Abstract
Global environmental concerns have recently accelerated interest in the usage of biodegradable polymers to replace petroleum-based conventional plastics. Lactic acid-based polymers are some of the most promising and widely studied biobased materials, which are suitable for packaging and biomedical applications. This is mainly due to their appealing characteristics such as relatively good mechanical properties, biocompatibility, and multiple end-of-life options such as recyclability and biodegradability in industrial composting conditions. However, the use of lactic acid-based polymers in advanced applications is constrained by their inherent brittleness, poor melt strength, and relatively high cost. These disadvantages can be remedied by reinforcement with cellulose nanomaterials which can enhance their mechanical properties while maintaining their biodegradability. This review provides an overview of recent studies on the development of biodegradable lactic acid-based polymer composites and nanocomposites reinforced with cellulose nanofibrils (CNFs), cellulose nanocrystals (CNCs) and microcrystalline cellulose (MCC). The different processing methods and chemical modification techniques utilised on modification and functionalisation of cellulosic nanomaterials for improving the properties of lactic acid-based polymer nanocomposites are also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The rising cost and eventual depletion of petrochemical feedstocks, concern about environmental pollution and greenhouse emissions, and a general shift in fundamental beliefs towards sustainable manufacturing, have all contributed to the recent interest in biodegradable polymers. Bio-based polymers have been considered as potential alternatives to conventional petroleum-based polymers because they are derived from renewable resources and possess technical properties that are similar to polyolefins (Kanakannavar et al. 2021). Additionally, the desire by different industrial sectors, such as the plastics, transport, and biomedical industries, to implement circular economy principles also compels the use of compostable polymers and organic fillers derived from biomass waste residues such as cellulose nanomaterials or cellulose micromaterials.
Among the various bio-based polymers, lactic acid-based polymers have numerous desirable qualities, including relatively high mechanical performance, biocompatibility, and multiple end-of-life disposal routes, such as recyclability and biodegradability under industrial composting settings (Agbakoba et al. 2023b). Furthermore, traditional polymer processing methods and additive manufacturing processes can be used to fabricate lactic acid-based polymer products (Kanakannavar et al. 2021). However, the wide scale utility of lactic acid-based polymers in cutting-edge applications is hindered by their intrinsic brittleness, poor melt strength, and relatively high cost. The characteristics, and ultimate cost effectiveness, of lactic acid-based polymers could be enhanced through blending them with biopolymers or by strengthening them with inexpensive bio-based fillers (Kanakannavar et al. 2021).
Bio-based reinforcements such as cellulose nanoparticles or cellulose microparticles are among the most abundant renewable materials that can be produced from a variety of biomass sources such as plants, animals, algae, bacteria, and tunicates. They have drawn wide interest as reinforcements due to their extraordinary features, which include high stiffness, low density, dimensional stability, low coefficient of thermal stability, chemical inertness, and biodegradability (Ren et al. 2022). Additionally, the incorporation of cellulose materials into plant-based polymers such as lactic acid-based polymers (John et al. 2013), xylan (Naidu and John 2021) and natural rubber latex (Abraham et al. 2013) helps to maintain the green credentials and biodegradability of the product.
As such, several reviews which focus on the extraction of nanocellulose (NC) (Trache et al. 2016; Cherian et al. 2022; Stepanova and Korzhikova-Vlakh 2022; Bangar et al. 2023) and the development of lactic acid-based polymer/cellulose bionanocomposites (Trivedi et al. 2023; Mahmud et al. 2023; Pesaranhajiabbas et al. 2023) have been published. However, most of the reviews focus on reinforcement effects of a specific form of NC, for instance cellulose nanofibrils (CNFs) or cellulose nanocrystals (CNCs) without any comparative evaluations or discussions on bionanocomposites from both. This review focuses on, and highlights, the most recent and up-to-date achievements in the field of lactic acid-based polymer/cellulose biocomposites and bionanocomposites, that have been published in the last five years. The studies that were reviewed were focused on CNFs, CNCs and microcrystalline cellulose (MCC) reinforced lactic acid-based polymers. A comparative study of the reinforcing effect of the cellulosic micromaterials and nanomaterials is also presented. The use of additive manufacturing and several processing methods to manufacture MCC, CNCs, and CNFs reinforced lactic acid-based polymer biocomposites and bionanocomposites is also discussed. Additionally, the different methods of NC modification to enhance adhesion between the lactic acid-based polymers and cellulose, are also examined.
Lactic acid-based polymers
Lactic acid-based polymers are derived from lactic acid (2-hydroxy propionic acid) and its derivatives. Lactic acid is an organic acid found in nature. Lactic acid can be produced through either chemical synthesis or fermentation of carbohydrates such as sugars such as sugar beets, corn, rice, wheat, sugar cane and sweet corn (Mehta et al. 2005; Zhou et al. 2021; Mehrpouya et al. 2021) (Rajan et al. 2014). The synthesis of lactic acid-based polymers can occur through two main pathways, polycondensation or ring-opening polymerization (ROP) (Södergård and Stolt 2002; Rajan et al. 2014). Various methods can be utilized in the synthesis of lactic acid based homopolymers, copolymers and cross-linked polymers such as poly(lactic acid) (PLA), poly(lactide), and poly(lactide-co-glycolide) (Rajan et al. 2014).
PLA and poly(lactide) are bio-based and biodegradable polymers that belong to the aliphatic polyester group. Polycondensation of lactic acid yields a low molecular weight polymer (PLA), with relatively poor mechanical properties (Södergård and Stolt 2002; Mehrpouya et al. 2021). Additionally, polycondensation requires relatively long polymerization times (more than 30 h) and requires that the water formed as a by-product be removed to avoid the reduction in the polymer molecular weight (Mehrpouya et al. 2021).
Lactic acid has a chiral carbon atom in its molecular structure and exhibits enantiomerism. It has two enantiomers: D- and L-lactic acid. Consequently, three forms of PLA can be obtained through polycondensation of the two enantiomers; poly(L-lactic acid) (PLLA), poly(D-lactic acid) (PDLA) and poly(D,L-lactic acid) (PDLLA), which is a racemic polymer containing a random sequence of the enantiomers (Mehrpouya et al. 2021). According to Mehta et al. (2005), various factors including the molecular weight of polymer, thermal and processing history, temperature, and duration of annealing treatment affect the degree of crystallinity. PDLA is a semi-crystalline polymer, while PDLLA is an amorphous polymer (Mehta et al. 2005). Wasanasuk et al. confirmed the crystalline structure of PLLA-α form through the use of two-dimensional wide-angle X-ray (WAXD) and neutron diffraction (WAND) studies (Wasanasuk et al. 2011).
Figure 1 shows the chemical structures of the various PLA isomers (Rivero et al. 2017). Stereoisomerism in PLA has an influence on the thermal, mechanical, and biodegradation properties. PLLA has a relatively high melting temperature (Tm) as shown in Table 1 (Teboho et al. 2018; DeStefano et al. 2020).
Chemical structures of PLA isomers (Rivero et al. 2017). [Reproduced with permission from Elsevier]
Lactide is produced via oligomerisation and cyclisation of the two lactic acid enantiomers, D- and L-lactic acid. This also produces three stereoisomers, L-lactide, D-lactide and meso(D,L)-lactide as illustrated in Fig. 2(a) (Murphy and Collins 2018; Vert et al. 2020; Mehrpouya et al. 2021). ROP of lactide yields polylactide with high molecular weight (Fig. 2(b)) (Vert et al. 2020). This technique involves the oligomerization of lactic acid into a low molecular weight prepolymer, and subsequent cyclization of the prepolymer into a cyclic dimer (lactide), which undergoes ring-opening polymerization into poly(lactide) (Vert et al. 2020). ROP is widely used commercially, and it produces polylactide with relatively high molecular weight. Furthermore, ROP allows the control of the chemistry during polymerisation, thereby allowing the tailor making of desired properties (Södergård and Stolt 2002; Mehrpouya et al. 2021).
a Chemical structure of the stereoisomers of lactic acid (or 2-hydroxy propionic acid) and lactide (b) ROP of lactide to produce poly(lactide) (Mehrpouya et al. 2021). [Republished with permission from Elsevier]
The leading producers of lactic acid-based polymers include NatureWorks (Ingeo), TotalEnergies Corbion (Luminy), Synbra (Biofoam), Futero, Hisun, and Weforyou (Mehrpouya et al. 2021). Due to the challenges encountered in the synthesis of PLA through polycondensation of lactic acid, which were highlighted earlier on, the manufacturers mentioned above mostly produce high molecular weight polylactide (Reddy et al. 2013; Mehrpouya et al. 2021). NatureWorks, the leading producer of polylactide, uses ROP of lactic acid to produce polylactide and has a capacity to produce up to 150 000 tonnes of polylactide per year (Jem and Tan 2020). Approximately 200 000 tonnes of polylactide are produced annually worldwide with the global market estimated to be US$2 23 billion in 2017. The annual growth rate in the production of polylactide is estimated to grow by 20.5% between the year 2018 and 2026 (Jem and Tan 2020; Mehrpouya et al. 2021). Table 2 shows the various applications of lactic acid-based polymers (Rajeshkumar et al. 2021).
Cellulose
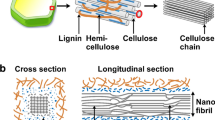
Cellulose is an abundant natural polymer accounting for approximately 1.5 × 1012 tonnes of the total biomass produced annually (Abraham et al. 2011; Reddy et al. 2013; Gauss et al. 2021). It is biodegradable, non-petroleum based, non-toxic to human and animal health, and it is environmentally friendly (Moon et al. 2011). Cellulose molecules are made up of repeating units of D-glucose units linked by β(1–4) glycosidic bonds which are organized to form a crystalline structure (Li et al. 2011; Trache et al. 2020; Kaur et al. 2021). Carbon 1 (C1) and carbon 4 (C4) form a 1–4 glycosidic bond that connects the two glucose rings. Cellulose is derived from wood, plants, algae, bacteria (bacterial cellulose (BC)), and tunicates and serves as an important structural component of plant cells (Ramires et al. 2020; Trache et al. 2020). Natural cellulose-based materials have been incorporated into several applications, including paints and coatings, electronic sensors, cosmetics, biomedical and pharmaceutical, pulp and paper, and composites (Moon et al. 2011; Mokhena and John 2020a, b).
Nanocellulose (NC) and microcrystalline cellulose (MCC) materials
The global NC market is predicted to grow at an annual growth rate of 20% from US$294.9 million in 2019 to US$1.08 billion in 2027 (Peter et al. 2022; Joe 2023). This envisaged growth is attributed to NC's superior properties such as biodegradability, biocompatibility, exceptional mechanical qualities, availability, low toxicity, low density, high aspect ratio, and high surface area. Cellu Force (Canada), Fibre Lean (Germany), Nippon Paper Industries Co. Ltd. (Japan), Kruger Inc. (Canada), Borregaard AS (Norway), CelluComp (UK), Melodea Ltd. (Israel), Blue Goose Refineries (Canada), GranBio Technologies (Brazil), and Stora Enso Biomaterials (Finland) are among the major companies that manufacture NC (Joe 2023).
Cellulose nanomaterials are classified according to the ISO/TS 20477:2017 guidelines set by the International Organization for Standardization (ISO). Table 3 displays the properties of different cellulose materials, with tunicate-cellulose nanocrystals (t-CNCs) having the highest crystallinity (Moon et al. 2011; Trache et al. 2020). The source or origin, part of biomass, polymerization, and crystallinity of the NC influences its shape, surface chemistry, degree of crystallinity, aspect ratio, and crystal structure (Trache et al. 2016; Gan et al. 2020). The degree of crystallinity can vary depending on the determination method such as nuclear magnetic resonance or X-ray diffraction spectroscopy etc. (Ling et al. 2019; Mokhena et al. 2021). NC extracted from oil palm (44–49%) was found to have tensile strengths of between 227–278 MPa and Young's moduli of between 2.7–3.2 GPa, whereas NC extracted from sisal (43–57%) exhibited tensile strengths of between 511–635 MPa and Young's moduli of between 9–22 GPa (Peter et al. 2022). Similarly, NC extracted from agro-waste residues exhibited tensile strengths of ~ 95.56 MPa for CNFs and ~ 32.77 MPa for CNCs (Mtibe et al. 2015).
MCC
MCC is microstructured cellulose derived from depolymerised α-cellulose which has been partially purified (Trache et al. 2016). In MCC, both paracrystalline and crystalline regions are present. The crystalline regions are formed by intermolecular and intramolecular interaction in-between adjacent cellulose chains in the form of hydrogen bonding, weak Van der Waal forces and London dispersion forces (Li et al. 2011; Trache et al. 2016; Nishiyama 2018). Structurally, MCC is very rigid and has a highly crystalline structure, making it insoluble in water (Bangar et al. 2023). The crystallinity of MCC is between 80—85% (Moon et al. 2011), however, the crystallinity of the MCC depends on the source of the cellulosic material and the extraction methods utilised. MCC has a diameter which ranges between 10—15 μm (Trache et al. 2016; Stepanova and Korzhikova-Vlakh 2022).
Industrially, MCC is derived from cotton and wood, but can also be extracted from non-woody plants such as herbaceous plants, grass, aquatic plants, and agricultural plants (Trache et al. 2016). As non-woody plants contain less lignin, the MCC extraction process requires less intensive bleaching. There are different pre-treatment extraction processes that are used to obtain cellulose pulp, including biological, physical, chemical processes or a combination of these (Trache et al. 2016). After pre-treatment, the MCC can be extracted from the cellulose pulp using a hydrolysis reaction, followed by neutralization, washing and drying as illustrated in Fig. 3. MCC is frequently extracted using freeze drying on an industrial scale (Trache et al. 2016).
Scheme of the common steps needed to produce MCC from cellulose source materials. (Trache et al. 2016). [Reproduced with permission from Elsevier]
CNFs
CNFs are long, flexible cellulose nanomaterials containing both amorphous and crystalline regions which co-exist in an alternative order (Cherian et al. 2022). CNFs can be entangled or individualized, with relatively large aspect ratios, high surface areas and large numbers of hydroxyl groups (Cherian et al. 2022). CNFs have diameters of between 1- 100 nm and lengths of between 100 nm to µm (Cherian et al. 2022). The majority of CNFs are sourced from wood, although they can be extracted from plant fibres such as jute fibres and pineapple; both of which result in CNFs with crystallinity 60–70% (Rojas et al. 2015; Peter et al. 2022). CNFs are extracted using several techniques as illustrated in Fig. 4. Techniques such as microfluidization and high-intensity ultrasonic treatment tend to produce strong shear gradients, which causes cellulose to cleave transversely along its longitudinal axis (Rojas et al. 2015). The microfibril structure is harmed by the transverse cleavage, which lowers the degree of polymerization and crystallinity (Rojas et al. 2015).
Conventional treatments to obtain cellulose nanoparticles (Rojas et al. 2015; Mokhena). [Reproduced with permission from IntechOpen]
CNCs
CNCs are obtained by the removal of amorphous regions in the CNFs, thereby promoting the formation of highly crystalline regions as illustrated in Fig. 5 (Trache et al. 2020; Gauss et al. 2021). The crystalline domains are resistant to mechanical, chemical, and enzymatic treatments (Gauss et al. 2021). CNCs are rod-like in shape and have high aspect ratios of between 10–100. CNCs have the ability to degrade faster than any other nanoparticles (Cherian et al. 2022; Abdelhamid and Mathew 2022).
Process for NC production through enzymatic hydrolysis. A Pretreatments of the lignocellulosic biomass for cellulose extraction; B Controlled enzymatic hydrolysis for production of CNFs and CNCs (rod-like and spherical) and their respective sizes; C Indication of the possible application of mechanical treatment after enzymatic hydrolysis, usually employed to obtain more uniform particles.(Michelin et al. 2020). [Reproduced with permission from MDPI]
In the production of CNCs from cellulosic materials, pre-treatment is used to remove hemicellulose, lignin, and other extractable materials before the extraction of CNCs (Trache et al. 2020). The next phase is the removal of amorphous regions using an acid hydrolysis agent such as H2SO4, leaving behind highly crystalline regions (Trache et al. 2020). The most widely used acid hydrolysis technique for producing CNCs uses H2SO4, because CNCs that were extracted using other chemicals, such as hydrochloric acid, lacks electrostatic repulsion between crystals, which leads to the aggregation of CNCs (Trache et al. 2020).
Surface modification of cellulose
NC materials have poor adhesion with thermoplastic materials because the hydroxyl groups on their surfaces render them hydrophilic, whereas most thermoplastic materials are hydrophobic (Bhagia et al. 2021). Weak adhesion has a significant impact on the mechanical strength and dimensional stability of composites, thus various types of modifications have been used to improve the compatibility between cellulose and thermoplastics. Chemical modifications include physicochemical pre-treatments such as acid treatment, esterification, etherification, crosslinking, and grafting, chemical modifications such as silanization and acetylation, and grafting reactions, all of which involve covalent bonding (Trache et al. 2020).
Figure 6 shows the different cellulose modification pathways (Trache et al. 2020). Silylation and esterification impart hydrophobicity to the cellulose surface, thus creating compatibility with hydrophobic thermoplastics. Silylation uses organosilanes such as n-propyl-, methacryloxy-, and acryloxyaminopropyltriethoxysilane, while esterification replaces the hydroxyl group with an acetyl group (Trache et al. 2020; Bhagia et al. 2021). Oxidation mediated by the 2,2,6,6-tetramethylpiperidine-1-oxyl radical (TEMPO) introduces carboxyl groups to the surface of cellulose, while cellulose retains hydrophilicity (Zhou et al. 2021). Pickering emulsion supports the dispersion of hydrophilic TEMPO-oxidised cellulose into a hydrophobic polymer matrix (Zhou et al. 2021). Li et al. (2019) used TEMPO-mediated oxidation to modify BC and produce TEMPO-oxidised BC (TOBC). In the study, it was observed that the TOBC functioned as a nucleating agent and promoted the crystallization of poly(lactide) (grade Ingeo 4043D, NatureWorks LLC, USA) leading to improved mechanical properties. Sulfonation modifies the cellulose surface with a strong acid. Borkotoky et al. (2018) prepared CNCs with H2SO4 and also observed that the CNCs acted as a nucleating agent at lower loadings in polylactide (grade: 4032 D, NatureWorks LLC, USA)/CNCs microcellular composite foams. Furthermore, Borkotoky et al. (2018) observed that the CNCs hindered chain folding of the polylactide at higher loadings.
Schematic representations of the different routes of modifying cellulose (Trache et al. 2020). [Reproduced with permission from National Library of Medicine]
Esterification is a common modification technique used for modifying the surfaces of CNCs. Minggang et al. (2022) used esterification in order to chemically modify NC to produce acetylated NC (ANC) by suspending CNCs in a solution of acetic anhydride and acetic acid. The modified ANC was incorporated into poly(lactide) (grade 4032, NatureWorks LLC, USA). The improvement in the crystallization of the polylactide was attributed to the nucleating effect of the ANC (Minggang et al. 2022). Gauss and Pickering (2023a) grafted L-lactide monomer onto CNFs using a solvothermal reaction. The grafted CNFs (g-CNFs) were blended with poly(lactide) (grade 2003D NatureWorks LLC, USA) polylactide/g-CNFs nanocomposites for 3D printing applications. The incorporation of g-CNFs into the poly(lactide) matrix resulted in 3D printed nanocomposites with improved the mechanical properties (Gauss and Pickering 2023). An et al. (2023) developed a biocomposite for antimicrobial food packaging film applications by blending poly(lactide) (grade 3052D, NatureWorks LLC, USA) with N-halamine modified MCC. The MCC was modified by grafting N-halamine 1-chloro-2,2,5,5-tetramethyl-4-imidazol-dinone to produce g-MCC, which was subsequently blended with the poly(lactide). The resulting polymer composites exhibited improved mechanical properties compared to those of composites fabricated from neat polylactide and MCC. For instance, the polylactide/g-MCC composite exhibited a modulus of 1030.99 MPa, which was relatively close to that of the neat poly(lactide) (1190.47 MPa), whereas that of the poly(lactide)/MCC was less than 1000 MPa. The tensile strength of the composites exhibited the same trend. These observations were attributed to good compatibility between the poly(lactide) and the g-MCC leading too even distribution of g-MCC within the poly(lactide) matrix (An et al. 2023).
Pornbencha et al. (2023) extracted CNCs from pineapple leaves, which were subsequently grafted with cinnamates to produce cinnamates-CNCs (Cin-CNCs). The Cin-CNCs were then blended with poly(lactide) (grade 3052D, NatureWorks, Thailand) to form a bionanocomposite. When compared to bionanocomposites produced using the untreated CNCs, the poly(lactide)/Cin-CNCs bionanocomposites demonstrated superior CNCs dispersion and transparency. The incorporation of the CNCs and Cin-CNCs into the poly(lactide) enhanced the mechanical properties of the poly(lactide), with the Cin-CNCs improving the tensile strength and Young’s modulus of the poly(lactide) by 70% and 30%, respectively. In comparison, the neat CNCs improved the tensile strength and Young’s modulus by 56% and 30%, respectively (Pornbencha et al. 2023).
Poly(lactide)-cellulose biocomposites and bionanocomposites
Extrusion, solvent casting, compression moulding, injection moulding, and the popular additive manufacturing (3D printing) can all be used to manufacture PLA composites. 3D printing has gained popularity over other manufacturing techniques due its ability to fabricate prototypes with complex geometries, relatively low costs and its use of few chemicals (Puppi and Chiellini 2020; Rigotti and Pegoretti 2022). In the following sections, studies on poly(lactide)-biocomposites and nanocomposites produced using different compounding methods and their resulting properties will be reviewed.
MCC reinforced poly(lactide) composites
MCC is of great interest due to its relatively high specific surface area and its ability to improve thermal and mechanical properties of polymers (Bangar et al. 2023). Cisneros-López et al. (2020) utilised injection moulding and 3D printing to develop composites using poly(lactide) (grade 4043D, NatureWorks LLC USA), an epoxy-based chain extender (Jonacryl ADR-4368), and MCC. Figure 8 showcases the distinct morphologies of the poly(lactide) composites obtained in this study using various procedures, as well as highlighting issues such as agglomeration and insufficient dispersion within the MCC-loaded poly(lactide) matrix. The injection-moulded samples exhibited a consistent appearance with subtle patterns from the moulding process. The poly(lactide) composites that exhibited voids were indicative of weak adhesion and inadequate compatibility between the materials. The presence of pores and consistent gaps in the 3D-printed samples was attributed to layering deposition during printing. This behaviour was also observed in the study by Cisneros-López et al. (2020) as shown in Fig. 7 (Cisneros-López et al. 2020). Agbakoba et al. (2023c) studied the mechanical recycling process of waste poly(lactide) resulting from unsuccessful fused filament fabrication (FFF) 3D prints that contained MCC and CNCs. When 20% of the recycled polymer that contained both the MCC and CNCs was blended with the neat poly(lactide), the resulting composite exhibited enhanced ultimate tensile strength (39.1 MPa) when compared to the ultimate strength of the virgin poly(lactide) material (31.6 MPa). The improvement in strength was attributed to the presence of the functionalized MCC and CNCs in the recycled poly(lactide) (Agbakoba et al. 2023c).
Scanning Electron Microscopy (SEM) micrographs of the microstructure of (a) and (b) Poly(lactide)/recycled poly(lactide); c and (d Poly(lactide)/recycled poly(lactide)/0.5 J; and (e) and (f) Poly(lactide)/recycled poly(lactide)/5MCC/0.5 J, produced by 3D printing and injection moulding, respectively (Cisneros-López et al. 2020). [Reproduced with permission from Elsevier]
Dos Santos et al. (2017) studied the effect of MCC on properties of poly(lactide) films which were prepared from blending poly(lactide) (grade 2002D, NatureWorks LLC, USA), MCC and R972 (organophilic silica) using the solution casting technique. The authors observed improved dispersion and mechanical properties in the composites with the addition of R972. This improvement was attributed to the R972, which functioned as a compatibiliser between MCC and the poly(lactide), resulting in the enhanced dispersion of MCC particles in the polymer matrix (dos Santos et al. 2017).
Iulianelli et al. (2023) developed biodegradable biocomposites by combining poly(lactide) (grade 2002D, NatureWorks LLC), poly(3-hydroxybutyrate) PHB, and MCC. In the study, the MCC was modified through sonication, leading to a comparative analysis of blends containing sonicated MCC and untreated MCC. Both the neat MCC and sonicated MCC functioned as nucleating agents, triggering crystallization in the poly(lactide)/PHB blends. This led to the emergence of a crystallization temperature (Tc) on the differential scanning calorimetry (DSC) cooling curve, whereas the neat poly(lactide) did not exhibit any crystallinity. The inclusion of the neat MCC or sonicated MCC at a loading of 7 wt.% led to a decline in the thermal stability of the blends. Specifically, the initial temperature for degradation decreased from 314 °C to 30 °C when neat MCC was incorporated into the blends at 7 wt.% content, whereas when the sonicated MCC was incorporated at the same composition, the onset degradation temperature decreased to 305 °C. This reduction in thermal stability was attributed to insufficient interaction between the filler and polymer matrix (Iulianelli et al. 2023).
Almasri et al. (2023) investigated the effect of gamma irradiation on properties of polylactide (Nature 3D, Japan)/MCC composites. A 3D printed biocomposite specimen was developed from the addition of 3% MCC to the poly(lactide) to produce a filament for 3D printing. The 3D printed specimen was exposed to 50 kGy irradiation under vacuum or air environment. Exposure to irradiation in the presence of air led to a decline in tensile strength of the biocomposites. After being subjected to a 50 kGy dose of irradiation, the tensile strength of the biocomposites dropped from 60 MPa to approximately 20 MPa. Interestingly, when the same radiation dosage was administered under vacuum conditions instead of an exposed air environment, an increase in tensile strength occurred from its initial value of 60 MPa to around 70 MPa. The observed enhancement in tensile strength was attributed to induced cross-linking phenomena that took place during irradiation. The process involved the formation of free radicals along with crosslinking between the poly(lactide) and MCC chains throughout the material structure (Almasri et al. 2023).
A study on the thermal stability of poly(lactide) (grade 3251, NatureWorks LLC, USA) reinforced with cellulose components (MCC and CNCs) was conducted by Bhiogade and Kannan (2021). Bhiogade and Kannan (2021) showed that CNCs degrades at a lower temperature than MCC. This is because CNCs have bigger surface areas when compared to MCC, which allows for faster heat trnafer (Bhiogade and Kannan 2021). In the study, the first thermal degradation step in the CNCs occurred at temperatures below 150 °C and it was attributed to the release of water molecules. The second CNCs thermal degradation step occurred between 200—35 °C and this was due the breakdown of cellulose into char residues. Bhiogade and Kannan (2021) also observed that the maximum degradation temperature of poly(lactide) decreased significantly from 365 °C to lower temperatures of between 220—265 °C when either CNCs or MCC were incorporated into the poly(lactide) matrix.
CNFs reinforced poly(lactide) nanocomposites
In this section, studies on the use of CNFs as a reinforcing agent for poly(lactide) are reviewed. John et al. (2021) investigated the effect of functionalised CNFs on properties of poly(lactide) (grade 1001, Cereplast Inc, Hawthorne, CA, USA)/poly(butylene succinate) blends The authors observed that canola oil modification of CNFs resulted in better dispersion leading to an increase in mechanical properties. One of the formulations was successfully used for 3D printing a crate, as illustrated in Fig. 8 (John et al. 2021).
a Representation of the CAD model of crate after slicing; b 3D-printed crate prototype from poly(lactide) and CNFs (John et al. 2021). [Reproduced with permission from MDPI]
Ren et al. (2022) studied the effects of acetylated CNFs on properties of poly(lactide) (grade TP-4000, UNITIKA Ltd., Japan) composites. They observed that acetylated CNFs exhibited increased hydrophobicity which led to better dispersion in the poly(lactide) matrix. The FTIR spectra in Fig. 9 shows that the peak at 1750 cm−1, which was attributed to the C = O vibration, was as a result of the acetic anhydride treatment. This peak was not observed in the FTIR spectrum of the unmodified CNFs. The inclusion of 2% modified CNFs in the poly(lactide) led to an increase its complex viscosity. This phenomenon was attributed to the ability of CNFs to create molecular entanglements within poly(lactide) matrix (Ren et al. 2022).
FTIR spectra of unmodified CNFs and modified CNFs (Ren et al. 2022). [Reproduced with permission from Elsevier]
Meng et al. (2018) investigated the properties of CNFs reinforced poly(lactide) (grade 4043D, NatureWorks LLC, USA) blended with epoxidised soyabean oil (ESO). The authors observed that the tensile strengthof the poly(lactide) increased by 3.13% from 63.9 MPa to 65.95 MPa and Young’s modulus increased from 3.2 GPa to 4.1G Pa, with the addition of 10% CNFs. Blending the poly(lactide) with ESO decreased the tensile strength and modulus of the poly(lactide) while increasing ductility. Poly(lactide)/CNFs/ESO nanocomposites outperformed poly(lactide)/ESO blends with respect to mechanical properties, with the nanocomposite with 30 wt.% CNFs and 5 wt.% ESO exhibiting the highest mechanical properties among the composites studied (Meng et al. 2018).
Clarkson et al. (2020a) investigated the thermal behaviour of poly(lactide) (grade Ingeo-3001D, NatureWorks LLC)/CNF/polyethylene glycol (PEG) nanocomposites. When compared to neat polylactide, the glass transition temperature (Tg) of the nanocomposites showed a significant decrease, demonstrating an increase in the poly(lactide) matrix mobility due to the addition of CNFs and PEG. The CNFs also acted as a nucleating agent, encouraging crystallization at lower temperatures compared to the neat poly(lactide), as evidenced by the decrease in the cold crystallization temperature (Tcc) shown in Table 4 (Clarkson et al. 2020a).
Composting and recycling are the most popular ways to dispose of poly(lactide), but there are other possibilities as well. Anaerobic digestion, incineration or thermal treatment, are some of the other poly(lactide) disposal options (Ghomi et al. 2021). Introducing agents which promote processes such as hydrolysis, heat activation, photolysis, biological activity, or oxidation can cause polymer degradation. Polymer degradation happens when the main chain or side chains of macromolecules split apart. Crystallinity, purity, molecular weight, temperature, and pH all have an impact on the degradation of poly(lactide) (Rajeshkumar et al. 2021).
Zabidi et al. (2022a) studied the biodegradation of poly(lactide) (grade Ingeo 7001D, NatureWorks LLC, USA)-nanofibrillated cellulose (NFC) composite sheets containing essential oils and anthocyanins. Figure 10 depicts the deterioration of the poly(lactide) and poly(lactide)-NFC composite sheets treated with 15% thymol (T), curry (C), and essential oil (EO) after being subjected to a composting medium for two weeks. The disintegration of samples containing 15% T was higher compared to the other samples. This was attributed to the presence of thymol hydroxide groups which form hydrogen bonds with the poly(lactide) matrix and therefore increase the disintegration rate. The presence of NFC also accelerated the degradation rate of the poly(lactide) (Zabidi et al. 2022b).
Poly(lactide)/NFC bionanocomposite films containing T, C and EO at loading 15% after 2 weeks (Zabidi et al. 2022b). [Modified and reproduced with permission from Elsevier]
CNCs reinforced poly(lactide) nanocomposites
Fortunati et al. (2012) studied the effect of the incorporation of CNCs into poly(lactide) (grade 3051D, NatureWorks LLC, USA) on the properties of poly(lactide)/CNCs nanocomposites using a solvent casting method. Higher crystallinity was observed at a DSC heating rate of 2 °C/min for the nanocomposites that contained CNCs modified with a surfactant (s-CNCs), this was attributed to the enhanced dispersion of the s-CNCs in the poly(lactide) matrix. It was observed in the study that increasing the heating rate decreased the melting temperature of the poly(lactide)/CNCs nanocomposites, whereas the neat poly(lactide) exhibited the opposite. The addition of s-CNCs into the poly(lactide) matrix resulted in an increase in the Tcc with increases in the DSC heating rate except for the 2 °C/min heating rate, as observed in Table 5 (Fortunati et al. 2012). The increase in the Tcc was attributed to the s-CNCs effectivelyacting as a nucleating agent for the poly(lactide). The addition of the CNCs also resulted in a decrease in the Tg, indicating increased chain mobility in the poly(lactide) polymer matrix.
Agbakoba et al. (2023a, b, c) analysed how the addition of CNCs affected the properties of polylactide (grade 6202D, NatureWorks LLC, USA) for its application in 3D printing. By incorporating CNCs into poly(lactide), they improved the crystallization behaviour due to their nucleating effect. This led to a higher degree of crystallinity in the bionanocomposite during cooling. Whereas neat poly(lactide) attained only 12.26% crystallinity, adding just 1% CNCs into the poly(lactide) resulted in an increase up to 23.33% crystallinity.
The mechanical properties of poly(lactide)/CNCs nanocomposites are affected by the addition of other polymers, plasticisers, compatibilisers, tougheners, natural additives, and copolymers (Fortunati et al. 2012; Cisneros-López et al. 2020). Table 6 shows mechanical properties of poly(lactide)/CNCs nanocomposites reported by numerous authors. The results obtained in these studies suggest that by modifying the surface of cellulose nanomaterials, the properties of the poly(lactide)/CNCs nanocomposites can be significantly improved. Modification of the surface of the cellulose nanomaterial aids in the even distribution and dispersion of the nanomaterials throughout the poly(lactide) matrix, which is a prerequisite for enhancing the properties of the nanocomposites. However, it was observed that when CNCs were incorporated into the poly(lactide) matrices at more than 2 wt.% content, there was a decrease in mechanical properties due to the increased agglomeration of the nanoparticles.
Gois et al. (2023) investigated the biodegradability of poly(lactide) (grade Ingeo 3251D, Cargil S.A., USA)-CNCs composite sheets as per ASTM G160-12 wherein the samples were exposed to garden soil for 190 days. The poly(lactide)/CNCs nanocomposites were treated with four different surfactants, which increased electrostatic repulsion or steric hindrance between the CNCs, preventing their agglomeration. Figure 11 shows the pictures of the poly(lactide)/CNCs nanocomposite sheets before and after being exposed to garden soil. A colour change was observed in the nanocomposite sheets, which was attributed to absorption of water and the presence of microorganisms. The susceptibility of nanocomposites to microbial attack was observed to increase in the presence of the surfactants. This was attributed to how the surfactants reduce the steric hindrance caused by the poly(lactide)’s alkyl group, thereby making it vulnerable to hydrolysis attacks on its ester groups. The sheets made from the poly(lactide)/CNCs nanocomposites that were enhanced with the sorbitan monolaurate SP20 and sorbitan monooleate SP80 surfactants, showed faster disintegration compared to those containing polyoxyethylene sorbitan monooleate (TW80) and polyoxyethylene sorbitan monooleate (TW20). This was explained by the fact that both SP20 and SP80 have lower molecular weights and longer alkyl chains, making them more susceptible to hydrolysis (Gois et al. 2023).
Photographs of poly(lactide), poly(lactide)/CNCs and poly(lactide)/CNCs/surfactant films before and after 90, 120, 150 and 180 days of exposure to garden soil (Gois et al. 2023). [Modified and reproduced with permission from Springer Nature]
Huang et al. (2023) studied the disintegration of poly(lactide) (grade Ingeo 4043D, NatureWorks LLC, USA)/CNCs nanocomposites under ISO-20200 composting conditions for 14 days. Methacrylamide (MAM), quaternary ammonium salt (CTAB), and metal oxide (ZnO) were used to modify the CNCs. The poly(lactide) nanocomposites with modified CNCs turned opaque and began to disintegrate on day 7, whereas the neat poly(lactide) did not disintergrate. Poly(lactide)/CNCs nanocomposites modified with ZnO (PLA-ZC8) exhibited weight loss after 14 days compared to other composites, as observed in Fig. 12 (Huang et al. 2023).
a Photos and (b) weight loss of neat and coated poly(lactide) films after incubating under composting conditions (ZC8:8 wt.% ZnO-CNCs, CC8:8 wt.% CTAB-CNCs and MC8: 8 wt.% MAM-CNCs) (Huang et al. 2023). [Modified and reproduced with permission from Elsevier]
Conclusions and perspectives
NC in various forms (CNCs and CNFs) and MCC have emerged as the most popular biobased reinforcements for biocomposites due to their properties such as biodegradability, biocompatibility, exceptional mechanical qualities, availability, low toxicity, low density, high aspect ratios, and high surface areas. Their reinforcing ability has resulted in the development of biocomposites and bionanocomposites for applications in the packaging, biomedical and transport sectors. Amongst the different nanocellulosic materials, studies have shown that CNFs imparts superior mechanical and barrier properties to poly(lactide). This is mainly due to the formation of hydrogen bonded networks between CNFs and poly(lactide).
Nanocomposites of poly(lactide), CNFs and CNCs can be developed using various processing techniques such as melt compounding, conventional processes, compression moulding and 3D printing. Additionally, the recent advances in both 4D and 5D printing techniques of biopolymers can also be considered as promising routes for the manufacturing of smart biomaterials for designing customised biomedical and pharmaceutical products.
The formation of agglomerates in NC materials poses a major challenge during the development of bionanocomposites. However, various studies have demonstrated that this issue can potentially be resolved by employing diverse chemical modification techniques on these materials. Notably, silyation, esterification, and TEMPO-mediated modifications show significant potential as they greatly improve both dispersion and the performance of CNCs and CNFs in the polymer matrices. Future studies can focus on the usage of biobased reagents to modify cellulose rather than the traditional approaches that use harsh chemicals while being less expensive.
Data availability
Not Applicable.
References
Abdelhamid HN, Mathew AP (2022) Cellulose-Based Nanomaterials Advance Biomedicine: A Review. Int J Mol Sci 23:5405. https://doi.org/10.3390/ijms23105405
Abraham E, Deepa B, Pothan LA et al (2011) Extraction of nanocellulose fibrils from lignocellulosic fibres: A novel approach. Carbohydr Polym 86:1468–1475. https://doi.org/10.1016/j.carbpol.2011.06.034
Abraham E, Deepa B, Pothan LA et al (2013) Physicomechanical properties of nanocomposites based on cellulose nanofibre and natural rubber latex. Cellulose 20:417–427. https://doi.org/10.1007/s10570-012-9830-1
Agbakoba VC, Hlangothi P, Andrew J, John MJ (2022) Mechanical and Shape Memory Properties of 3D-Printed Cellulose Nanocrystal (CNC)-Reinforced Polylactic Acid Bionanocomposites for Potential 4D Applications. Sustainability 14:12759. https://doi.org/10.3390/su141912759
Agbakoba VC, Hlangothi P, Andrew J, John MJ (2023) Preparation of cellulose nanocrystal (CNCs) reinforced polylactic acid (PLA) bionanocomposites filaments using biobased additives for 3D printing applications. Nanoscale Adv 5:4447–4463. https://doi.org/10.1039/D3NA00281K
Agbakoba VC, Mokhena TC, Ferg EE et al (2023) PLA bio-nanocomposites reinforced with cellulose nanofibrils (CNFs) for 3D printing applications. Cellulose 30:11537–11559. https://doi.org/10.1007/s10570-023-05549-2
Agbakoba VC, Webb N, Jegede E et al (2023) Mechanical Recycling of Waste PLA Generated From 3D Printing Activities: Filament Production and Thermomechanical Analysis. Macromol Mater Eng. https://doi.org/10.1002/mame.202300276
Ahmed MJ, Balaji MS, Saravanakumar S et al (2019) Characterization of Areva javanica fiber – A possible replacement for synthetic acrylic fiber in the disc brake pad. J Ind Text 49:294–317. https://doi.org/10.1177/1528083718779446
Almasri R, Akiyama Y, Manabe Y, Sato F (2023) A study on the prospects of vacuum gamma irradiation to enhance crosslinking for 3D-Printing PLA/MCC biocomposite filaments. Physics Open 15:100154. https://doi.org/10.1016/j.physo.2023.100154
An L, Perkins P, Yi R, Ren T (2023) Development of polylactic acid based antimicrobial food packaging films with N-halamine modified microcrystalline cellulose. Int J Biol Macromol 242:124685. https://doi.org/10.1016/j.ijbiomac.2023.124685
Bangar SP, Esua OJ, Nickhil C, Whiteside WS (2023) Microcrystalline cellulose for active food packaging applications: A review. Food Packag Shelf Life 36:101048. https://doi.org/10.1016/j.fpsl.2023.101048
Bhagia S, Bornani K, Agrawal R et al (2021) Critical review of FDM 3D printing of PLA biocomposites filled with biomass resources, characterization, biodegradability, upcycling and opportunities for biorefineries. Appl Mater Today 24:101078. https://doi.org/10.1016/j.apmt.2021.101078
Bhiogade A, Kannan M (2021) Studies on thermal and degradation kinetics of cellulose micro/nanoparticle filled polylactic acid (PLA) based nanocomposites. Polym Polym Compos 29:S85–S98. https://doi.org/10.1177/0967391120987170
Borkotoky SS, Chakraborty G, Katiyar V (2018) Thermal degradation behaviour and crystallization kinetics of poly (lactic acid) and cellulose nanocrystals (CNC) based microcellular composite foams. Int J Biol Macromol 118:1518–1531. https://doi.org/10.1016/J.IJBIOMAC.2018.06.202
Castro-Aguirre E, Iñiguez-Franco F, Samsudin H et al (2016) Poly(lactic acid)—Mass production, processing, industrial applications, and end of life. Adv Drug Deliv Rev 107:333–366. https://doi.org/10.1016/j.addr.2016.03.010
Cherian RM, Tharayil A, Varghese RT et al (2022) A review on the emerging applications of nano-cellulose as advanced coatings. Carbohydr Polym 282:119123. https://doi.org/10.1016/j.carbpol.2022.119123
Cisneros-López EO, Pal AK, Rodriguez AU et al (2020) Recycled poly(lactic acid)–based 3D printed sustainable biocomposites: a comparative study with injection molding. Mater Today Sustain 7–8:100027. https://doi.org/10.1016/j.mtsust.2019.100027
Clarkson CM, El Awad Azrak SM, Schueneman GT et al (2020) Crystallization kinetics and morphology of small concentrations of cellulose nanofibrils (CNFs) and cellulose nanocrystals (CNCs) melt-compounded into poly(lactic acid) (PLA) with plasticizer. Polymer (guildf) 187:122101. https://doi.org/10.1016/j.polymer.2019.122101
DeStefano V, Khan S, Tabada A (2020) Applications of PLA in modern medicine. Eng Regen 1:76–87. https://doi.org/10.1016/J.ENGREG.2020.08.002
Dinesh Kumar S, Venkadeshwaran K, Aravindan MK (2020) Fused deposition modelling of PLA reinforced with cellulose nano-crystals. Mater Today Proc 33:868–875. https://doi.org/10.1016/J.MATPR.2020.06.404
dos Santos FA, Iulianelli GCV, Tavares MIB (2017) Effect of microcrystalline and nanocrystals cellulose fillers in materials based on PLA matrix. Polym Test 61:280–288. https://doi.org/10.1016/J.POLYMERTESTING.2017.05.028
Farrington DW, Lunt J, Davies S, Blackburn RS (2005) Poly(lactic acid) fibers. Biodegradable and Sustainable Fibres. Elsevier, pp 191–220
Fortunati E, Armentano I, Zhou Q et al (2012) Microstructure and nonisothermal cold crystallization of PLA composites based on silver nanoparticles and nanocrystalline cellulose. Polym Degrad Stab 97:2027–2036. https://doi.org/10.1016/j.polymdegradstab.2012.03.027
Foss SW, Turra J-M (2014) Teabags and Coffee/Beverage Pouches Made From Mono-component, Mono-constituent Polylactic Acid (PLA) Fibers
Gan PG, Sam ST, Abdullah MFB, Omar MF (2020) Thermal properties of nanocellulose-reinforced composites: A review. J Appl Polym Sci 137:48544. https://doi.org/10.1002/app.48544
Gauss C, Pickering KL (2023) A new method for producing polylactic acid biocomposites for 3D printing with improved tensile and thermo-mechanical performance using grafted nanofibrillated cellulose. Addit Manuf 61:103346. https://doi.org/10.1016/j.addma.2022.103346
Gauss C, Pickering KL, Muthe LP (2021) The use of cellulose in bio-derived formulations for 3D/4D printing: A review. Compos Part C: Open Access 4:100113. https://doi.org/10.1016/j.jcomc.2021.100113
Ghomi ER, Khosravi F, Ardahaei AS et al (2021) polymers The Life Cycle Assessment for Polylactic Acid (PLA) to Make It a Low-Carbon Material. Polymers 13(11):1854. https://doi.org/10.3390/polym13111854
Gois GDS, Santos ASF, Hernandéz EP et al (2023) Biodegradation of PLA/CNC composite modified with non-ionic surfactants. Polymer Bull 80:11363–11377. https://doi.org/10.1007/s00289-022-04618-z
Hayes DG, Dharmalingam S, Wadsworth LC, et al (2012) Biodegradable Agricultural Mulches Derived from Biopolymers. pp 201–223. http://doi.org/10.1021/bk-2012-1114.ch013
Huang S, Zou S, Wang Y (2023) Construction of compostable packaging with antibacterial property and improved performance using sprayed coatings of modified cellulose nanocrystals. Carbohydr Polym 305:120539. https://doi.org/10.1016/j.carbpol.2023.120539
Iulianelli GCV, Costa LV, da Silva PSC, dos Santos FA (2023) Evaluation of Fully Biodegradable PLA/PHB Blend Filled with Microcrystalline Celluloses. Mater Res 26:e20220433. https://doi.org/10.1590/1980-5373-mr-2022-0433
Jandas PJ, Mohanty S, Nayak SK, Srivastava H (2011) Effect of surface treatments of banana fiber on mechanical, thermal, and biodegradability properties of PLA/banana fiber biocomposites. Polym Compos 32:1689–1700. https://doi.org/10.1002/pc.21165
Jem KJ, Tan B (2020) The development and challenges of poly (lactic acid) and poly (glycolic acid). Adv Ind Eng Polym Res 3:60–70. https://doi.org/10.1016/j.aiepr.2020.01.002
Joe C (2023) Top Companies in Nanocellulose Market by Size, Share, Historical and Future Data & CAGR | Report by Vantage Market Research. In: Vantage Market Research
John MJ, Anandjiwala R, Oksman K, Mathew AP (2013) Melt-spun polylactic acid fibers: Effect of cellulose nanowhiskers on processing and properties. J Appl Polym Sci 127:274–281. https://doi.org/10.1002/app.37884
John MJ, Dyanti N, Mokhena T et al (2021) Design and Development of Cellulosic Bionanocomposites from Forestry Waste Residues for 3D Printing Applications. Materials 14:3462. https://doi.org/10.3390/ma14133462
Jonoobi M, Harun J, Mathew AP, Oksman K (2010) Mechanical properties of cellulose nanofiber (CNF) reinforced polylactic acid (PLA) prepared by twin screw extrusion. Compos Sci Technol 70:1742–1747. https://doi.org/10.1016/J.COMPSCITECH.2010.07.005
Kanakannavar S, Pitchaimani J, Thalla A, Rajesh M (2021) Biodegradation properties and thermogravimetric analysis of 3D braided flax PLA textile composites. J Ind Text 51:1066S-1091S. https://doi.org/10.1177/15280837211010666
Kaur P, Sharma N, Munagala M et al (2021) Nanocellulose: Resources, Physio-Chemical Properties, Current Uses and Future Applications. Front Nanotechnol 3:747329. https://doi.org/10.3389/fnano.2021.747329
Kelnar I, Kaprálková L, Krejčíková S et al (2023) Effect of Polydopamine Coating of Cellulose Nanocrystals on Performance of PCL/PLA Bio-Nanocomposites. Materials 16:1087. https://doi.org/10.3390/ma16031087
Lasprilla AJR, Martinez GAR, Lunelli BH et al (2012) Poly-lactic acid synthesis for application in biomedical devices — A review. Biotechnol Adv 30:321–328. https://doi.org/10.1016/j.biotechadv.2011.06.019
Levy Y, Paz A, Ben YR et al (2009) Biodegradable inflatable balloon for reducing radiation adverse effects in prostate cancer. J Biomed Mater Res B Appl Biomater 91B:855–867. https://doi.org/10.1002/jbm.b.31467
Li Y, Lin M, Davenport JW (2011) Ab Initio Studies of Cellulose I: Crystal Structure, Intermolecular Forces, and Interactions with Water. J Phys Chem C 115:11533–11539. https://doi.org/10.1021/jp2006759
Li L, Chen Y, Yu T et al (2019) Preparation of polylactic acid/TEMPO-oxidized bacterial cellulose nanocomposites for 3D printing via Pickering emulsion approach. Compos Commun 16:162–167. https://doi.org/10.1016/j.coco.2019.10.004
Ling Z, Wang T, Makarem M et al (2019) Effects of ball milling on the structure of cotton cellulose. Cellulose 26:305–328. https://doi.org/10.1007/s10570-018-02230-x
Madhavan Nampoothiri K, Nair NR, John RP (2010) An overview of the recent developments in polylactide (PLA) research. Bioresour Technol 101:8493–8501. https://doi.org/10.1016/j.biortech.2010.05.092
Maharana T, Mohanty B, Negi YS (2009) Melt–solid polycondensation of lactic acid and its biodegradability. Prog Polym Sci 34:99–124. https://doi.org/10.1016/j.progpolymsci.2008.10.001
Mahmud MdA, Abir N, Anannya FR et al (2023) Coir fiber as thermal insulator and its performance as reinforcing material in biocomposite production. Heliyon 9:e15597. https://doi.org/10.1016/j.heliyon.2023.e15597
Mehrpouya M, Vahabi H, Janbaz S et al (2021) 4D printing of shape memory polylactic acid (PLA). Polymer (guildf) 230:124080. https://doi.org/10.1016/J.POLYMER.2021.124080
Mehta R, Kumar V, Bhunia H, Upadhyay SN (2005) Synthesis of Poly(Lactic Acid): A Review. J Macromol Sci Part C: Polym Rev 45:325–349. https://doi.org/10.1080/15321790500304148
Meng X, Bocharova V, Tekinalp H et al (2018) Toughening of nanocelluose/PLA composites via bio-epoxy interaction: Mechanistic study. Mater Des 139:188–197. https://doi.org/10.1016/j.matdes.2017.11.012
Michelin M, Gomes DG, Romaní A et al (2020) Nanocellulose Production: Exploring the Enzymatic Route and Residues of Pulp and Paper Industry. Molecules 25:3411. https://doi.org/10.3390/molecules25153411
Minggang F, Luo C, Guo X et al (2022) The Effect of Cellulose Nanocrystals and Acetylated Nanocellulose on the Crystallization Kinetics and Thermal Stability of Polylactic Acid. Polym Sci, Ser A 64:802–817. https://doi.org/10.1134/S0965545X22700523
Mokhena TC, John MJ (2020) Cellulose nanomaterials: new generation materials for solving global issues. Cellulose 27:1149–1194. https://doi.org/10.1007/s10570-019-02889-w
Mokhena TC, John MJ (2020) Esterified cellulose nanofibres from saw dust using vegetable oil. Int J Biol Macromol 148:1109–1117. https://doi.org/10.1016/j.ijbiomac.2020.01.278
Mokhena TC, Sadiku ER, Mochane MJ et al (2021) Mechanical properties of cellulose nanofibril papers and their bionanocomposites: A review. Carbohydr Polym 273:118507. https://doi.org/10.1016/j.carbpol.2021.118507
Moon RJ, Martini A, Nairn J et al (2011) Cellulose nanomaterials review: structure, properties and nanocomposites. Chem Soc Rev 40:3941. https://doi.org/10.1039/c0cs00108b
Mtibe A, Linganiso LZ, Mathew AP et al (2015) A comparative study on properties of micro and nanopapers produced from cellulose and cellulose nanofibres. Carbohydr Polym 118:1–8. https://doi.org/10.1016/j.carbpol.2014.10.007
Murphy CA, Collins MN (2018) Microcrystalline cellulose reinforced polylactic acid biocomposite filaments for 3D printing. Polym Compos 39:1311–1320. https://doi.org/10.1002/pc.24069
Naidu DS, John MJ (2021) Cellulose nanofibrils reinforced xylan-alginate composites: Mechanical, thermal and barrier properties. Int J Biol Macromol 179:448–456. https://doi.org/10.1016/j.ijbiomac.2021.03.035
Nishiyama Y (2018) Molecular interactions in nanocellulose assembly. Philos Trans R Soc A: Math Phys Eng Sci 376:20170047. https://doi.org/10.1098/rsta.2017.0047
Obuchi S, Ogawa S (2010) Packaging and Other Commercial Applications. Poly(Lactic Acid). Wiley, Hoboken, NJ, USA, pp 457–467
Papenburg BJ, Liu J, Higuera GA et al (2009) Development and analysis of multi-layer scaffolds for tissue engineering. Biomaterials 30:6228–6239. https://doi.org/10.1016/j.biomaterials.2009.07.057
Pesaranhajiabbas E, Misra M, Mohanty AK (2023) Recent progress on biodegradable polylactic acid based blends and their biocomposites: A comprehensive review. Int J Biol Macromol 253:126231. https://doi.org/10.1016/j.ijbiomac.2023.126231
Peter S, Lyczko N, Gopakumar D et al (2022) Nanocellulose and its derivative materials for energy and environmental applications. J Mater Sci 57:6835–6880. https://doi.org/10.1007/s10853-022-07070-6
Pornbencha K, Sringam S, Piyanirund S et al (2023) Functionalization of cellulose nanocrystals extracted from pineapple leaves as a UV-absorbing agent in poly(lactic acid). RSC Adv 13:15311–15321. https://doi.org/10.1039/D3RA02693K
Puppi D, Chiellini F (2020) Biodegradable Polymers for Biomedical Additive Manufacturing. Appl Mater Today 20:100700. https://doi.org/10.1016/J.APMT.2020.100700
Rajak D, Pagar D, Menezes P, Linul E (2019) Fiber-Reinforced Polymer Composites: Manufacturing, Properties, and Applications. Polymers (basel) 11:1667. https://doi.org/10.3390/polym11101667
Rajan R, Skrifvars M, Järvelä P (2014) Lactic Acid Polymers: Synthesis, Properties, and Applications. pp 49–65. https://doi.org/10.1142/9789814566469_0050
Rajeshkumar G, Arvindh Seshadri S, Devnani GL et al (2021) Environment friendly, renewable and sustainable poly lactic acid (PLA) based natural fiber reinforced composites – A comprehensive review. J Clean Prod 310:127483. https://doi.org/10.1016/J.JCLEPRO.2021.127483
Ramires EC, Megiatto JD, Dufresne A, Frollini E (2020) Cellulose Nanocrystals versus Microcrystalline Cellulose as Reinforcement of Lignopolyurethane Matrix. Fibers 8:21. https://doi.org/10.3390/fib8040021
Reddy MM, Vivekanandhan S, Misra M et al (2013) Biobased plastics and bionanocomposites: Current status and future opportunities. Prog Polym Sci 38:1653–1689. https://doi.org/10.1016/j.progpolymsci.2013.05.006
Ren Q, Wu M, Wang L et al (2022) Cellulose nanofiber reinforced poly (lactic acid) with enhanced rheology, crystallization and foaming ability. Carbohydr Polym 286:119320. https://doi.org/10.1016/j.carbpol.2022.119320
Rigotti D, Pegoretti A (2022) Additive manufacturing with biodegradable polymers. Biodegradable Polymers, Blends and Composites 611–679. https://doi.org/10.1016/B978-0-12-823791-5.00026-0
Rivero CP, Hu Y, Kwan TH, et al (2017) Bioplastics From Solid Waste. In: Current Developments in Biotechnology and Bioengineering. Elsevier, pp 1–26. https://doi.org/10.1016/B978-0-444-63664-5.00001-0
Rojas J, Bedoya M, Ciro Y (2015) Current Trends in the Production of Cellulose Nanoparticles and Nanocomposites for Biomedical Applications. In: Cellulose - Fundamental Aspects and Current Trends. InTech. https://doi.org/10.5772/61334
Södergård A, Stolt M (2002) Properties of lactic acid based polymers and their correlation with composition. Prog Polym Sci 27:1123–1163. https://doi.org/10.1016/S0079-6700(02)00012-6
Stepanova M, Korzhikova-Vlakh E (2022) Modification of Cellulose Micro- and Nanomaterials to Improve Properties of Aliphatic Polyesters/Cellulose Composites: A Review. Polymers (basel) 14:1477. https://doi.org/10.3390/polym14071477
Tan ML, Choong PFM, Dass CR (2010) Recent developments in liposomes, microparticles and nanoparticles for protein and peptide drug delivery. Peptides (NY) 31:184–193. https://doi.org/10.1016/j.peptides.2009.10.002
Teboho M, Sefadi J, Sadiku R et al (2018) Thermoplastic Processing of PLA/Cellulose Nanomaterials Composites. Polymers (basel) 10:1363. https://doi.org/10.3390/polym10121363
Trache D, Hussin MH, Hui Chuin CT et al (2016) Microcrystalline cellulose: Isolation, characterization and bio-composites application—A review. Int J Biol Macromol 93:789–804. https://doi.org/10.1016/J.IJBIOMAC.2016.09.056
Trache D, Tarchoun AF, Derradji M et al (2020) Nanocellulose: From Fundamentals to Advanced Applications. Front Chem 8:392. https://doi.org/10.3389/fchem.2020.00392
Trivedi AK, Gupta MK, Singh H (2023) PLA based biocomposites for sustainable products: A review. Adv Ind Eng Polym Res 6:382–395. https://doi.org/10.1016/j.aiepr.2023.02.002
Uribe-Calderón J, Rodrigue D, Hirschberg V et al (2022) Influence of surface-modified cellulose nanocrystal on the rheological, thermal and mechanical properties of PLA nanocomposites. Polym Bull 80:10193–10213. https://doi.org/10.1007/s00289-022-04556-w
Valantin M-A, Aubron-Olivier C, Ghosn J et al (2003) Polylactic acid implants (New-Fill)® to correct facial lipoatrophy in HIV-infected patients. AIDS 17:2471–2477. https://doi.org/10.1097/00002030-200311210-00009
Vert M, Chen J, Hellwich K-H et al (2020) Nomenclature and terminology for linear lactic acid-based polymers (IUPAC Recommendations 2019). Pure Appl Chem 92:193–211. https://doi.org/10.1515/pac-2017-1007
Wasanasuk K, Tashiro K, Hanesaka M et al (2011) Crystal Structure Analysis of Poly( <scp>l</scp> -lactic Acid) α Form On the basis of the 2-Dimensional Wide-Angle Synchrotron X-ray and Neutron Diffraction Measurements. Macromolecules 44:6441–6452. https://doi.org/10.1021/ma2006624
Wuisman PIJM, Smit TH (2006) Bioresorbable polymers: heading for a new generation of spinal cages. Eur Spine J 15:133–148. https://doi.org/10.1007/s00586-005-1003-6
Yu F, Fei X, He Y, Li H (2021) Poly(lactic acid)-based composite film reinforced with acetylated cellulose nanocrystals and ZnO nanoparticles for active food packaging. Int J Biol Macromol 186:770–779. https://doi.org/10.1016/J.IJBIOMAC.2021.07.097
Zabidi NA, Nazri F, Tawakkal ISMA et al (2022) Characterization of active and pH-sensitive poly(lactic acid) (PLA)/nanofibrillated cellulose (NFC) films containing essential oils and anthocyanin for food packaging application. Int J Biol Macromol 212:220–231. https://doi.org/10.1016/j.ijbiomac.2022.05.116
Zabidi NA, Nazri F, Tawakkal ISMA et al (2022) Characterization of active and pH-sensitive poly(lactic acid) (PLA)/nanofibrillated cellulose (NFC) films containing essential oils and anthocyanin for food packaging application. Int J Biol Macromol 212:220–231. https://doi.org/10.1016/J.IJBIOMAC.2022.05.116
Zhou L, Ke K, Yang M-B, Yang W (2021) Recent progress on chemical modification of cellulose for high mechanical-performance Poly(lactic acid)/Cellulose composite: A review. Compos Commun 23:100548. https://doi.org/10.1016/j.coco.2020.100548
Funding
Open access funding provided by Council for Scientific and Industrial Research. Financial support from the Department of Science and Innovation (DSI) Bioinnovation (DSI CON 2265/2021) and the Council for Scientific and Industrial Research (CSIR), is acknowledged.
Author information
Authors and Affiliations
Contributions
Conceptualisation: [Maya Jacob John]; Writing—original draft preparation: [Clara N. Nkuna]; Writing—review and editing: [Clara N. Nkuna], [Maya Jacob John], [Washington Mhike], [Mxolisi B. Shongwe]; Supervision: [Maya Jacob John], [Washington Mhike], [Mxolisi B. Shongwe], [Vincent Ojijo].
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nkuna, C.N., Mhike, W., Ojijo, V. et al. Biocomposites and bionanocomposites from poly(lactide) and cellulosic materials – a review. Cellulose 31, 4709–4732 (2024). https://doi.org/10.1007/s10570-024-05913-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-024-05913-w