under salinity conditions protects plants from ionic imbalances, thus improving growth under salinity. Thus, commercial HA added to nutrient solution reduced Na+/K+ ratio in pepper plants subjected to salt stress (Bacilio et al. 2016). Besides, radicular-HA reduced Na+ accumulation in shoots, while enhancing K+ in two cultivars of strawberry (Saidimoradi et al. 2019). Similar results were subsequently obtained in citrus subjected to FA (Zhang et al. 2023) and in quinoa plants treated with HA (Rekaby et al. 2023), under salt stress conditions. Khaleda et al. (2017) suggested the induction of a sodium influx transporter HIGH-AFFINITY K+ TRANSPORTER (HKT1) as a possible mechanism underlying the reduction in Na+ translocation to shoots after HS application. In this way, HA induced HKT1 in Arabidopsis thaliana under saline conditions, reducing the Na+ flux to shoots, and improving salt tolerance. In addition, Na+ compartmentalization in vacuoles or apoplast through the induction of membranes transporters, has also been proposed as HS-mechanism of action to mitigate salt stress. Thus, Souza et al (2021) found that HA induced SOS3 (Salt Overlay Sensitive-3) expression in maize plants, suggesting that it could prepare a molecular response to salt stress, reducing cytosol Na+ content. Although BLACKJAK® reduced Na+ translocation (Fig. 2C), increased leaves K+ accumulation (Fig. 2D), and decreased Na+/K+ ratio (Fig. 2G), the differences in relation to the negative saline control were not excessively high. For this reason, BALCKJAK® could promote Na+ compartmentalization in vacuoles or apoplast. Nevertheless, to test this hypothesis, future studies focusing on the HS induction of membranes Na+ and K+ transporters, such as HKT or SOS, are needed to elucidate the mechanism of action of HS to alleviate salt stress.
Photosynthesis performance
Photosynthetic activity reduction is common in plants exposed to salinity due to the inhibition of photosynthetic pigments synthesis, damages to photosynthetic machinery, decreasing electron (e−) transport flux between photosystem II (PSII) and I (PSI), affecting Rubisco activity and CO2 assimilation efficiency (Acosta-Motos et al. 2017; Yang et al. 2020; Zahra et al. 2022). In this context, photosynthetic pigments (Chls a and b, and carotenoids) concentrations could be reduced under salinity (Hamani et al. 2020; Shen et al. 2022; Singh et al. 2022b). However, in the present study, the application of 100 mM NaCl did not affect pigments concentration with respect to the control conditions. Besides, HS applied at R-HS at 0.60 mL/L enhanced Chls and carotenoids compared with control plants (Table 2). As has been previously observed, depending on plant species or NaCl concentration, pigments may not be affected by salinity (Cantabella et al. 2017). Thus, in some mint ecotypes, salinity did not affect pigments concentration (Hosseini et al. 2021). Akladious and Mohamed (2018) and Hassanein et al. (2022) also found that HA addition enhanced pigments concentration in salt-stressed pepper and coriander, respectively, with respect to control conditions, which agrees with our results for R-HS 0.60 mL/L.
A correct CO2 supply is crucial for optimal Rubisco activity, the main enzyme of the photosynthetic process. Under stress conditions, Rubisco activity may be negatively affected. Thus, CO2 fixation and assimilation and, ultimately, the net photosynthetic rate could be reduced, thereby affecting biomass production and plant development (Yan et al. 2022; Zahra et al. 2022). For this reason, measuring leaf gas exchange parameters through IRGARs analyzers offers us an approximation of the photosynthetic status of plants, which is of great importance, especially in abiotic stress studies (Navarro-León et al. 2023). In the present experiment, plants subjected to root or foliar-HS showed a lower reduction of A (Table 3). Similarly, it has been reported that HA enhance the photosynthetic rate in tomato plants under 200 mM NaCl (Souza et al. 2021), as well as in perennial ryegrass subjected to 250 mM NaCl (Meng et al. 2023), resulting in higher plant tolerance to salt stress. Additionally, salt-tolerant species display higher A under salt conditions with respect to salt sensitive (El-Hendawy et al. 2017; Mahlooji et al. 2018; Yang et al. 2020). A possible explanation for this improved photosynthetic activity is the reduction of toxic Na+ translocation to leaves through apoplastic barriers (Yang et al. 2020). Hence, the decrease in Na+ DC observed after HS application (Fig. 2C) could contribute to maintain a higher A (Table 3). Particularly, F-HS 10.00 mL/L was the dose that most reduced Na+ DC and, consequently, did not present differences in A with respect to control, showing these plants higher salt tolerance.
In addition, the increased photosynthetic rate in salinity-tolerant plants is associated with enhanced CO2 fixation efficiency through Rubisco activity (Acosta-Motos et al. 2017; Smith et al. 2023). Thus, in the present experiment, although no differences were observed in stomatal fluxes (E or gs) among all the plants subjected to salinity treatments, the increased Vcmax found after HS application, especially with F-HS (Table 3) could contribute to maintaining A under these conditions. The physiological mechanisms through which HS stimulate plant growth have also been related to the enhancement of Rubisco activity, enabling higher photosynthetic rates and increased crop productivity (Ertani et al. 2019), which could be critical for plant growth under saline conditions (Kaushal 2020. Furthermore, enhanced A may explain the increased WUE in plants subjected to the leonardite-SC-based product (Table 3), contributing to better water status, which is crucial under salinity stress (Sheteiwy et al. 2022). Our results are in line with other studies where HS helped maintain an efficient photosynthetic rate and WUE under abiotic stress, improving Rubisco activity, and conferring plant tolerance. In this way, Rady et al. (2016) reported a significant improvement in WUE and photosynthesis efficiency in cotton plants treated with HA under salt stress. Similarly, Chen et al. (2022a, b) found an increase in Rubisco activity and, consequently, an enhancement in A and WUE in two maize genotypes exposed to water deficit.
Photochemical reactions may also be affected by high NaCl concentrations (Zahra et al. 2022). An approximation to PSII activity could be achieved by Chl a fluorescence parameters analysis (Garab et al. 2023). Under stress conditions, Chl a fluorescence emission is increased and some parameters such as Area, Fv/Fm, and PIABS are decreased. In this way, Lucini et al. (2015) observed a reduction in the Fv/Fm ratio after the application of 25 mM NaCl to lettuce plants. Kalhor et al. (2018) also found that PIABS was reduced in lettuce subjected to 80 mM NaCl, whereas 40 and 80 mM NaCl also decreased Fv/Fm. Besides, in salt-tolerant plants, this reduction is lower than in salt-sensitive plants. For this reason, these parameters are usually employed as indicators of salt tolerance (Song et al. 2023). In addition, some Chl a fluorescence parameters indicate the efficiency of e− transport through PSII, as ΦEo, Sm, N, or ETo/RC, which are decreased under abiotic stress (Kalhor et al. 2018). In the present study, the application of radicular and foliar-HS conferred a maintenance of PSII performance, enhancing e− transport between PSII and PSI, and increasing QA reduction rate (Fig. 3, Table S1), thereby contributing to improved growth under salt stress. The role of HS in PSII status protection under salinity conditions was shown in Plantago ovata L. (Gholami et al. 2013), cotton (Rady et al. 2016), and maize plants (Kaya et al. 2018), where HS enhanced maximum quantum yield and performance index, which is in line with our results. In addition, it has also been reported that HA could protect PSII components, such as D1 protein, by decreasing excitation energy (Ozfidan-Konakci et al. 2018). Hence, our data suggest the protection of photosynthesis performance as a possible action mechanism to mitigate salt stress in lettuce by HS, although future research is required to uncover the fundamental molecular mechanisms behind this improvement.
Oxidative stress indicators and antioxidants
Salinity causes oxidative stress through an increase in ROS (i.e., O2.− and H2O2) production, which trigger damage to cell membranes, resulting in MDA generation and EL (Mousavi et al. 2022; Bai et al. 2023). As previously found, lettuce exposed to salt stress presented an increase in EL (Hniličková et al. 2019) and MDA (Bai et al. 2023), which reduced plant growth. HS application resulted in reduced ROS generation and lipid peroxidation, showing F-HS doses the lowest O2.− and MDA levels (Fig. 4A, D). The maintenance of higher K+ concentrations is usually linked to lower membrane lipid oxidation (Cantabella et al. 2017; Navarro-León et al. 2020b). Therefore, the largest values of shoot K+ and K+ DC (Fig. 2D, F), found in lettuce treated with HS compared with stressed plants without HS, could counteract the cell membrane damage caused by salinity. Besides, our data align with other studies that demonstrate a reduction in ROS generation and lipid peroxidation under salinity induced by HS applications (Saidimoradi et al. 2019; Shukry et al. 2023; Zhang et al. 2023).
The generation of ROS under abiotic stress conditions, such as salinity, usually leads to an enhancement in enzymatic antioxidant activity and the biosynthesis of non-enzymatic antioxidants (Acosta-Motos et al. 2017). SOD and APX contribute to detoxify O2− and H2O2, respectively, whereas phenolic compounds, AsA, and GSH may also detoxify ROS and reduce lipid peroxidation (Hasanuzzaman et al. 2021; Chourasia et al. 2022). Overall, the application of root and foliar-HS did not enhance the antioxidant capacity of lettuce subjected to salinity stress (Fig. 5A, B, Table 4). As previously reported, salt-tolerant plants present different physiological strategies to cope with salinity: avoid ionic imbalances, improve antioxidant capacity, enhance photosynthetic activity, or biosynthesis of compatible solutes (Acosta-Motos et al. 2017; Feng et al. 2019; Yang et al. 2020). In our experiment, the enhanced tolerance of lettuce treated with HS to salinity caused a reduction in antioxidants activity and biosynthesis, which indicates that salinity effects in these plants could be counteracted by other mechanisms such as improvement in K+ homeostasis and photosynthetic activity. Similar results were observed by other studies, which demonstrated lower antioxidant enzymes activity (Saidimoradi et al. 2019) and antioxidant biosynthesis (Shukry et al. 2023) in HS-treated plants under saline conditions, showing these plants higher growth and lower oxidative damage.
Pro concentration
Pro concentration is usually increased after salinity stress exposed (De la Torre-González et al. 2018). Pro is an amino acid with multifunctionality under abiotic stress: it is an osmoprotectant, stabilizes protein structure, contributes to maintain cell turgor, and acts as a ROS scavenger (Alvarez et al. 2022). For this reason, Pro concentration is widely used as a salt stress indicator (Cantabella et al. 2017). The highest Pro accumulation was found in lettuce not treated with HS, whereas F-HS did not increase Pro levels (Fig. 6). Higher Pro concentration could indicate plant damage and negatively impact plant growth (Shukry et al. 2023). Likewise, different studies agreed with our results. Thus, Saidimoradi et al. (2019) showed an increase in plant growth and a reduction in Pro accumulation in strawberry subjected to salinity + HA, suggesting that reduced Pro may indicate salinity stress alleviation. In addition, Shukry et al. (2023) found reduced Pro in maize plants subjected to salt stress and treated with HA, with respect to plants without biostimulant. These authors reported that plants subjected to HA presented higher biomass, despite reduced Pro, and concluded that these plants invested more energy in growth than in Pro biosynthesis. This fact could explain the results obtained in our experiment for both Pro accumulation and antioxidant capacity.
Conclusions
Our results indicate that root and foliar-HS applications at the doses employed enhance salt stress tolerance in lettuce, as evidenced by higher biomass production, shoot RGR, leaf area, and STI. This tolerance could be induced by HS´s contribution to ionic homeostasis, reducing Na+ translocation to the shoot, and increasing shoot K+ concentration, as well as the improvement in photosynthesis performance. In addition, both root and foliar-HS applications reduced oxidative damage through lower ROS generation and lipid peroxidation. Comparing both modes of application, F-HS caused a better improvement in the analyzed parameters than R-HS, especially at 10.00 mL/L dose. Hence, our data suggest that BLAKCJAK® enhances lettuce growth under salinity stress with both radicular and foliar applications, being foliar mode slightly better. Future research, especially focusing on molecular aspects, would be crucial to understand the physiological mechanisms through which BLACKJAK® induces salt stress tolerance.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Abd El-Mageed TA, Mekdad AAA, Rady MOA, Abdelbaky AS, Saudy HS, Shaaban A (2022) Physio-biochemical and agronomic changes of two sugar beet cultivars grown in saline soil as influenced by potassium fertilizer. J Soil Sci Plant Nutr 22:3636–3654. https://doi.org/10.1007/s42729-022-00916-7
Abd-Elrahman ShH, Saudy HS, Abd El–Fattah DA, Hashem FA (2022) Effect of irrigation water and organic fertilizer on reducing nitrate accumulation and boosting lettuce productivity. J Soil Sci Plant Nutr 22:2144–2155. https://doi.org/10.1007/s42729-022-00799-8
Abou El-Enin MM, Sheha AM, El-Serafy Rasha S, Ali OAM, Saudy HS, Shaaban A (2023) Foliage-sprayed nano-chitosan-loaded nitrogen boosts yield potentials, competitive ability, and profitability of intercropped maize-soybean. Inter J Plant Prod 17:517–542. https://doi.org/10.1007/s42106-023-00253-4
Abu-Ria M, Shukry W, Abo-Hamed S, Albaqami M, Almuqadam L, Ibraheem F (2023) Humic acid modulates ionic homeostasis, osmolytes content, and antioxidant defense to improve salt tolerance in rice. Plants 12:1834. https://doi.org/10.3390/plants12091834
Acosta-Motos JR, Ortuño MF, Bernal-Vicente A, Diaz-Vivancos P, Sanchez-Blanco MJ, Hernandez JA (2017) Plant responses to salt stress: adaptive mechanisms. Agronomy 7:18. https://doi.org/10.3390/agronomy7010018
Akladious SA, Mohamed HI (2018) Ameliorative effects of calcium nitrate and humic acid on the growth, yield component and biochemical attribute of pepper (Capsicum annuum) plants grown under salt stress. Sci Hortic 236:244–250. https://doi.org/10.1016/j.scienta.2018.03.047
Ali IAA, Hassan Soheir E, Abdelhafez AA, Hewidy M, Nasser MA, Saudy HS, Hassan KM, Abou Hadid AF (2024a) Modifying the growing media and bio stimulants supply for healthy gerbera (Gerbera jamesonii) flowers. Gesunde Pflanzen. 76(337):345. https://doi.org/10.1007/s10343-023-00943-z
Ali MAA, Nasser MA, Abdelhamid AN, Ali IAA, Saudy HS, Hassan KM (2024b) Melatonin as a key factor for regulating and relieving abiotic stresses in harmony with phytohormones in horticultural plants—a Review. J Soil Sci Plant Nutr 24:54–73. https://doi.org/10.1007/s42729-023-01586-9
Alvarez ME, Savouré A, Szabados L (2022) Proline metabolism as regulatory hub. Trends Plant Sci 27:39–55. https://doi.org/10.1016/j.tplants.2021.07.009
Atero-Calvo S, Magro F, Masetti G, Navarro-León E, Rios JJ, Ruiz JM (2023) Assaying the use of a leonardite-suspension concentrate-based product as a potential biostimulant to enhance growth, NPK use efficiency, and antioxidant capacity in Lactuca sativa L. Agronomy 14:64. https://doi.org/10.3390/agronomy14010064
Bacilio M, Moreno M, Bashan Y (2016) Mitigation of negative effects of progressive soil salinity gradients by application of humic acids and inoculation with Pseudomonas stutzeri in a salt-tolerant and a salt-susceptible pepper. Appl Soil Ecol 107:394–404. https://doi.org/10.1016/j.apsoil.2016.04.012
Bai Y, Zhou Y, Yue T et al (2023) Plant growth-promoting rhizobacteria Bacillus velezensis JB0319 promotes lettuce growth under salt stress by modulating plant physiology and changing the rhizosphere bacterial community. Environ Exp Bot 213:105451. https://doi.org/10.1016/j.envexpbot.2023.105451
Behera TK, Krishna R, Ansari WA et al (2022) Approaches involved in the vegetable crops salt stress tolerance improvement: present status and way ahead. Front Plant Sci 12:1–20. https://doi.org/10.3389/fpls.2021.787292
Cantabella D, Piqueras A, Acosta-Motos JR et al (2017) Salt-tolerance mechanisms induced in Stevia rebaudiana Bertoni: effects on mineral nutrition, antioxidative metabolism and steviol glycoside content. Plant Physiol Biochem 115:484–496. https://doi.org/10.1016/j.plaphy.2017.04.023
Chen TC, Chou SY, Chen MC, Lin JS (2022a) IbTLD modulates reactive oxygen species scavenging and DNA protection to confer salinity stress tolerance in tobacco. Plant Sci 323:111415. https://doi.org/10.1016/j.plantsci.2022.111415
Chen Q, Qu Z, Ma G et al (2022b) Humic acid modulates growth, photosynthesis, hormone and osmolytes system of maize under drought conditions. Agric Water Manag 263:107447. https://doi.org/10.1016/j.agwat.2021.107447
Chourasia KN, More SJ, Kumar A et al (2022) Salinity responses and tolerance mechanisms in underground vegetable crops: an integrative review. Planta 255:1–25. https://doi.org/10.1007/s00425-022-03845-y
De la Torre-González A, Montesinos-Pereira D, Blasco B, Ruiz JM (2018) Influence of the proline metabolism and glycine betaine on tolerance to salt stress in tomato (Solanum lycopersicum L.) commercial genotypes. J Plant Physiol 231:329–336. https://doi.org/10.1016/j.jplph.2018.10.013
Doklega SMA, Saudy HS, El-Sherpiny MA, Abou El-Yazied A, Abd El-Gawad HG, Ibrahim MFM, Abd El-Hady MAM, Omar MMA, Metwally AA (2024) Rhizospheric addition of hydrogel polymer and zeolite plus glutathione mitigate the hazard effects of water deficiency on common bean plants through enhancing the defensive antioxidants. Gesunde Pflanzen 76:235–249. https://doi.org/10.1007/s10343-023-00947-9
Duursma RA (2015) Plantecophys-An R package for analysing and modelling leaf gas exchange data. PLoS ONE 10:1–13. https://doi.org/10.1371/journal.pone.0143346
El-Hendawy SE, Hassan WM, Al-Suhaibani NA, Refay Y, Abdella KA (2017) Comparative performance of multivariable agro-physiological parameters for detecting salt tolerance of wheat cultivars under simulated saline field growing conditions. Front Plant Sci 8:1–15. https://doi.org/10.3389/fpls.2017.00435
El-Metwally IM, Geries L, Saudy HS (2022a) Interactive effect of soil mulching and irrigation regime on yield, irrigation water use efficiency and weeds of trickle–irrigated onion. Archiv Agron Soil Sci 68:1103–1116. https://doi.org/10.1080/03650340.2020.1869723
El-Metwally IM, Saudy HS, Elewa TA (2022b) Natural plant by-products and mulching materials to suppress weeds and improve sugar beet (Beta vulgaris L.) yield and quality. J Soil Sci Plant Nutr 22:5217–5230. https://doi.org/10.1007/s42729-022-00997-4
Ertani A, Nardi S, Francioso O et al (2019) Metabolite-targeted analysis and physiological traits of Zea mays L. in response to application of a leonardite-humate and lignosulfonate-based products for their evaluation as potential biostimulants. Agronomy. https://doi.org/10.3390/agronomy9080445
Farquhar GD, Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149:78–90
Feng H, Tang Q, Cai J, Xu B, Xu G, Yu L (2019) Rice OsHAK16 functions in potassium uptake and translocation in shoot, maintaining potassium homeostasis and salt tolerance. Planta 250:549–561. https://doi.org/10.1007/s00425-019-03194-3
Fu J, Huang B (2001) Involvement of antioxidants and lipid peroxidation in the adaptation of two cool-season grasses to localized drought stress. Environ Exp Bot 45:105–114. https://doi.org/10.1016/S0098-8472(00)00084-8
Garab G, Magyar M, Sipka G, Lambrev PH (2023) New foundations for the physical mechanism of variable chlorophyll a fluorescence. Quantum efficiency versus the light-adapted state of photosystem II. J Exp Bot 74:5458–5471. https://doi.org/10.1093/jxb/erad252
García AC, Santos LA, de Souza LGA et al (2016) Vermicompost humic acids modulate the accumulation and metabolism of ROS in rice plants. J Plant Physiol 192:56–63. https://doi.org/10.1016/j.jplph.2016.01.008
Gholami H, Samavat S, Ardebili ZO (2013) The alleviating effects of humic substances on photosynthesis and yield of Plantago ovate in salinity conditions. Int Res J Appl Basic Sci 4:1683–1686
Hadid ML, Ramadan KhMA, El Beltagi HS, Ramadan AA, El Metwally IM, Shalaby TA, Bendary ESA, Saudy HS (2023) Modulating the antioxidant defense systems and nutrients content by proline for higher yielding of wheat under water deficit. Not Bot Horti Agrobo 51(3):13291
Hamani AKM, Wang G, Soothar MK (2020) Responses of leaf gas exchange attributes, photosynthetic pigments and antioxidant enzymes in NaCl-stressed cotton (Gossypium hirsutum L.) seedlings to exogenous glycine betaine and salicylic acid. BMC Plant Biol 20:1–14. https://doi.org/10.1186/s12870-020-02624-9
Hasanuzzaman M, Raihan MRH, Masud AAC et al (2021) Regulation of reactive oxygen species and antioxidant defence in plants under salinity. Int J Mol Sci 22:9326. https://doi.org/10.3390/ijms22179326
Hassanein RA, Hussein OS, Farag IA et al (2022) Salt-stressed coriander (Coriandrum sativum L.) responses to potassium silicate, humic acid and gamma irradiation pretreatments. Agronomy 12:2268. https://doi.org/10.3390/agronomy12102268
Hidalgo-Santiago L, Navarro-León E, López-Moreno FJ et al (2021) The application of the silicon-based biostimulant Codasil® offset water deficit of lettuce plants. Sci Hortic 285:110177. https://doi.org/10.1016/j.scienta.2021.110177
Hniličková H, Hnilička F, Orsák M, Hejnák V (2019) Effect of salt stress on growth, electrolyte leakage, Na+ and K+ content in selected plant species. Plant Soil Environ 65(90):96
Hosseini SJ, Tahmasebi-Sarvestani Z, Pirdashti H et al (2021) Investigation of yield, phytochemical composition, and photosynthetic pigments in different mint ecotypes under salinity stress. Food Sci Nutr 9:2620–2643. https://doi.org/10.1002/fsn3.2219
Hussain S, Ali B, Ren X, Chen X, Li Q, Saqib M, Ahmad N (2021) Recent progress in understanding salinity tolerance in plants: story of Na+/K+ balance and beyond. Plant Physiol Biochem 160:239–256. https://doi.org/10.1016/j.plaphy.2021.01.029
Irigoyen JJ, Einerich DW, Sánchez-Díaz M (1992) Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol Plant 84:55–60. https://doi.org/10.1111/j.1399-3054.1992.tb08764.x
Javed SA, Shahzad SM, Ashraf M (2022) Interactive effect of different salinity sources and their formulations on plant growth, ionic homeostasis and seed quality of maize. Chemosphere 291:132678. https://doi.org/10.1016/j.chemosphere.2021.132678
Junglee S, Urban L, Sallanon H, Lopez-Lauri F (2014) Optimized assay for hydrogen peroxide determination in plant tissue using potassium iodide. Am J Anal Chem 5:730–736. https://doi.org/10.4236/ajac.2014.511081
Kalhor MS, Aliniaeifard S, Seif M et al (2018) Title: Enhanced salt tolerance and photosynthetic performance: Implication of ɤ-amino butyric acid application in salt-exposed lettuce (Lactuca sativa L.) plants. Plant Physiol Biochem 130:157–172. https://doi.org/10.1016/j.plaphy.2018.07.003
Kaushal M (2020) Insights into microbially induced salt tolerance and endurance mechanisms (STEM) in plants. Front Microbiol 11:530189. https://doi.org/10.3389/fmicb.2020.01518
Kaya C, Akram NA, Ashraf M, Sonmez O (2018) Exogenous application of humic acid mitigates salinity stress in maize (Zea mays L.) plants by improving some key physico-biochemical attributes. Cereal Res Commun 46:67–78. https://doi.org/10.1556/0806.45.2017.064
Khaleda L, Park HJ, Yun DJ et al (2017) Humic acid confers HIGH-AFFINITY K+ TRANSPORTER 1-mediated salinity stress tolerance in arabidopsis. Mol Cells 40:966–975
Kubiś J (2008) Exogenous spermidine differentially alters activities of some scavenging system enzymes, H2O2 and superoxide radical levels in water-stressed cucumber leaves. J Plant Physiol 165:397–406. https://doi.org/10.1016/j.jplph.2007.02.005
Lasheen FF, Hewidy M, Abdelhamid AN, Thabet RS, Abass MMM, Fahmy Asmaa A, Saudy HS, Hassan KM (2024) Exogenous application of humic acid mitigates salinity stress on pittosporum (Pittosporum tobira) plant by adjusting the osmolytes and nutrient homeostasis. Gesunde Pflanzen 76:317–325. https://doi.org/10.1007/s10343-023-00939-9
Law MY, Charles SA, Halliwell B (1983) Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. The effect of hydrogen peroxide and of Paraquat. Biochem J 210:899–903. https://doi.org/10.1042/bj2100899
Leyva R, Sánchez-Rodríguez E, Ríos JJ et al (2011) Beneficial effects of exogenous iodine in lettuce plants subjected to salinity stress. Plant Sci 181:195–202. https://doi.org/10.1016/j.plantsci.2011.05.007
Liu C, Mao B, Yuan D, Chu C, Duan M (2022) Salt tolerance in rice: Physiological responses and molecular mechanisms. Crop J 10:13–25. https://doi.org/10.1016/j.cj.2021.02.010
Liu X, Ji H, Zhang C, Sun N, **a T, Wang Z, Wang X (2024) The poly-γ-glutamic acid-producing bacterium Bacillus amyloliquefaciens W25 enhanced the salt tolerance of lettuce by regulating physio-biochemical processes and influencing the rhizosphere soil microbial community. Environ Exp Bot 220:105679. https://doi.org/10.1016/j.envexpbot.2024.105679
Lucini L, Rouphael Y, Cardarelli M et al (2015) The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci Hortic 182:124–133. https://doi.org/10.1016/j.scienta.2014.11.022
Mahlooji M, Sharifi RS, Razmjoo J et al (2018) Effect of salt stress on photosynthesis and physiological parameters of three contrasting barley genotypes. Photosynthetica 56:549–556. https://doi.org/10.1007/s11099-017-0699-y
Makhlouf BSI, Khalil SRA, Saudy HS (2022) Efficacy of humic acids and chitosan for enhancing yield and sugar quality of sugar beet under moderate and severe drought. J Soil Sci Plant Nutr. https://doi.org/10.1007/s42729-022-00762-7
Mangal V, Lal MK, Tiwari RK et al (2023) Molecular insights into the role of reactive oxygen, nitrogen and sulphur species in conferring salinity stress tolerance in plants. J Plant Growth Regul 42:554–574. https://doi.org/10.1007/s00344-022-10591-8
Man-hong Y, Lei Z, Sheng-tao X (2020) Effect of water soluble humic acid applied to potato foliage on plant growth, photosynthesis characteristics and fresh tuber yield under different water deficits. Sci Rep 10:1–10. https://doi.org/10.1038/s41598-020-63925-5
Marriboina S, Attipalli RR (2020) Hydrophobic cell-wall barriers and vacuolar sequestration of Na+ ions are among the key mechanisms conferring high salinity tolerance in a biofuel tree species, Pongamia pinnata L. pierre. Environ Exp Bot 171:103949
Meng Q, Yan M, Zhang J et al (2023) Humic acids enhance salt stress tolerance associated with pyrroline 5-carboxylate synthetase gene expression and hormonal alteration in perennial ryegrass (Lolium perenne L.). Front Plant Sci 14:1272987. https://doi.org/10.3389/fpls.2023.1272987
Moubarak K, Gabr S, Aboukila E, Brengi S (2022) Possibility of overcoming salt stress of lettuce plants using humic acid and mycorrhiza. J. Adv. Agric. Res. 27:193–211
Mousavi SS, Karami A, Maggi F (2022) Photosynthesis and chlorophyll fluorescence of Iranian licorice (Glycyrrhiza glabra L.) accessions under salinity stress. Front Plant Sci 13:1–19. https://doi.org/10.3389/fpls.2022.984944
Mubarak M, Salem EMM, Kenawey MKM, Saudy HS (2021) Changes in calcareous soil activity, nutrient availability, and corn productivity due to the integrated effect of straw mulch and irrigation regimes. J Soil Sci Plant Nutr 21:2020–2031. https://doi.org/10.1007/s42729-021-00498-w
Navarro-León E, López-Moreno FJ, Atero-Calvo S (2020a) CAX1a TILLING mutations modify the hormonal balance controlling growth and ion homeostasis in Brassica rapa plants subjected to salinity. Agronomy 10:1699. https://doi.org/10.3390/agronomy10111699
Navarro-León E, López-Moreno FJ, de la Torre-González A et al (2020b) Study of salt-stress tolerance and defensive mechanisms in Brassica rapa CAX1a TILLING mutants. Environ Exp Bot 175:104061. https://doi.org/10.1016/j.envexpbot.2020.104061
Navarro-León E, Ruiz JM, Albacete A, Blasco B (2020c) Tolerance to cadmium toxicity and phytoremediation potential of three Brassica rapa CAX1a TILLING mutants. Ecotoxicol Environ Saf 189:109961. https://doi.org/10.1016/j.ecoenv.2019.109961
Navarro-León E, Paradisone V, López-Moreno FJ et al (2021) Effect of CAX1a TILLING mutations on photosynthesis performance in salt-stressed Brassica rapa plants. Plant Sci. https://doi.org/10.1016/j.plantsci.2021.111013
Navarro-León E, Grazioso A, Atero-Calvo S et al (2023) Evaluation of the alkalinity stress tolerance of three Brassica rapa CAX1 TILLING mutants. Plant Physiol Biochem. https://doi.org/10.1016/j.plaphy.2023.107712
Noctor G, Foyer CH (1998) Simultaneous measurement of foliar glutathione, γ-glutamylcysteine, and amino acids by high-performance liquid chromatography: Comparison with two other assay methods for glutathione. Anal Biochem 264:98–110. https://doi.org/10.1006/abio.1998.2794
Olaetxea M, Mora V, Bacaicoa E et al (2019) Root ABA and H+-ATPase are key players in the root and shoot growth-promoting action of humic acids. Plant Direct 3:e00175. https://doi.org/10.1002/pld3.175
Ore OT, Adeola AO, Fapohunda O et al (2023) Humic substances derived from unconventional resources: extraction, properties, environmental impacts, and prospects. Environ Sci Pollut Res 30:59106–59127. https://doi.org/10.1007/s11356-023-26809-5
Ozfidan-Konakci C, Yildiztugay E, Bahtiyar M, Kucukoduk M (2018) The humic acid-induced changes in the water status, chlorophyll fluorescence and antioxidant defense systems of wheat leaves with cadmium stress. Ecotoxicol Environ Saf 155:66–75. https://doi.org/10.1016/j.ecoenv.2018.02.071
Pizzeghello D, Schiavon M, Francioso O (2020) Bioactivity of size-fractionated and unfractionated humic substances from two forest soils and comparative effects on N and S metabolism, nutrition, and root anatomy of Allium sativum L. Front Plant Sci 11:1203. https://doi.org/10.3389/fpls.2020.01203
Rady MM, El-Mageed TAA, Abdurrahman HA, Mahdi AH (2016) Humic acid application improves field performance of cotton (Gossypium barbadense L.) under saline conditions. J Ani Plant Sci 26:487–493
Ramadan KMA, El-Beltag HS, Abd El Mageed TA, Mazrou KE, Mohamed GF, El-Saadony MT, El-Saadony FMA, Roby MHH, Saudy HS, Abou-Sreea AIB (2023a) Significance of selenium in ameliorating the effects of irrigation deficit via improving photosynthesis efficiency, cell integrity, osmo-protectants, and oil profile of anise crop. Not Bot Horti Agrobo 51(4):13437
Ramadan KMA, El-Beltagi HS, Abd El-Mageed TAA, Saudy HS, Al-Otaibi HH, Mahmoud MAA (2023b) The changes in various physio-biochemical parameters and yield traits of faba bean due to humic acid plus 6-benzylaminopurine application under deficit irrigation. Agron 13:1227. https://doi.org/10.3390/agronomy13051227
Rao MV, Paliyath G, Ormrod DP (1996) Ultraviolet-B- and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol 110:125–136. https://doi.org/10.1104/pp.110.1.125
Rekaby SA, AL-Huqail AA, Gebreel M, Alotaibi SS, Ghoneim AM (2023) Compost and humic acid mitigate the salinity stress on quinoa (Chenopodium quinoa Willd L.) and improve some sandy soil properties. J Soil Sci Plant Nutr 23:2651–2661. https://doi.org/10.1007/s42729-023-01221-7
Rivero RM, Ruiz JM, García PC, Lopez-Lefebre LR, Sánchez E, Romero L (2001) Resistance to cold and heat stress: accumulation of phenolic compounds in tomato and watermelon plants. Plant Sci 160:315–321. https://doi.org/10.1016/S0168-9452(00)00395-2
Saidimoradi D, Ghaderi N, Javadi T (2019) Salinity stress mitigation by humic acid application in strawberry (Fragaria x ananassa Duch). Sci Hortic 15:108594
Salem EMM, Kenawey MKM, Saudy HS, Mubarak M (2021) Soil mulching and deficit irrigation effect on sustainability of nutrients availability and uptake, and productivity of maize grown in calcareous soils. Commun Soil Sci Plant Anal 52:1745–1761. https://doi.org/10.1080/00103624.2021.1892733
Sardar H, Khalid Z, Ahsan M et al (2023) Enhancement of salinity stress tolerance in lettuce (Lactuca sativa L.) via foliar application of nitric oxide. Plants 12:1115
Sary DH, Hamed E (2021) Effect of salicylic, humic and fulvic acids application on the growth, productivity and elements contents of two wheat varieties grown under salt stress. J Soil Sci Agric Eng 12:657–671
Saudy HS, Hamed MF, Abd El–Momen WR, Hussein H (2020) Nitrogen use rationalization and boosting wheat productivity by applying packages of humic, amino acids and microorganisms. Commun Soil Sci Plant Anal 51:1036–1047. https://doi.org/10.1080/00103624.2020.1744631
Saudy HS, El-Bially MA, Hashem FA, Shahin MG, El–Gabry YA (2023a) The changes in yield response factor, water use efficiency, and physiology of sunflower owing to ascorbic and citric acids application under mild deficit irrigation. Gesunde Pflanzen 75:899–909. https://doi.org/10.1007/s10343-022-00736-w
Saudy HS, Salem EMM, Abd El–Momen WR (2023b) Effect of potassium silicate and irrigation on grain nutrient uptake and water use efficiency of wheat under calcareous soils. Gesunde Pflanzen 75:647–654. https://doi.org/10.1007/s10343-022-00729-9
Shaaban A, Abd El-Mageed TA, Abd El-Momen WR, Saudy HS, Al-Elwany OAAI (2023a) The integrated application of phosphorous and zinc affects the physiological status, yield and quality of canola grown in phosphorus-suffered deficiency saline soil. Gesunde Pflanzen 75:1813–1821. https://doi.org/10.1007/s10343-023-00843-2
Shaaban A, Mahfouz H, Megawer EA, Saudy HS (2023b) Physiological changes and nutritional value of forage clitoria grown in arid agro-ecosystem as influenced by plant density and water deficit. J Soil Sci Plant Nutr 23:3735–3750. https://doi.org/10.1007/s42729-023-01294-4
Shah AN, Tanveer M, Abbas A (2021) Targeting salt stress co** mechanisms for stress tolerance in Brassica: a research perspective. Plant Physiol Biochem 158:53–64. https://doi.org/10.1016/j.plaphy.2020.11.044
Shahzad B, Rehman A, Tanveer M et al (2021) Salt stress in Brassica: Effects, tolerance mechanisms, and management. J Plant Growth Regul 41:781–795. https://doi.org/10.1007/s00344-021-10338-x
Shen Z, Pu X, Wang S et al (2022) Silicon improves ion homeostasis and growth of liquorice under salt stress by reducing plant Na+ uptake. Sci Rep 12:5089. https://doi.org/10.1038/s41598-022-09061-8
Sheteiwy MS, Ulhassan Z, Qi W et al (2022) Association of jasmonic acid priming with multiple defense mechanisms in wheat plants under high salt stress. Front Plant Sci 13:886862. https://doi.org/10.3389/fpls.2022.886862
Shukry WM, Abu-Ria ME, Abo-Hamed SA et al (2023) The efficiency of humic acid for improving salinity tolerance in salt sensitive rice (Oryza sativa): Growth responses and physiological mechanisms. Gesunde Pflanz 75:2639–2653. https://doi.org/10.1007/s10343-023-00885-6
Singh P, Choudhary KK, Chaudhary N et al (2022a) Salt stress resilience in plants mediated through osmolyte accumulation and its crosstalk mechanism with phytohormones. Front Plant Sci 13:1006617. https://doi.org/10.3389/fpls.2022.1006617
Singh P, Kumar V, Sharma J et al (2022b) Silicon supplementation alleviates the salinity stress in wheat plants by enhancing the plant water status, photosynthetic pigments, proline content and antioxidant enzyme activities. Plants 11:2525. https://doi.org/10.3390/plants11192525
Smith J, Wijewardene I, Cai Y et al (2023) Co-overexpression of RCA and AVP1 in cotton substantially improves fiber yield for cotton under drought, moderate heat, and salt stress conditions. Curr Res Biotechnol 5:100123. https://doi.org/10.1016/j.crbiot.2023.100123
Soloklui AAG, Ershadi A, Fallahi E (2012) Evaluation of cold hardiness in seven iranian commercial pomegranate (Punica granatum L.) cultivars. HortScience 47:1821–1825
Song J, Yang H, Qiao C et al (2023) Natural variations of chlorophyll fluorescence and ion transporter genes influenced the differential response of japonica rice germplasm with different salt tolerances. Front Plant Sci 14:1095929. https://doi.org/10.3389/fpls.2023.1095929
Souza AC, Zandonadi DB, Santos MP et al (2021) Acclimation with humic acids enhances maize and tomato tolerance to salinity. Chem Biol Technol Agric 8:40. https://doi.org/10.1186/s40538-021-00239-2
Strasser RJ, Tsimilli-Michael M, Srivastava A (2004) Analysis of the Chlorophyll a fluorescence transient. Chlorophyll a fluorescence. Springer, Dordrecht, pp 321–362
Tan WK, Goenadie V, Lee HW et al (2020) Growth and glucosinolate profiles of a common Asian green leafy vegetable, Brassica rapa subsp chinensis var parachinensis (choy sum), under LED lighting. Sci Hortic 261:108922. https://doi.org/10.1016/j.scienta.2019.108922
Tiwari J, Ramanathan AL, Bauddh K, Korstad J (2023) Humic substances: Structure, function and benefits for agroecosystems—a review. Pedosphere 33:237–249. https://doi.org/10.1016/j.pedsph.2022.07.008
Valenzuela FJ, Reineke D, Leventini D (2022) Plant responses to heterogeneous salinity: agronomic relevance and research priorities. Ann Bot 129:499–518. https://doi.org/10.1093/aob/mcac022
Wellburn AR (1994) The Spectral Determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313. https://doi.org/10.1016/S0176-1617(11)81192-2
Wolf B (2008) A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Commun Soil Sci Plant Anal 13:1035–1059. https://doi.org/10.1080/00103628209367332
Yan S, Chong P, Zhao M (2022) Effect of salt stress on the photosynthetic characteristics and endogenous hormones, and: a comprehensive evaluation of salt tolerance in Reaumuria soongorica seedlings. Plant Signal Behav 17:e2031782. https://doi.org/10.1080/15592324.2022.2031782
Yang Z, Li JL, Liu LN, **e Q, Sui N (2020) Photosynthetic regulation under salt stress and salt-tolerance mechanism of sweet Sorghum. Front Plant Sci 10:1722. https://doi.org/10.3389/fpls.2019.01722
Yu Q, Osborne L, Rengel Z (1998) Micronutrient deficiency changes activities of superoxide dismutase and ascorbate peroxidase in tobacco plants. J Plant Nutr 21:1427–1437. https://doi.org/10.1080/01904169809365493
Zahra N, Al Hinai MS, Hafeez MB (2022) Regulation of photosynthesis under salt stress and associated tolerance mechanisms. Plant Physiol Biochem 178:55–69. https://doi.org/10.1016/j.plaphy.2022.03.003
Zhang M, Li X, Wang X, Feng J, Zhu S (2023) Potassium fulvic acid alleviates salt stress of citrus by regulating rhizosphere microbial community, osmotic substances and enzyme activities. Front Plant Sci 14:1161469. https://doi.org/10.3389/fpls.2023.1161469
Zuluaga MYA, Monterisi S, Rouphael Y (2023) Different vegetal protein hydrolysates distinctively alleviate salinity stress in vegetable crops: a case study on tomato and lettuce. Front Plant Sci 14:1077140. https://doi.org/10.3389/fpls.2023.1077140
Funding
Funding for open access publishing: Universidad de Granada/CBUA. This research was supported by the PAI program (Plan Andaluz de Investigación, Grupo de Investigación AGR282) and by a Grant from the FPU of the Ministerio de Universidades awarded to S.A.C. Grant number [FPU20/05049].
Author information
Authors and Affiliations
Contributions
FM, GM, BB, and JMR conceived and designed research. SAC and ENL conducted experiments. FM and GM provided biostimulant. SAC and ENL analyzed data. SAC wrote the manuscript. FM, GM, BB, and JMR did a critical revision of the article. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Atero-Calvo, S., Magro, F., Masetti, G. et al. Salinity stress mitigation by radicular and foliar humic substances application in lettuce plants. Plant Growth Regul (2024). https://doi.org/10.1007/s10725-024-01151-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10725-024-01151-z